2023 Volume 63 Issue 12 Pages 1927-1940
2023 Volume 63 Issue 12 Pages 1927-1940
The calcium treatment was the most popular and effective method to modify inclusions in the molten steel. For the modification of oxide inclusions, the partial liquid inclusion was suggested to avoid the nozzle clogging caused by solid inclusions, as well as the formation of hardly removed large liquid inclusions in the Al-killed Ca-treated steel. During the calcium treatment process, the added Ca firstly modified Al2O3 inclusions to liquid CaO–Al2O3 for T.Ca<T.O in steel, while the composition of liquid CaO–Al2O3 was little influenced by the T.Ca/T.O ratio for T.Ca>T.O in steel. Meanwhile, the CaS/(CaO+Al2O3) in inclusions increased with a higher (T.Ca-T.O)/T.S ratio in steel. For the sulfide inclusion modification, the calcium treatment retarded the precipitation of linear sulfides along the grain boundary of the steel matrix and lower the deformability of CaS during the rolling process due to the higher hardness of CaS. The length of sulfides increased with a higher T.Ca content, while it decreased with lower T.O and T.S contents in steel. Influence factors of the calcium yield of the calcium treatment were summarized to steadily increase the calcium yield, including steel composition, temperature, feeding speed, etc. An empirical equation between the [Ca], T.Ca, T.O, T.S, and [Al] was fitted to predict the [Ca] content in steel. During solidification and cooling processes of Al-killed Ca-treated steels, inclusions transformed from liquid CaO–Al2O3–MgO in the molten steel to Al2O3–MgO–CaS in the solid steel due to the equilibrium change. Moreover, the focus on the calcium treatment in the future was proposed.
The control of non-metallic inclusions is one of the most important issues of the clean steel.1,2,3,4,5,6,7,8) Fragile inclusions in alloy structural steels lead to the cracking failure of steel products.9,10,11,12,13) Sulfide inclusions precipitate along grain boundaries in free-cutting steels resulted in the fatigue damage of products.14,15,16) Large inclusions formed by the collision and growth in the molten steel may lead to defect detection incompatibility of steel products.17) Therefore, the characteristic of non-metallic inclusions in steel are directly related to the quality and performance of steel products. Moreover, high melting point inclusions result in the clogging of the submerged entry nozzle during the continuous casting process.18,19,20,21,22) Many alloy treatment technologies were developed to modify inclusions in the molten steel. However, the magnesium treatment modifies inclusions to MgO-rich inclusions to retard the collision of inclusions and refine the grain size, while it lead to the splash of the molten steel due to its lower boiling point.23,24,25) The rare earth treatment can modify inclusions and improve the steel performance, while it lead to the nozzle clogging due to the formation of solid rare earth-containing inclusions.26,27,28,29) Thus, the calcium treatment is the most popular and effective method to modify inclusions in the molten steel.
It was reported the calcium addition in the molten steel as early as 1906.30) The calcium treatment began to attract more and more attention in the 1960s.31,32) Since the 1980s, extensive experiments and calculations were conducted to better understand the effect of calcium treatment.33,34,35,36) The calcium treatment was introduced for the steel refining process based on two reasons. On one hand, calcium can effectively reduce alumina inclusions to calcium aluminates in the molten steel due to its strong deoxidation ability.37,38) On the other hand, there were two liquid inclusions of 12CaO·7Al2O3 and 3CaO·Al2O3 among calcium aluminates in steel,39) which rarely led to the nozzle clogging at the continuous casting temperature. Thus, the original purpose of the calcium treatment is the modification of solid Al2O3-rich inclusions to liquid calcium aluminates to avoid the nozzle clogging during the continuous casting process.40) Another key role of the calcium treatment is that it effectively modified the morphology of sulfide inclusions due to the strong desulfurization ability of the calcium, improving the performance of sulfur-containing steels.41,42)
The boiling point of calcium is roughly 1756 K, which was lower than the steelmaking temperature.43) Besides, the solubility of calcium in the molten steel is very low.44) When calcium is fed into the molten steel, calcium is vaporized rapidly, resulting in a low and unstable yield of calcium.45) Thus, the stable control of the calcium yield is always one of key issues in the precise modification of inclusions by the calcium treatment.46) During the refining process, Ca-containing wires are injected into the molten steel by the wire-feeding technology to improve the calcium yield.47) Meanwhile, the gasification of calcium in the calcium treatment process cause a strong fluctuation of the slag-steel interface, resulting in the reoxidation of the molten steel and the slag entrainment.48) In addition, active physical and chemical properties of calcium lead to the deviation of thermodynamic parameter measurement of calcium in the molten steel.49)
In this review, the control target of oxide and sulfide inclusions was proposed. Influence factors of the calcium yield during the calcium treatment were summarized to steadily increase calcium yield. Experimental and calculated results were compared to better understand the modification mechanism of oxide and sulfide inclusions in the molten steel by calcium treatment. The precipitation and transformation of inclusions in Al-killed Ca-treated steels during solidification, cooling, and heating processes were introduced. At last, opportunities and challenges of calcium treatment in the future were proposed and discussed.
Solid inclusions in the molten steel lead to the clogging of the submerged entry nozzle.20,27) The most important role of the calcium treatment is to modify solid aluminate inclusions to liquid ones at the continuous casting temperature. The relationship between the liquid fraction and contact angle of Al2O3–MgO–CaO inclusions is given in Fig. 1. The contact angle of inclusions was collected from literatures reported by Shinozak,50) Yoshikawa,51) Kapilashrami,52) Zhao,53) Cramb,54) Takahisa,55) Monaghan,56) Gaye,57) Adachi,58) and Bretonnet.59) The liquid fraction of inclusions at 1873 K was calculated using FactSage software.60) It is found that the contact angle between liquid inclusions and the molten steel is larger than 110°, promoting the inclusion collision and nozzle clogging. The contact angle between liquid inclusions and the molten steel is smaller than 80°, which can hardly lead to the nozzle clogging. However, it is found that the removal of liquid inclusions is much slower than solid ones.61) After soft blowing and ladle standing processes, remained liquid inclusions in the molten steel are much larger than solid inclusions, leading to the string shape inclusion after the rolling process. Thus, it is concluded that solid Al2O3 inclusions lead to the nozzle clogging, while full liquid CaO–Al2O3 inclusions result in large inclusions remained in the steel.

