2023 Volume 63 Issue 11 Pages 1817-1824
2023 Volume 63 Issue 11 Pages 1817-1824
High-proportion pellet smelting is the direction of optimization of blast furnace burden structures in the future. In this paper, the effect of SiO2 content on the softening and dropping properties of magnesia flux pellets was studied. And the influence of SiO2 content on the mineralogical composition and microstructure of magnesia flux pellets was analyzed, which clarified the reason for the air permeability of the middle and lower columns of magnesia flux pellets becoming poor during smelting. The results show that with increasing SiO2 content, the T10 (softening start temperature) and TS (melting start temperature) of the column gradually decrease, and the melting range increases; in particular, T10 and Ts decrease dramatically when the SiO2 content exceeds 6%. The maximum pressure difference increases from 15.0 kPa to 20.7 kPa, and the characteristic value increases from 644.8 kPa·°C to 1334.8 kPa·°C when the SiO2 content of magnesia flux pellets increases from 6% to 8%. The mineralogical composition difference between low-silica magnesia flux pellets (SiO2=3.5%) and high-silica magnesia flux pellets (SiO2=7.0%) at different temperatures is caused by a large amount of unreacted SiO2 in high-silica magnesia flux pellets. SiO2 and silicate produce a large amount of liquid phase at low temperature, which reduces TS. In addition, the dropping temperature of high-silica magnesia flux pellets is higher than that of low-silica magnesia flux pellets. It’s due to the existence of particulate SiO2 in the primary slag, which causes the slag to become sticky and makes it difficult to separate the slag from iron.
The Chinese iron and steel industry is mainly based on the blast furnace-converter long process that relies on coal fossil energy,1,2) which is a typical high carbon emission industry. Every ton of steel produced emits approximately 1.8 tons of carbon dioxide,3,4,5) accounting for 16% of the country’s total carbon emissions.6) In the whole process, blast furnace ironmaking is the link with the highest energy consumption and the largest SiO2 emission, accounting for approximately 75% of the whole steel production process.7) Therefore, energy conservation and emission reduction of blast furnaces is an important guarantee to achieve the strategic goal of “carbon peak and carbon neutrality” in China.
Magnesia flux pellets have become the high-quality raw material of blast furnaces and have high grade, high efficiency, low energy consumption and environmental friendliness because of their good metallurgical properties.8,9,10,11) For the international situation of blast furnace ironmaking, countries such as North America and Europe mainly use flux based pellets in their blast furnace burden structure, and have achieved good results.12) The large proportion of magnesia flux pellets used in the blast furnace can not only optimize the burden structure of the blast furnace but also help to reduce the source and process of pollutants in the steel industry, effectively reduce the coke ratio and fuel ratio of the blast furnace, and improve the gas utilization rate. Thus, high-efficiency and low-consumption smelting of blast furnaces is realized.13,14,15) However, the SiO2 content of magnesia flux pellets produced is higher than that of foreign countries due to the complex raw materials and high silicon content of domestic pelletizing. When the proportion of high-silica magnesia flux pellets is increased, it is easy to cause changes in the structure of the soft melting band of the blast furnace. This will cause an increase in the blast furnace fuel ratio and the pressure difference of column. These problems seriously restrict the increase in the proportion of magnesia flux pellets in the furnace. Therefore, the effect of SiO2 on the softening-melting and dropping behavior of magnesia flux pellets should be studied, which clarifies the mechanism of poor permeability caused by high-silica magnesia flux pellets.
