2022 Volume 62 Issue 8 Pages 1725-1730
2022 Volume 62 Issue 8 Pages 1725-1730
Modelling of nitride precipitation during isothermal heating was conducted in duplex stainless steel which was rapidly cooled from high temperature before isothermal heating. Nitride precipitation is frequently harmful for the properties such as toughness or corrosion resistance in duplex stainless steels. The heat affected zone (HAZ) in the vicinity of fusion boundary in weldments tends to have more remarkable nitride precipitation because of rapid cooling from the ferrite phase enriched temperature. It was taken into considered in modelling of nitride precipitation phenomena that the growth of austenite phase from overcooled ferrite phase occurs concurrently in the HAZ in weldment of duplex stainless steel. Because the austenite phase has extremely higher solubility of nitrogen than the ferrite.
Employing 25%Cr duplex stainless steels containing the levels of nitrogen of 0.1% and 0.3%, the measurement and observation of nitride precipitation were conducted in the specimens isothermally heated at 873 K to 1073 K after rapidly cooled from 1653 K. The model to describe the change of nitride precipitation during isothermal heating considering the effect of growth of austenite phase was proposed and that was confirmed to be good fit to the experimental results.
Recently duplex stainless steels consisting of ferrite and austenite phase are widely used for various structural materials because of high performance for cost.1,2,3,4) The high performance in terms of mechanical property and corrosion resistance is obtained by the proper solution treatment without formation of harmful precipitates such as sigma phase or nitride during the manufacturing process in the duplex stainless steel.5,6,7,8,9)
However, in the welded portion it is indispensable to be heated at the high temperature where the phase transformation is remarkable, therefore the fraction of austenite phase can be changed to lower in heat affected zone (HAZ) by weld thermal cycles. The HAZ consisting of the small fraction of austenite phase can deteriorate in the mechanical performance and corrosion resistance due to that changing in microstructure. Therefore it is important to obtain the optimum austenite phase fraction in weldments. Many research works10,11,12,13,14,15,16) have been conducted about the microstructure change in HAZ of duplex stainless steels from the view point of chemical composition in steels and welding conditions. The deterioration of performance in the HAZ is mainly caused by the precipitation of harmful phase such as nitride or intermetallic compounds.15,16)
It is known that the dominant factors of nitride precipitation in the HAZ of duplex stainless steel is potential of austenite phase formation and cooling rate. Nitride precipitation phenomena in the HAZ occurs concurrently with the transformation of austenite phase from overcooled ferrite phase.17,18,19) Therefore the analysis by modelling or simulation of this phenomena is not always conducted easily. For example, a conventional software such as TC-PRISMA20) consisting of thermo-dynamic model dealing with multi alloying elements is employed for the simulation of various precipitation. Almost of simulation using such software is usefully applied for the precipitation phenomena in the single phase matrix because of difficulty to apply to concurrent precipitation. Therefore it is important to obtain the experimental findings regarding nitride precipitation with the concurrent decrease or increase in austenite phase during thermal cycles. In the previous work21) as the first step to the investigation of nitride precipitation in continuous cooling process in the HAZ, the amount of nitride precipitation during isothermal heating after rapidly cooled from the high temperature, where ferrite phase is enriched in duplex stainless steel, has been experimentally clarified. Subsequently in this work modelling of nitride behavior during isothermal heating after rapidly cooled from high temperature in duplex stainless steel was investigated. That is considered to be the changing process of the quenched microstructure to the equilibrium state at each temperature. In the next step this model can be applicable to obtain the microstructure in the HAZ cooled continuously from high temperature by the integration of the transformation in isothermal heating at each temperature along the cooling process.