The relationship between the composition of inclusions and the clogging possibility of nozzle clogging was investigated by plant trials,61) as shown in Fig. 2. Inclusions in the Al-killed Ca-treated steel in tundish are liquid CaO–Al2O3–MgO, while clogging materials on the nozzle wall are Al2O3-rich inclusions with the liquid fraction less than 20%. Percentages of clogging materials and inclusions with the range of the liquid fraction are compared. The nozzle clogging possibility gradually reduces with a higher liquid fraction of inclusions. The critical range of the liquid fraction of inclusions extends from 100% liquid phase to larger than 20% liquid phase to avoid the nozzle clogging. It is suggested to modify solid inclusions to partial liquid ones rather than full liquid ones to avoid the nozzle clogging and improve the inclusion removal.

Figure 3 shows the phase diagram of CaO–Al2O3 inclusions calculated using FactSage software.60) The melting temperature of inclusions decreases first then increases with the CaO content in CaO–Al2O3 inclusions. In zones A and E, Al2O3-rich and CaO-rich inclusions are fully solid at the steelmaking temperature, resulting in the nozzle clogging. In zone C, 12CaO·7Al2O3 and 3CaO·Al2O3 inclusions are fully liquid at the steelmaking temperature, which lead to remaining large inclusions due to their low removal rate. Thus, partial liquid inclusions in zones B and D are suggested to avoid the nozzle clogging and remained large inclusions in the Al-killed steel. Moreover, inclusions in zone D contain lower CaO content, which need less Ca wire injection.

The calcium treatment is widely used to modify MnS inclusions to CaS–MnS complex inclusions to avoid the precipitation of linear MnS in the high sulfur steel.62) Figure 4 shows the effect of the calcium treatment on the tool life of high sulfur steels in literatures reported by JSPE,63) Ito,64) Yamada.65) With the increase of the T.Ca content, the tool life increases first, then decreases. On one hand, the calcium treatment retards the precipitation of linear sulfides along the grain boundary of the steel matrix.66) On the other hand, the deformability of CaS is much lower than MnS during the rolling process.67) However, the excessive addition of calcium in steel results in superfluous CaS formed in high sulfur steels, which is detrimental to the steel performance of steel. There is a maximum tool life of the high sulfur steel with the T.Ca content of roughly 40 ppm. The calcium treatment also effectively modifies MnS inclusions in the low sulfur steel.