At present, many scholars have mainly studied the reduction behavior of flux pellets and the difference between acid pellets and magnesia acid pellets in terms of softening-melting performance. Srinivas Dwarapudi et al.16,17) studied the effect of basicity and MgO content on the quality and microstructure of magnetite pellets. They found that the reduction degree of pellets at high temperature was significantly improved after the added flux was obtained. Geassy et al.18) studied the reduction behavior of flux pellets at 1023–1273 K. The results showed that the reduction rate of flux pellets was significantly higher than that of acid pellets in the early stage, and the reduction rate decreased in the later stage due to the formation of magnesia wustite. Kemppainen et al.19) studied the softening behavior of acid pellets and magnesia acid pellets. The results showed that the main reason for pellet deformation is the dissolution of the wustite phase and the separation of the wustite phase into a single wustite phase particle in the pellet. The separation of the wustite is inhibited because of the addition of flux. Iljana et al.20) compared the softening-melting performance of sinter, magnesia pellet, and acid pellet. It was found that the reason for the reduction degree and softening performance of sinter being better than that of pellets is that the slag phase formed by the reduction of sinter at high temperature is mainly dicalcium silicate and magnesia wustite. The melting point of wustite is significantly improved because most MgO is dissolved in the wustite. In addition, the sinter particles can still maintain a good porosity during the softening process. Therefore, the softening property and reduction degree of sinter are better. The reduction degree and softening resistance of acidic pellets are worse than those of magnesia pellets because the high content of SiO2 in acid pellets and the absence of magnesia fluxes form a large number of low melting point olivine phases. And the dissolution of wustite can be stopped by MgO. Therefore, the softening starting temperature of magnesia pellets is higher than that of acid pellets. It can be seen that MgO can improve the softening-melting property, while SiO2 has the opposite effect. Silva et al.21) studied 16 pellets and analyzed the role of CaO and MgO in the pellets. The results showed that CaO and MgO promote the formation of dicalcium silicate and wustite and improve the melting point of the slag phase. In addition, pellet pore plugging is also avoided. Semberg et al.22,23) studied the mineralization reaction and evolution law of quartz, olivine and other minerals in the process of pellet reduction and the change rule of iron oxide. Wang et al.24) analyzed the softening-melting behavior of the burden column based on the burden structure of a high proportion high-silica flux pellet. It was found that the softening and melting behavior of sinter and high-silica flux pellets can be synergistically optimized when used together, which could improve the permeability of the burden column. The smelting behavior of ordinary acid pellets, magnesia acid pellets, and flux pellets in the soft melting zone of blast furnaces have been studied by previous scholars. But the research on the softening-melting and dropping of magnesia flux pellets is weak, especially research on the smelting of high-silica magnesia flux pellets in China. Therefore, the influence of SiO2 on the softening-melting property of magnesia flux pellets and the evolution law of mineral phase structure in the process of reduction were studied by using a melting-dropping furnace. That clarified the reason for the worsening softening-melting property of high-silica magnesia flux pellets. This will lay a theoretical foundation for the development of a large proportion of high-silica magnesia flux pellet smelting technology suitable for Chinese resource structures.
The iron concentrate powder, flux and bentonite used in the experiment are all from an iron and steel enterprise in Hebei. Their chemical compositions are presented in Table 1. The proportion of Yanshan is fixed at 5.0%. The SiO2 content in the pellet is adjusted to 3.5%, 4.0%, 5.0%, 6.0%, 7.0% and 8.0% by Qian’an refined powder and then Zunhua refined powder. The MgO content in the pellet is adjusted to 2.0% using dolomite. The basicity is adjusted to 1.0 using limestone. The ball is made using a disc pelletizer, and the green ball is preheated and roasted. The preheating temperature is 900°C, and the roasting temperature is 1250°C. It is taken out and cooled for standby after the roasting is completed. The chemical composition of the roasted pellets is shown in Table 2.