The 25%Cr duplex stainless steels containing 0.1% and 0.3% nitrogen listed in Table 1 were used. Those were melted in laboratory, hot forged and hot rolled to plates of 12 mm in thickness in the range of 1373 to 1523 K. Then those were solution treated at 1373 K for 1800 s. Those plates were machined to the specimens of 11 mm in thickness, 11 mm in width and 60 mm in length. The specimens were heated at 1653 K for 3 s and cooled rapidly with argon gas shower to the various test temperatures. And then those are held for various durations at a constant temperature of 873 K to 1073 K and quenched with argon gas shower. Some of those were cooled directly to room temperature from 1653 K. The average cooling rate was approximately 100 K/s.
| C | Si | Mn | Ni | Cr | W | Mo | N | |
|---|---|---|---|---|---|---|---|---|
| Steel A | 0.02 | 0.17 | 0.47 | 7.11 | 25.20 | 1.95 | 2.97 | 0.33 |
| Steel B | 0.02 | 0.16 | 0.48 | 7.10 | 25.29 | 1.98 | 3.03 | 0.11 |
The heat treated specimens were evaluated in terms of nitride analysis. The amount of nitride was measured by the analysis of residue extracted using the chips obtained from the homogenously heated zone of the heat treated specimens. The chips with about 1 mm2 in cross section and 5 mm in length produced by machining were dissolved in 10%bromine alcohol solution to obtain extracted residue. The amount of N as nitride was measured by analyzing the amount of nitrogen included in the extracted residue. The amount of N as nitride [N]as Cr2N (mass%) was defined as the ratio of the amount of nitrogen included in the extracted residue to the mass of dissolved chips of steel. The volume fraction of nitride V(t)/V0 is obtained from the measured data of [N]as Cr2N by the above manner using the following equation.
| (1) |
Because it has been clarified the nitride in those specimens was identified as Cr2N and the shape was stick like in the previous report,21) a model was proposed which the nitride grows in just longitudinal direction with the constant area of cross section. Assuming that longitudinal growth is controlled by the diffusion of Cr atom in the ferrite phase, the length L(t) and the volume of nitride V(t) are shown by the following equations23) respectively.
| (2) |
| (3) |
When the nitride with the average volume of V(t) are dispersed with the number of density NP in the volume V0 of steel, the volume fraction of nitride is calculated by the following.
| (4) |
| (5) |
It was assumed that the number of density Np is according to the Boltzmann’s distribution23) shown as the following equation.
| (6) |
| (7) |
The model which the effect of the austenite phase growth during isothermal heating was taken in to consideration was proposed to be applied for the later stage where the austenite phase growth rather progressed. The growth austenite phase with large solubility of nitrogen during the isothermal heating contributes to reduce the supersaturation of nitrogen in ferrite phase in the duplex steel rapidly cooled from high temperature. The model of austenite phase growth has been already reported by the authors,22) where the volume fraction of austenite phase fγ(t) at a time t is described as the follows equation.22)
| (8) |
| (9) |
| Cr | Ni | N | kp0 (m−2) | Δum (J/mol) | Teq (K) | fo (%) |
|---|---|---|---|---|---|---|
| 25 | 7 | 0.3 | 4.0×1014 | 1950 | 1668 | 50 |
| 25 | 7 | 0.1 | 1.1×1014 | 1950 | 1618 | 0 |
Regarding the change of partitioning of alloying elements between the phases by the transformation of the ferrite to austenite phase, just nitrogen was taken into account in the model. Because nitrogen atom has larger mobility than the substitutional atoms such as chromium or molybdenum and has smaller partition coefficient between the phases than chromium. In addition in the model it was assumed that nitrogen partitioning between ferrite and austenite phase can instantly reach equilibrium condition because nitrogen atom has extremely large diffusiblility at high temperature in the ferrite phase where nitrogen was oversaturated.