To achieve the target composition of inclusions by calcium treatment, the calcium content is necessary to be stably controlled in an optimal range. However, the low and unstable calcium yield restricts the precision of the calcium treatment due to its low boiling point, low density, low calcium solubility in steel, and active chemical properties. Then, the alloy-cored wire-feeding technology was introduced to improve the calcium yield.45) Consequently, the calcium addition in the molten steel caused a strong fluctuation of the steel interface in the ladle, resulting in the reoxidation of the molten steel and the entrainment of the refining slag.48) Thus, the control of the yield of inclusions is one of the most important issues for the precise calcium treatment.
Figure 5 shows the effect of influence factors on the solubility of the calcium in the iron. Experimental data was collected from literatures reported by Kohler,44) Sponseller,68) and Engell.69) With the increase of the temperature from 1540°C to 1660°C, the solubility of calcium in the liquid iron increases from 290 ppm to 405 ppm. The solubility of calcium is also related to alloy element concentrations in the liquid iron. The calcium solubility obviously increases to nearly 1% with a higher silicon content in steel. The addition of aluminum or nickel is also beneficial to increase the calcium solubility in steel. The calcium solubility changes little with the increase of the manganese content in steel, while it slightly decreases with the addition of chromium in the steel.

The dissolved calcium content ([Ca]) and total calcium (T.Ca) content are widely used to predict the formation of inclusions in the molten steel. During the production process, the T.Ca content in steel is measured using spark source atomic emission spectrometry. The [Ca] in steel can be measured through the sample acid solution analysis using induction coupled plasma (ICP) emission spectrometry,38) whose analysis time is much longer than the measurement of T.Ca. Thus, Liu et al. reported the relationship between the [Ca] and T.Ca in steel, as shown in Fig. 6.70) The content of dissolved calcium in steel is closely related to the total calcium, total oxygen (T.O), total sulfur (T.S), the dissolved aluminum ([Al]), and temperature. The evolution of [Ca] content exhibits a similar tend with T.Ca content. The increase of T.O content promotes the formation of CaO, lowering the content of [Ca]. The increase of T.S content reduces the [Ca] content in steel due to the formation of CaS. The [Ca] content is little influenced by [Al] content in the steel. The decrease in temperature is conducive to the precipitation of CaS, which decreases [Ca] content in steel. In the current study, according to thermodynamic calculation results by Liu et al., an empirical equation is fitted to predict the [Ca] content in steel based on T.Ca, T.O, T.S, and [Al], as given in Eqs. (1) and (2) with 0.001% < T.Ca < 0.002% and Eq. (2) with 0.002%≤T.Ca.
| (1) |
| (2) |
where [Ca] and [Al] are the dissolved calcium content and dissolved aluminum content, %; T.O, T.Ca, and T.S are total oxygen, total calcium, and total sulfur contents, %.

It is necessary to study influence factors of the calcium yield in the calcium treatment process to achieve the accurate modification of inclusions by the calcium treatment. Influence factors of feeding speed,71,72,73) temperature,71,74,75) alloy elements,71,73) the initial T.Ca,72) and [O]75) on the calcium yield during the calcium treatment are summarized in Fig. 7. The calcium yield decreases with the increase of the feeding speed from 1.5 m/s to 4.0 m/s. The slower feeding speed is beneficial to increase the calcium yield due to the low calcium solubility in the molten steel. With the increase of temperature from 1540°C to roughly 1590°C, the calcium yield first increases due to the higher solubility of calcium in the molten steel, while it decreases at a higher temperature owing to the rigidity decrease of the calcium alloy wire at the higher temperature. The addition of aluminum and silicon increases the calcium yield in steel since the calcium solubility is higher with the increase of silicon or aluminum content, as shown in Fig. 5(b). The calcium yield decreases with the higher initial T.Ca content, while it increases with the increase of the initial dissolved oxygen ([O]) in steel due to the formation of CaO inclusions.