| Sample | TFe | FeO | SiO2 | CaO | MgO | Al2O3 | TiO2 | Burning loss |
|---|---|---|---|---|---|---|---|---|
| Qian’an refined powder | 61.20 | 26.59 | 11.18 | 0.70 | 0.90 | 0.73 | 0.19 | −2.00 |
| The proportion of Yanshan | 65.36 | 26.05 | 7.29 | 0.62 | 0.36 | 0.34 | 0.059 | −2.90 |
| Zunhua refined powder | 67.50 | 29.77 | 2.83 | 0.40 | 0.49 | 1.03 | 0.57 | −2.78 |
| Dolomite | – | – | 0.96 | 29.86 | 22.32 | 0.24 | 0.045 | 30.00 |
| Limestone | – | – | 1.72 | 53.71 | 0.80 | 0.74 | 0.02 | 42.58 |
| Bentonite | – | – | 55.96 | 4.16 | 2.01 | 11.73 | – | 12.46 |
| Pellet | TFe | SiO2 | CaO | MgO | Al2O3 | TiO2 | R |
|---|---|---|---|---|---|---|---|
| 1#SiO2=3.5% | 61.46 | 3.47 | 3.51 | 2.00 | 1.05 | 0.50 | 1.01 |
| 2#SiO2=4.0% | 60.78 | 3.99 | 3.98 | 2.00 | 1.04 | 0.47 | 1.00 |
| 3#SiO2=5.0% | 59.41 | 5.01 | 4.98 | 2.00 | 1.01 | 0.42 | 0.99 |
| 4#SiO2=6.0% | 58.04 | 5.98 | 6.02 | 2.00 | 0.98 | 0.37 | 1.01 |
| 5#SiO2=7.0% | 56.67 | 6.97 | 7.02 | 2.00 | 0.95 | 0.32 | 1.01 |
| 6#SiO2=8.0% | 55.30 | 8.03 | 7.99 | 2.00 | 0.92 | 0.27 | 1.00 |
To simulate the actual production conditions of the blast furnace, graphite crucibles with an inner diameter size of 75 mm and a depth of 175 mm and a drip hole of 19–8 mm at the bottom were required. In the process of sample loading, 40 g and 80 g coke with particle sizes of 10.0–12.5 mm should be loaded into the upper and lower parts of the graphite crucible, respectively. And 500 g magnesia flux pellets with particle sizes of 10–12.5 mm should be loaded into the middle of the graphite crucible, that sample heights of approximately 60 mm. The graphite crucible is put into the melting-dropping furnace for the experiment after the sample is loaded. The furnace volume is 100 mm×650 mm, the load is 2 kg/cm2, and the heating rate and gas composition are shown in Fig. 1. The melting-dropping furnace measuring device is shown in Fig. 2. Throughout the experiment, the temperature, shrinkage, and differential pressure of the specimen are recorded.


In the experiment, the parameters for evaluating the soft melt drop performance of iron ore under high temperature load reduction were mainly as follows: the temperature at which the volume shrinkage rate is 10% is defined as the beginning of softening temperature (T10). The temperature at which the volume shrinkage rate is 40% is defined as the end of softening temperature (T40). And the temperature difference between the two was the softening interval. The temperature at the inflection point of the sharp rise in pressure difference is defined as the start melting temperature (TS). The temperature at which the first drop of iron drops falls is the drop temperature (Td). And the temperature difference between the two is the melting interval. The S characteristic value is the integration of the pressure difference in the melting interval over temperature, which is used to characterize the air permeability.
2.3. Microscopic InspectionThe magnesia flux pellets with 3.5% and 7.0% SiO2 content are put into the melting-dropping furnace. The heating rate and gas composition are shown in Fig. 1. After the temperature rises to 1150°C, 1250°C, 1300°C, 1350°C and 1390°C, the temperature stops rising. Then, N2 at a rate of 10 L/min is sent through the melting-dropping furnace for rapid cooling to room temperature. The mineral phase and microstructure of samples at different temperatures are analyzed by a D/MAX2500PC X-ray diffractometer and Quanta 650 SEM‒EDS.
Figure 3(a) shows that the T10 of magnesia flux pellets decreases gradually with increasing SiO2 content. When the SiO2 content is not more than 6%, T10 changes little. However, when the SiO2 content is 7% and 8%, T10 decreases greatly and is 1144°C and 1124°C, respectively, which are 23°C and 43°C lower than that of magnesia flux pellets with 3.5% SiO2 content. The main reason is that during the roasting process, the pellets with high SiO2 had poor crystallization capacity, a more liquid phase and more pores.25) It shrinks more easily under high temperature and load conditions.

The TS of magnesia flux pellets decreases gradually with increasing SiO2 content. Especially when the SiO2 content is 7% and 8%, the TS is 1243°C and 1229°C, respectively. The magnesia flux pellet with 3.5% SiO2 content decreases by 74°C and 88°C with increasing SiO2 content. The main reason is that the formation of a large amount of silicate phase is promoted by the increase in SiO2 content. The silicate undergoes a peritectic reaction with unreacted SiO2 to generate a large amount of liquid phase. That makes the deformation of the high-silica magnesia flux pellet larger. Therefore, TS is lower. The drop temperature decreases first and then increases with increasing SiO2 content, and the melting range shows a trend of widening. This shows that the slag becomes sticky, which inhibits the carburization and polymerization of metallic iron and makes it difficult to separate slag and iron. This is also the main reason why the shrinkage rate curve of the column with a SiO2 content of 7.0% slows down in the later stage, and the pressure difference fluctuates near the peak, which results in the poor permeability of the blast furnace, as shown in Figs. 3(c) and 3(d).