To maintain the conservation of nitrogen mass in the whole of steel the nitrogen content in each phase have to satisfy the following equation perpetually.
| (10) |
Assuming partition ratio of nitrogen
| (11) |
| (12) |
Nitride precipitation concurrent with the austenite phase growth during isothermal heating process is calculated by applying ω(t) to Eq. (5) and using Eq. (4). The parameters necessary for calculation shown in Table 3 were employed, which are determined using thermodynamic data base.24)
| N (mass%) | Xneα (mass%) | pN | |||
|---|---|---|---|---|---|
| 700°C | 800°C | 700°C | 800°C | ||
| Steel A | 0.33 | 2.35×10−3 | 7.05×10−3 | 2.30×102 | 6.17×101 |
| Steel B | 0.11 | 2.96×10−3 | 5.92×10−3 | 6.42×101 | 4.87×100 |
Figure 1 shows the volume fraction of nitride in the 25%Cr-7%Ni-3%Mo-2%W-0.3%N steel during isothermally heated at various temperatures of 873 K to 1073 K after rapidly cooled from 1653 K. That steel had no nitride immediately after rapidly cooled to the room temperature from 1653 K but that had the nitride precipitation after the isothermal heating. The solid line in the figure shows the proportional relation between the logarithm of V(t)/V0 and heating time with the inclination of 1/2 and the increase of nitride was accordance with that line in the early duration. However, in the later duration the volume fraction of nitride was less than that proportional relation. It is considered the growth of nitride increased with the accordance to Eq. (4) and that model is applicable within the early duration. The growth rate constant kN was obtained referring the inclinations in the relations of the experimental data. The square of growth rate constant
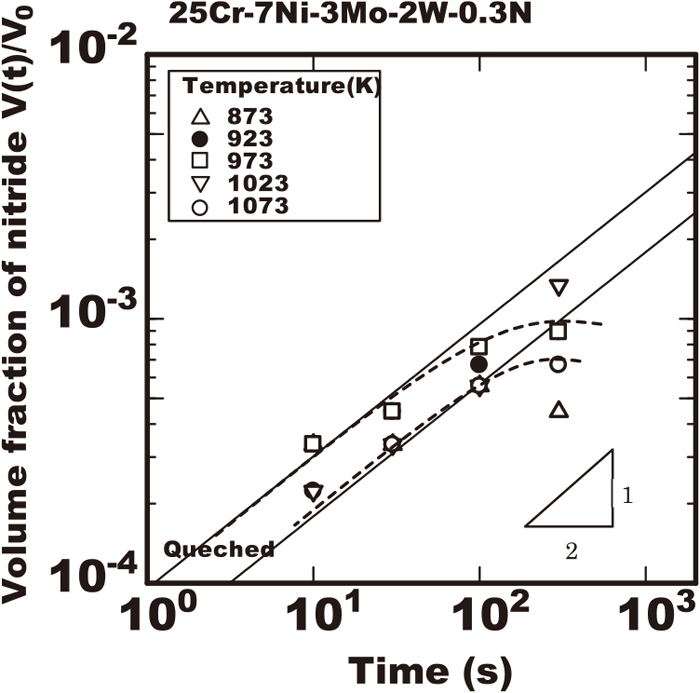
Change in amount of nitride measured with extracted residue during isothermal heating in 25Cr-7Ni-3Mo-2W-0.3N steel heated at various temperatures after rapid cooled from 1653 K.
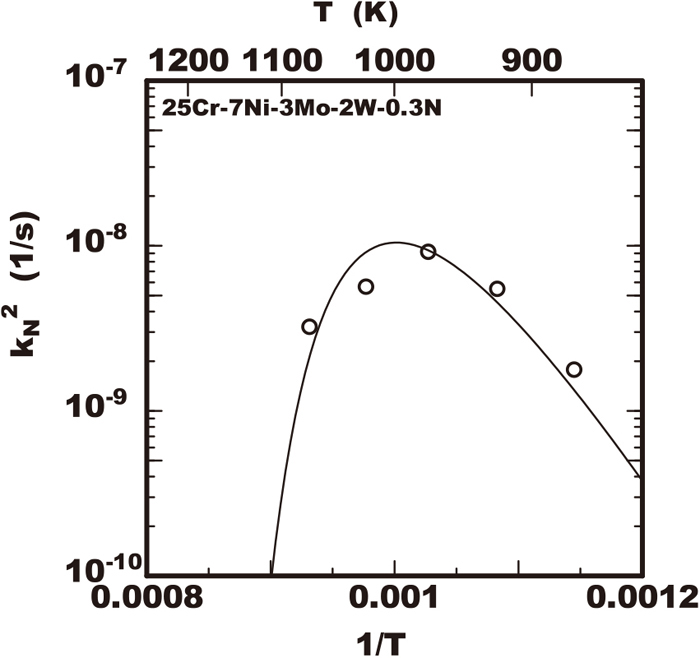
Growth rate constant of nitride determined with experimental data of 25Cr-7Ni-3Mo-2W-0.3N steel in various temperature.