There are a large number of influence factors on the calcium yield during the calcium treatment process, which is hardly predicted using the traditional mechanism model. With the development of data analysis technologies, the neural network technology is widely used to solve problems with influence of complex factors. Wang et al.45) established a calcium yield prediction model based on neural network technology, considering 23 influence factors of the composition, temperature, wire feeding speed, etc. After inputting calcium treatment parameters, the calcium yield during the actual calcium treatment process is effectively predicted, as shown in Fig. 8. The model can be used for the prediction and optimization of the calcium yield during the calcium treatment process.

Figure 9 shows the deoxidation and desulfurization curves of calcium in the liquid iron at 1873 K. Thermodynamic data76) of Ca–O in the liquid iron was reported by many researchers, including Han,77) Wakasugi,78) Miyashita,79) Gustafsson,80) Kimura,38) Hino,49) Ichise,81) Degawa,82) Kusakawa,83) Ozawa,84) Inoue,85) Taguchi,86) Kobayashi,87) JSPS,88) Itoh,37) Cho,89) Ohta.90) Equilibrium curves of Ca–S reported by Taguchi,86) Han,77) Inoue,85) JSPS88) are compared. It is noted that equilibrium curves obtained by various researchers are quite different. The error of results reaches two orders of magnitude. The oxygen or sulfur content generally decreases first and then increases with a higher Ca content in the molten steel. Thermodynamic parameters of calcium in steel are also debatable due to the low boiling point and active chemistry properties of calcium in the molten steel. A slight difference in experimental conditions can lead to a huge difference in measured results. It is suggested to consider the second-order interaction coefficient for the Ca–O and Ca–S thermodynamic calculation.91) More works are necessary to correct thermodynamic parameters of calcium in the molten steel.

The calculated stability diagram is widely used for the prediction of the inclusion formation. Figure 10 shows the calculated stability diagram of Al–Mg–Ca–O system in the molten steel at 1873 K. The used equilibrium constant logK of reactions and the interaction activity coefficient are summarized in Tables 1, 2, 3. Figure 11 shows the fitted curve of activity of oxides in the CaO–Al2O3 system at 1873 K. Activity data of CaO and Al2O3 in the CaO–Al2O3 system was reported by many researchers, including Ye,92) Fujisawa,93) Rein,94) Korousic,95) Visser,96) Yang,97) Zhang,98) Wang.99) Among various Al–Ca–O inclusions in the Al–Ca–O stability diagram, only Al2O3·3CaO and 7Al2O3·12CaO inclusions are liquid at 1873 K. For the Al-killed steel, the calcium addition is necessary to modify solid Al2O3 inclusions to liquid ones. As shown in the Al–Mg–O stability diagram, the formation of MgO·Al2O3 inclusions are inevitable in steel with several ppm Mg during the refining process due to the application of MgO-containing ladle refractory. The MgO·Al2O3 inclusion can be modified to liquid ones by 2 ppm Ca in steel, as shown in the Al-Mg-2 ppm Ca–O stability diagram.


Figure 12 shows the predicted composition of inclusions in the Al-killed steel with various T.Ca contents at 1873 K using FactSage software.60) With the increase of the calcium content, the solid Al2O3 inclusion is modified to solid CaO·6Al2O3, solid CaO·2Al2O3, and the liquid CaO–Al2O3. The excessive addition of calcium results in the formation of solid CaS and CaO inclusions. Thus, the inclusion modification process mainly includes following steps with the increase of the calcium content. In step 1, the added calcium firstly modifies Al2O3 to the liquid CaO–Al2O3 with the insufficient calcium addition in steel, as Eq. (3). The optimal T.Ca content is mainly related to the T.O in the Al-killed steel. In step 2, the excess calcium addition leads to the formation of solid CaO and CaS in the steel, as Eqs. (4) and (5). The optimal T.Ca content is related to the T.O and T.S in the Al-killed steel.
| (3) |
| (4) |
| (5) |