Figure 3(b) shows that the maximum pressure difference and characteristic value of magnesia flux pellets increase gradually with increasing SiO2 content. In particular, the maximum pressure difference and characteristic value increase sharply when the SiO2 content exceeds 6%. When the SiO2 content of the magnesia flux pellet increases from 6% to 8%, the maximum pressure difference increases from 15.0 kPa to 20.7 kPa and the characteristic value increases from 644.8 kPa·°C to 1334.8 kPa·°C, which significantly affects the permeability of the burden column. The main reason is that there are many primary slags generated, and the slag phase viscosity is large, which stays in the coke layer and does not easily drop. This results in a large increase in the maximum pressure difference and characteristic value of the magnesia flux pellet, which results in the deterioration of the permeability of the burden column. Therefore, the SiO2 content of magnesia flux pellets should not exceed 6%. Otherwise, it will cause the position of the soft melting zone to move up and the permeability of the burden column to become poor, thus making the operation of the blast furnace unstable.
3.2. XRD AnalysisFigure 4 shows that the reduced mineral phase composition of the magnesia flux pellet (SiO2=3.5%) is mainly metallic iron, magnesia wustite, iron olivine and silicate slag phase at 1150°C. In other words, the pellets generate a large amount of silicate mineral phase after CaO and MgO are added, which reduces the reaction between wustite and SiO2. That makes the amount of iron olivine mineral phase produced reduce and improves the porosity of the pellets. When the temperature rises to 1250°C, the eutectic phase melting reaction occurs in Fe2SiO4, CaMgSi2O6, Ca3MgSi2O8, CaAl2Si2O8, CaSiO3 and SiO2, such as Eqs. (1), (2) and (3). This causes the gangue phase to change from the solid phase to the liquid phase. Due to the low silica content of the magnesia flux pellet, the flux CaO and MgO gradually melt into the liquid phase during the extrusion flow process of the liquid phase, which makes the high melting point melilite solidify. This is consistent with the XRD results at 1250°C. The softening and melting properties are affected directly by the mineral phase of reaction formation. This is also the reason for the sharp rise in pressure difference and high temperature of the low-silica magnesia flux pellet. In addition, the liquid phase produced in the reduction process of magnesia flux pellets is not only the olivine phase but also the silicate phase. In the range of 1250°C–1390°C, the magnesia wustite is gradually reduced, and the viscosity of the primary slag is improved by MgO entering the liquid phase, while FexO is reduced to metallic iron. At 1390°C, the magnesia wustite is completely reduced, and only Fe, FeO and melilite remain in the mineral phase. The slag phase with melilite as the main mineral phase has good fluidity, and the slag and iron are easy to separate. Thus, the permeability of the burden column is improved.