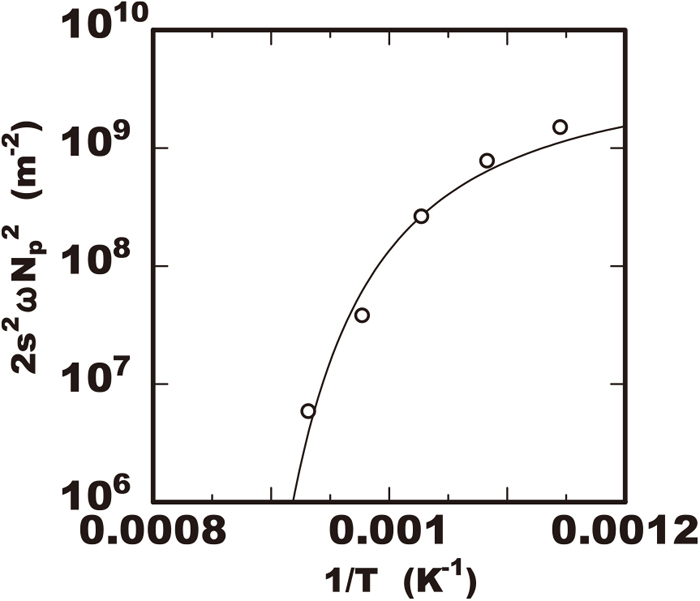
Term consisting of supersaturation and number of density of nitride in growth rate constant kN2 determined with experimental data of 25Cr-7Ni-3Mo-2W-0.3N steel in various temperature.
The following equation is obtained as a function of just temperature by transforming Eq. (4) to the formula with the time t in the left side.
| (13) |
The curve of the relation between the time and the temperature which the volume fraction of nitride reaches some critical value is calculated as a TTT (time-temperature-transformation) diagram using the above equation. The curve of solid line shown in Fig. 4 was the TTT curve calculated in the condition where volume fraction V(t)/V0 reaches 5×10−4 and that had approximately good fit to the experimental results plotted in that figure.
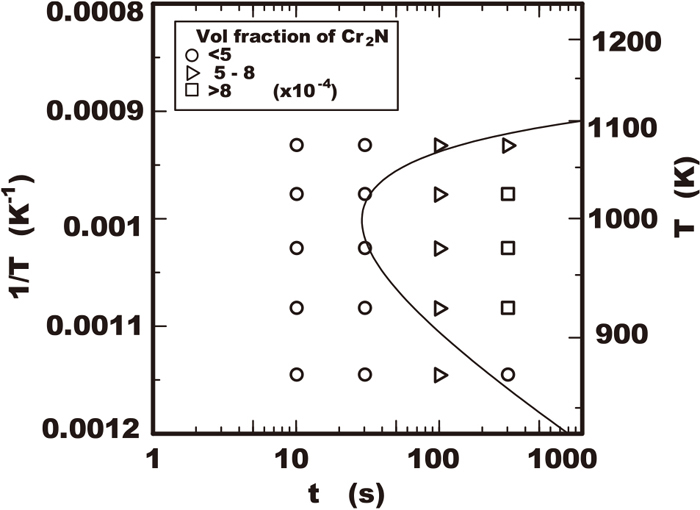
Calculated TTP curve of nitride fraction of 5×10−4 using proposed model with measured data of nitride precipitation in 25Cr-7Ni-3Mo-2W-0.3N steel.