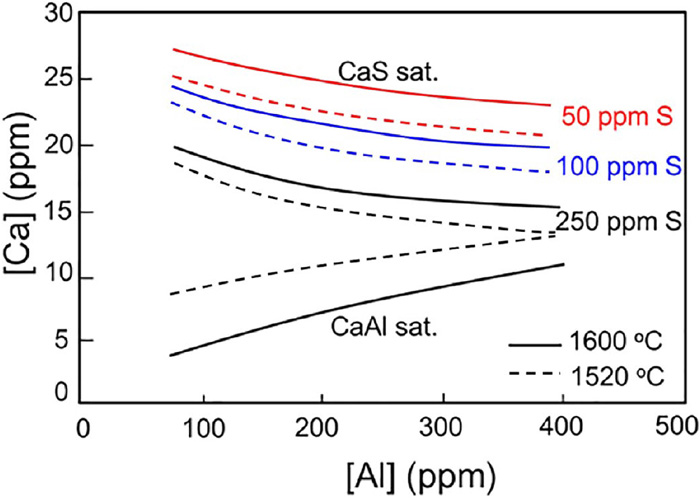
The insufficient and excess addition of calcium in steel can hardly modify inclusions to liquid inclusions. Holappa104) proposed there was a liquid window of the calcium content to achieve the modification of liquid inclusions, as shown in Fig. 13. To avoid the nozzle clogging, inclusions can be modified to liquid ones by adding the T.Ca amount in the liquid window. It is found that the liquid window is related to the T.O in steel. The needed T.Ca content increases with the T.O content in steel due to the larger number of inclusions. The increase of [Al] content slightly narrows the liquid window of inclusions.
Many kinetic models of the calcium treatment were established to predict the modification of inclusions from alumina or spinel to calcium aluminates in the molten steel, as summarized in Table 4. Inclusion components considered in the model are mainly Al2O3, MgO, CaO, and CaS. During the calcium treatment process, the calcium evaporates from the liquid steel due to its low boiling point, which has an obvious influence on the evolution of inclusion composition. The rate-limiting step considered in kinetic models includes the calcium wire feeding, the calcium evaporation, the diffusion of the unreacted core, etc. Recently, multiple reaction coupling calculation models are developed to better predict the inclusion composition, considering inclusion-steel-slag reaction, the calcium wire feeding, and the calcium evaporation.
| Authors | Year | Inclusion components | Calcium evaporation | Rate limiting step | Scale | Ref. |
|---|---|---|---|---|---|---|
| Heinke et al. | 1987 | Al2O3, CaO, CaS | No | Calcium wire feeding | Industrial | 105) |
| Lu et al. | 1994 | Al2O3, CaO, CaS | Yes | Calcium evaporation | Laboratorial | 106) |
| Ye et al. | 1996 | Al2O3, CaS | No | Unreacted core | Laboratorial | 92) |
| Ito et al. | 1996 | Al2O3, CaO | No | Unreacted core | Laboratorial | 107) |
| Higuchi et al. | 1996 | Al2O3, CaO, CaS | No | Coupled reactions | Laboratorial | 108) |
| Numata et al. | 1998, 2011 | Al2O3, CaO, CaS | Yes | Coupled reactions | Laboratorial | 110,119) |
| Visser et al. | 2008 | Al2O3, CaO, CaS | Yes | Coupled reactions | Industrial | 96) |
| Lind et al. | 2010 | Al2O3, CaO | No | Unreacted core | Laboratorial | 111,112) |
| Guo et al. | 2014 | Al2O3, CaO | No | Unreacted core | Industrial | 113) |
| Ren et al. | 2016 | Al2O3, CaO, CaS | No | Calcium wire feeding | Industrial | 114) |
| Zhang et al. | 2018 | Al2O3, MgO, CaO, CaS | Yes | Calcium evaporation | Laboratorial | 98) |
| Tabatabaei et al. | 2018 | Al2O3, MgO, CaO, CaS | Yes | Coupled reactions | Industrial | 115,116,117) |
| Xi et al. | 2021 | Al2O3, CaO | No | Unreacted core | Laboratorial | 118) |
The transient evolution of Al2O3 and spinel inclusions during the calcium treatment in the Al-killed steel was observed by Pistorius et al., as shown in Fig. 14.119) Angular shape Al2O3 and spinel inclusions are generated after the aluminum deoxidation of steel. The CaS outer layer is transiently formed on Al2O3 and spinel inclusion cores due to the excess Ca concentration in the local steel after the calcium addition. With the mixing of calcium in the molten steel, the formed CaS in inclusions gradually decreases and inclusions are fully modified to spherical liquid calcium aluminates. As shown in Fig. 11, the CaS is mainly formed in the molten steel with a high concentration of calcium. It is indicated that the formation of CaS in inclusions in the molten steel, reflecting the excessive calcium addition.