| (1) |
| (2) |
| (3) |
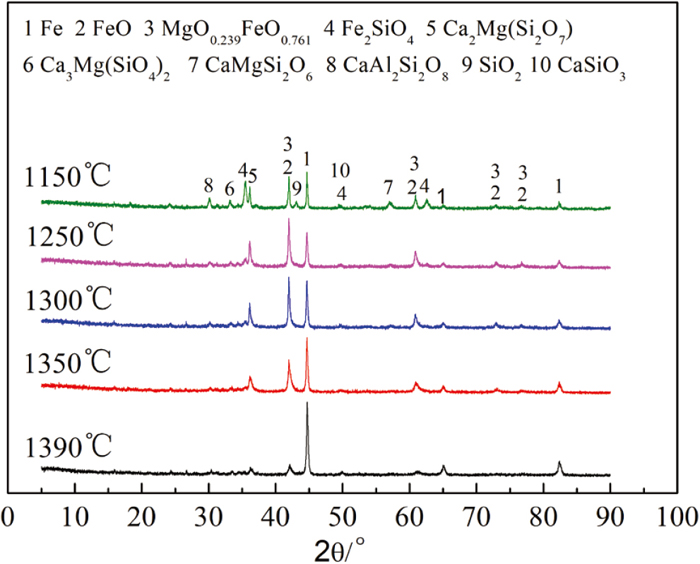
As shown in Fig. 5, at 1150°C, the mineral phase composition of the high-silica magnesia flux pellet (SiO2=7.0%) is the same, but the amount of silicate phase generated increases. And there is still a large amount of silicate phase in the range of 1250°C–1300°C. This is mainly because a large amount of unreacted SiO2 continues to react with surrounding oxides to generate a silicate phase, and the silicate phase continues to react with SiO2 to generate a large amount of liquid phase. Therefore, the permeability of the burden column is worse due to the formation of a large amount of liquid phase, and the pores are blocked by the flowing slag phase, which decreases the TS of the high-silica magnesia flux pellet. Continuing to raise the temperature, the reduction of magnesia wustite is completed, and a large amount of metallic iron is generated. At the same time, there is a large amount of SiO2 in the slag phase, which increases the primary slag viscosity and the difficulty of slag and iron separation. This will result in the rise of the drop temperature of the burden column, the sharp deterioration of the permeability of the burden column and the rise of the pressure difference, which makes the blast furnace unable to operate stably.

It can be seen from the Fig. 6 that the metallization rate of the high-silica magnesia flux pellet is significantly higher than that of the low-silica magnesia flux pellet at 1150°C. A large number of pores are generated by liquid phase shrinkage during cooling because many liquid phases are produced during the roasting process of high-silica magnesia flux pellets. Therefore, the reducibility of the high-silica magnesia flux pellet is better than that of the low-silica magnesia flux pellet in the early stage of reduction.
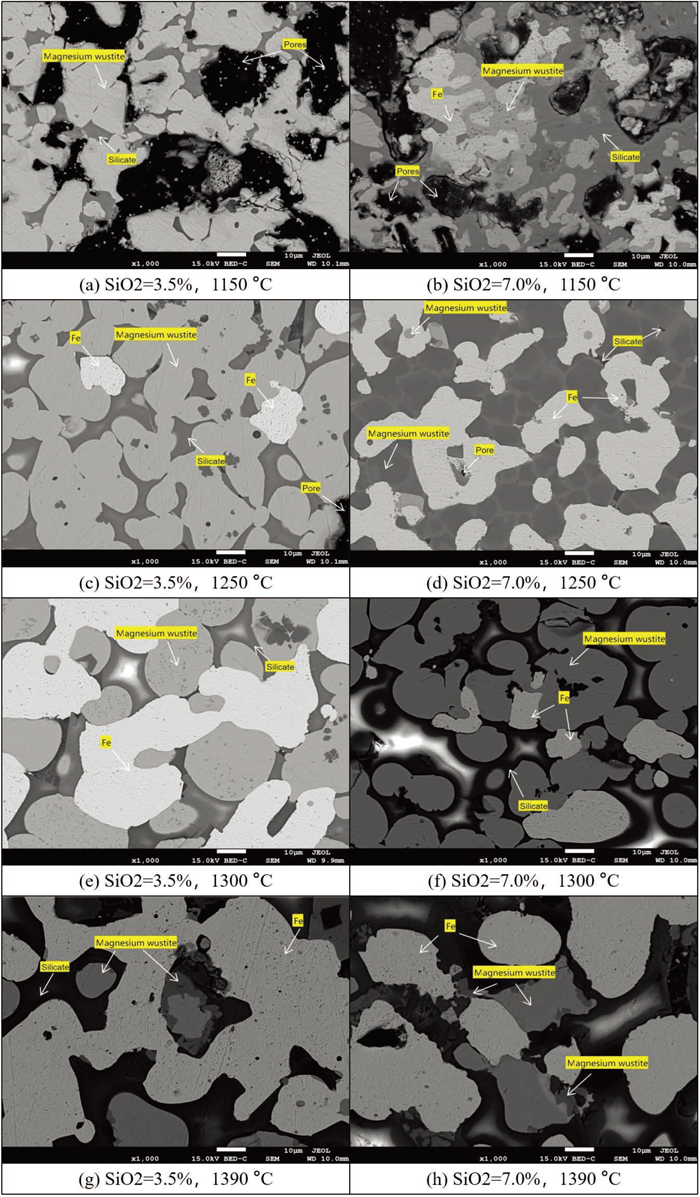
At 1250°C, the small pores and edge pores of the high-silica magnesia flux pellet begin to disappear due to high-temperature sintering. The content of Mg in the slag phase is higher, which causes the precipitation of the olivine phase in the slag phase. However, with increasing temperature, there is a large amount of liquid phase, which softens the pellets, worsens the permeability of the burden column, and reduces the reduction performance. According to EDS analysis, there is a large amount of Al in the magnesia wustite (Fig. 7), which reduces the melting point of the magnesia wustite. Thus, this can make a large number of magnesia flux pellets disappear. In addition, there is a small amount of magnesia wustite on the metallic iron, and the content of Mg in magnesia wustite is approximately 3.0–4.4%. And the content of Mg in magnesia wustite outside the metallic iron is approximately 1.0%. This indicates that Mg is enriched on the unreduced wustite when the magnesia wustite is reduced to metallic iron. The structure of magnesia wustite in low-silica magnesia flux pellets gradually becomes circular, and the slag phase is gradually filled around the perimeter, which further reduces the porosity in the internal structure of low-silica magnesia flux pellets. It also shows that the slag phase around the magnesia wustite has begun to melt.26)