In the later stage of isothermal heating the experimental data of volume fraction of nitride tended to be lower comparing to the calculated line by Eq. (4) with a constant kN. In that case the effect of austenite phase growth during isothermal heating was not taken in to consideration. It is understood the model including the effect of austenite phase growth is necessary for reproducing the experimental data of growth of nitride in the later stage.
The curves of solid line in Fig. 5 shows the calculated results of nitride growth at 973 K and 1073 K using the model concurrent with the austenite phase growth. The calculated results reproduced quantitatively the experimental data that in later stage the growth rate of nitride gradually reduced as well as that in early stage the amount of nitride increased proportionally to the heating time.
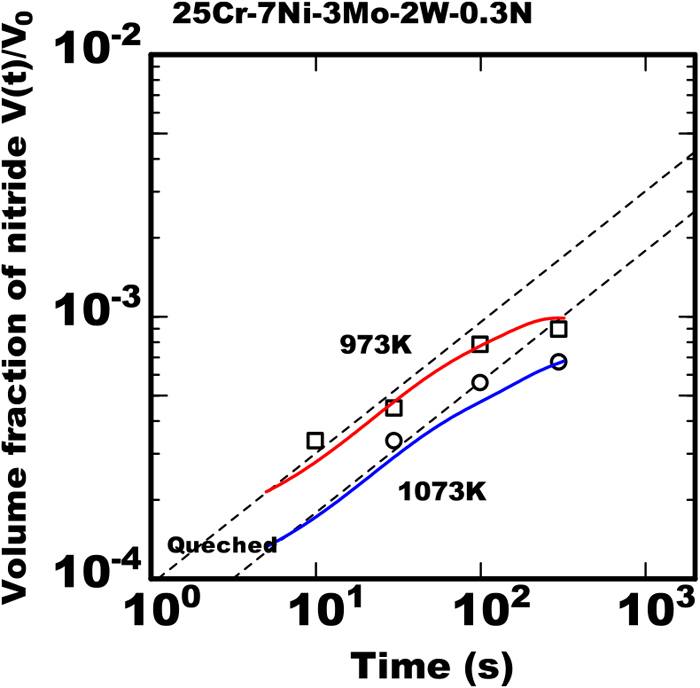
Calculated results with model proposed and measured data of change in amount of nitride during isothermal heating in 25Cr-7Ni-3Mo-2W-0.3N steel heated at various temperature after rapid cooled from 1653 K. (Online version in color.)
Figure 6 shows the experimental data of volume fraction of nitride during isothermal heating in the 25%Cr-7%Ni-3%Mo-2%W-0.1%N steel. That steel had much of the volume fraction of nitride of 4.2×10−3 after rapidly cooled to the room temperature from 1653 K even without isothermal heating. The amount of nitride fraction increased slightly and gradually saturated during heating at 973 K. That turned over to reduce after the gradual increase in the long heating duration at 1073 K.
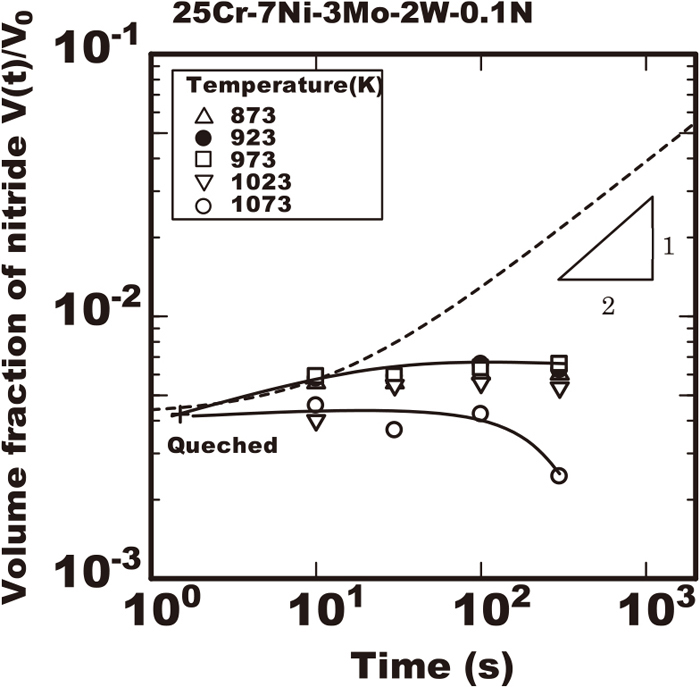
Change in amount of nitride measured with extracted residue during isothermal heating in 25Cr-7Ni-3Mo-2W-0.1N steel heated at various temperatures after rapid cooled from 1653 K.
In the 25%Cr-7%Ni-3%Mo-2%W-0.1%N steel containing the lower nitrogen as an alloying element, the experimental data of volume fraction of nitride had no fit to the model referring Eq. (4) even in the early stage of isothermal heating. That is considered to be due to the large effect of rapid growth of austenite phase during heating in the early stage. In that steel much of nitride was detected in the specimen just quenched to room temperature from the ferrite single phase temperature even without isothermal heating. From the results it is considered rather amount of nitride precipitated during cooling in that quenching process. Hence the growth rate constant kN′ was determined by fitting of experimental data of volume fraction of nitride between 0 and 10 s to Eq. (4) as a matter of convenience. The results of calculation by applying the value kN′ to Eq. (4) at 973 K and 1073 K are shown as the curves of broken line in Fig. 7 with experimental data. The curve was completely deviated from experimental dada after the heating time of 10 s. It is understood to be due to the remarkable decrease of ω by the effect of austenite phase growth. In that steel the model including the effect of austenite growth is necessary from the initial stage.
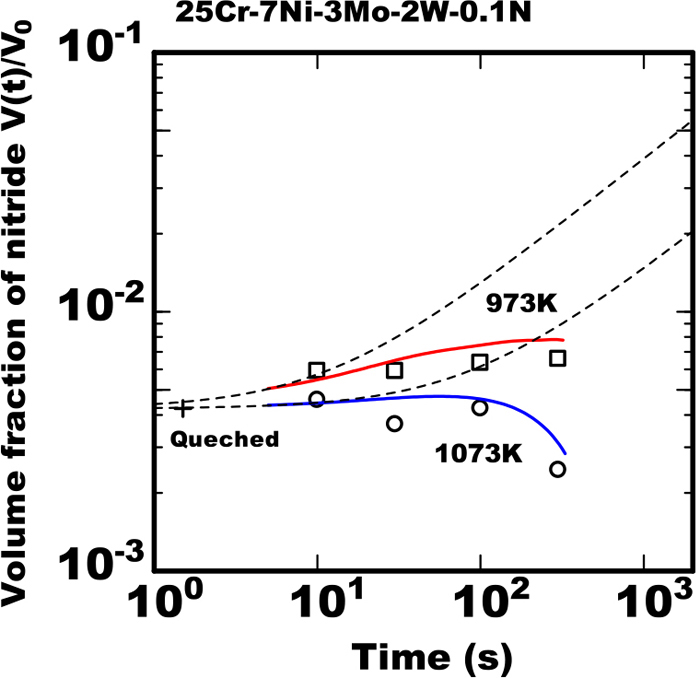
Calculated results with model proposed and measured data of change in amount of nitride during isothermal heating in 25Cr-7Ni-3Mo-2W-0.1N steel heated at various temperature after rapid cooled from 1653 K. (Online version in color.)