With the further increase in temperature, the structure of magnesia wustite of high-silica magnesia flux pellets and that of low-silica magnesia flux pellets are spherical and surrounded by slag phase. The magnesia wustite becomes larger than that at 1250°C, and its structure is very dense with almost no pores. However, in general, the porosity of low-silica magnesia flux pellets is higher than that of high-silica magnesia flux pellets, which makes the internal metallization rate of low-silica magnesia flux pellets significantly higher than that of high-silica magnesia flux pellets.
At 1390°C, the metallization rate of low-silica magnesia flux pellets is significantly higher than that of high-silica magnesia flux pellets. The metallic iron in low-silica magnesia flux pellets is connected and mature, which is easier to drop. However, the metallic iron of high-silica magnesia flux pellets is not connected. This is mainly because the existence of SiO2 particles in the slag phase causes the primary slag to become sticky, which is not favorable to the carburization and polymerization of metallic iron. This is consistent with the research results of Higuchi et al.27) In other words, the dropping temperature is affected by two factors: one is the crystal in the slag, and the other is the viscosity of the slag. Therefore, the dropping temperature of high-silica magnesia flux pellets is higher than that of low-silica magnesia flux pellets.
(1) With increasing SiO2 content, the softening-melting performance of magnesia flux pellets worsens. In particular, when the SiO2 content is greater than 6%, the TS of magnesia flux pellets changes abruptly, the characteristic value and the maximum pressure difference increase sharply, and the permeability of the burden column deteriorates significantly. Therefore, the SiO2 content of magnesia flux pellets should not exceed 6% in the smelting process of high-proportion pellets.
(2) There is a large amount of unreacted SiO2 in the high-silica magnesia flux pellets, which undergo a eutectic phase melting reaction with the silicate phase at high temperature to form a liquid phase. However, the low-silica magnesia flux pellets do not contain SiO2 particles at high temperature. The liquid phase generated at low temperature reacts with CaO and MgO to convert into the high melting point of the melilite phase. Therefore, the Ts of low-silica magnesia flux pellets is higher than that of high-silica magnesia flux pellets.
(3) The reason for the increase in the dripping temperature of high-silica magnesia flux pellets is the presence of SiO2 particles in the primary slag, which increases the viscosity of the primary slag, which is not conducive to carburizing and polymerization of metallic iron.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
No data were used for the research described in the article.
The authors give sincere thanks for the financial support from the National Natural Science Foundation of China (U20A20271); Natural Science Foundation of Hebei Province (E2020209184); Science and Technology Project of Tangshan City (20150217C); and Science and Technology Research Project of the University of Hebei Province (ZD2021084).