The calculated results using the model with the concurrent of the austenite phase growth is shown as solid curves in Fig. 7. In the 0.1%N steel the calculated results reproduced quantitatively the experimental data that in early stage the amount of nitride increased slightly during heating as well as that in the later stage the amount saturated at 973 K and turned over to reduce at 1073 K.
Nitride precipitation in duplex stainless steel rapidly cooled from high temperature can be promoted by the high supersaturation of nitrogen in ferrite phase. That precipitation can be prevented by the growth of austenite phase due to reducing that supersaturation of nitrogen. It was clarified the model proposed quantitatively reproduced the change in the experimental data of nitride precipitation during isothermal heating. Hence the following presupposed items in the model were considered to be supported by that fact.
a) The length in stick shaped nitride increases proportionally to the term of
b) The number of nucleation density and the area of cross section of nitride are constant during heating.
c)-1 Supersaturation of nitrogen ω(t) is constant in the ferrite phase in the early stage of precipitation.
c)-2 Supersaturation of nitrogen ω(t) in the ferrite phase is the function of the fraction of austenite phase in the later stage.
The above items a) and b) correspond to that the amount of nitride obtained as the experimental result increased proportionally to the square root of heating time as described in Eq. (4) with a constant kN in the 0.3%N steel. That means the value of coefficient in Eq. (4)
The reasons of validity those items was considered from view points of the dominant mechanism of nitride precipitation. The increase of the fraction of austenite phase was very slow in the early stage of isothermal heating in the 0.3%N steel because that steel already had sufficiently large fraction of about 50% of austenite phase immediately after rapidly cooled to the isothermal heating temperature. Therefore the condition of constant ω in the above item c)-1 was applied in the early stage in the 0.3%N steel. Contrary in the later stage of 0.3%N steel or in the almost stage of 0.1%N steel the growth rate of austenite phase was large. Hence applying the above item c)-2 was necessary to be applied and the effect of remarkable growth of austenite phase on the supersaturation of nitrogen in ferrite phase was reflected to the calculation. The item c)-2 means the combination of Eqs. (8), (11) and (12) are reflected to Eq. (4). The 0.1%N steel had almost no fraction of austenite phase immediately after rapidly cooled to the isothermal heating temperature and the austenite phase increased rapidly during the heating. Figure 8 shows the calculated result at 1073 K assuming without taking the austenite growth into consideration in the model proposed in the 0.1%N steel. Even without the austenite phase growth the gradual decrease of growth of nitride was reproduced but the turning over to the reduction of the amount of nitride was not reproduced in the calculated result. The difference of the amount of nitride in the calculated results between with and without the austenite phase growth indicates the quantitative effect of austenite phase growth on the nitride precipitation.
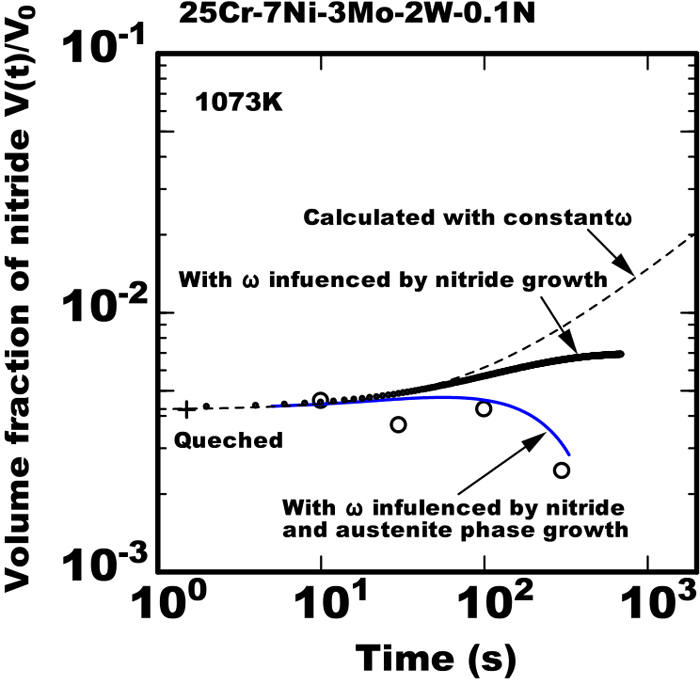
Calculated results using model with and without considering austenite phase growth and measured data of change in amount of nitride during isothermal heating in 25Cr-7Ni-3Mo-2W-0.1N steel heated at constant temperature after rapid cooled from 1653 K. (Online version in color.)
The mechanism of the turning over to the reduction of the amount of nitride is explained qualitatively as the following referring the other work17) reported. In case of the large supersaturation in ferrite phase in the early stage the nitride can precipitate rapidly but in the stage after the sufficient austenite phase growth the nitride precipitation can be excess than the equilibrium amount therefore some amount of nitride can be dissolved. This qualitative consideration is the same as the above item c)-2 in the model proposed in this work therefore this phenomena is understood to be reproduced quantitatively. As shown in Fig. 9 the value of the supersaturation ω calculated by the model decreased below 0 in the long term of heating at 1073 K. It is explained that is the reason the amount of nitride turned over to the reduction in the later stage using the model.
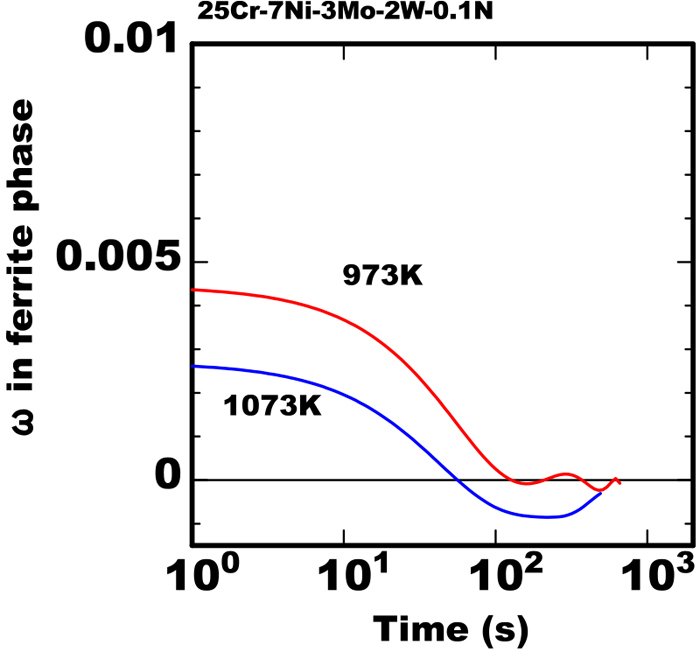
Calculated results of change in value of supersaturation ω during isothermal heating in 25Cr-7Ni-3Mo-2W-0.1N steel heated at constant temperature after rapid cooled from 1653 K. (Online version in color.)
From the results it was confirmed the validity of the reflection of the dominant factors of nitride precipitation in the model proposed was supported in the duplex steel rapid cooled from high temperature.
The following results were obtained by the investigation regarding the modelling of nitride precipitation during isothermal heating after rapidly cooled from ferrite temperature(1653 K) in the 25%Cr duplex stainless steels containing 0.1% and 0.3% nitrogen.
(1) The amount of nitride increased proportionally to the square root of heating time in the early stage in the 0.3%N steel. Contrary that amount during heating deviated from the proportional increase to the square root of heating time in the 0.1%N steel.
(2) The growth rate constant was obtained experimentally from the results at various heating temperatures in the early stage in the 0.3%N steel and the model as a function to describe the effect of temperature on the growth rate constant was proposed.
(3) The model considering the effect of growth of austenite phase to describe the change of nitride precipitation during isothermal heating was proposed and that was confirmed to be good fit to the experimental results.