The relationship between the T.Ca content and inclusion compositions in the Al-killed Ca-treated steel is shown in Fig. 15. Experimental data reported by Zhang,98) Yang,120) Xu,121) and Ren122) was summarized and compared. Besides, the optimal T.Ca content is related to the T.O content in the steel. Thus, the CaO/Al2O3 ratio in inclusions linearly increases with the increase T.Ca/T.O ratio in steel from 0 to 1. The added Ca firstly modifies Al2O3 inclusions to liquid CaO–Al2O3 for T.Ca<T.O, while the composition of liquid CaO–Al2O3 is little influenced by the T.Ca/T.O ratio for T.Ca>T.O. Meanwhile, it is noted that the CaS/(CaO+Al2O3) in inclusions increases with a higher (T.Ca-T.O/T.S) ratio in steel in the case of T.Ca>T.O. It is indicated that the rest of the calcium in steel after the modification of liquid calcium aluminates leads to the formation of solid CaS, agreeing well with results in Fig. 12.

Recently, it is found that there is a certain amount of calcium in the FeSi alloy, which has a significant influence on the modification of inclusions, as shown in Fig. 16. It is found that T.Ca rises up after the addition of the Ca-containing FeSi alloy, which is higher than that without the addition of FeSi alloy. For the Al-killed steel, the calcium in FeSi alloy effectively modifies spinel inclusions to liquid calcium aluminates, whose influence is similar to the calcium treatment of inclusions. Besides, the calcium yield of the Ca-containing FeSi alloy addition exceeds 85%, which provides a new method to achieve the precise control of the calcium treatment. For the Si-Mn-killed steel, the addition of pure FeSi alloys leads to the formation of SiO2–MnO inclusions, while the Ca-containing FeSi alloy addition modifies inclusions to Al2O3–SiO2–CaO. Thus, it is important to rigidly control the calcium content in ferroalloys.

Inclusions of MnS are highly deformable during the rolling process. The morphology of MnS has an obvious influence on the performance of steel products. The calcium treatment is an effective method to modify MnS inclusions in the steel. Haida et al.125,126) proposed the atomic concentration ratio (ACR) index of T.Ca, T.S, and T.O to evaluate the modification of MnS by the calcium treatment, as Eq. (6). The ACR index increases with a higher T.Ca content, while it decreases with higher T.O and T.S contents. The effect of ACR on the length of inclusions in high sulfur steels is shown in Fig. 17. The length of sulfides shows a decreasing tendency with the ACR index, indicating that the calcium treatment lowers the deformability of MnS inclusions.
| (6) |

The plasticity of MnS and CaS in steel during the hot rolling is shown in Fig. 18, which is modified from the schematic proposed by Luyckx et al.67) The plasticity of MnS increases from randomly dispersed globular Type I sulfides to rod-like fine Type II sulfides. Thus, it is necessary to avoid the precipitation of Type II sulfides along the grain boundary at the temperature between the liquidus and solidus of steel. Besides, the plasticity of MnS increases with the Mn/S ratio in steel. The plasticity of CaS is much lower than MnS. Thus, the calcium treatment lowers the deformability of MnS inclusions due to the higher hardness of CaS. Moreover, the formation of CaS retards the precipitation of MnS inclusions along the grain boundary.66) Thus, the calcium treatment plays an important role in the control of sulfides in steel.

During solidification and cooling processes of Al-killed Ca-treated steels, inclusions transform from liquid CaO–Al2O3–MgO in the molten steel to Al2O3–MgO–CaS in the solid steel due to the equilibrium change of reactions between steel and inclusions,127,128) as shown in Fig. 19.129) With the decrease of the cooling rate, the CaO content in inclusions decreases while the CaS content increases due to a longer transformation time of inclusions with a lower cooling rate. The thermal expansion coefficient of CaO–Al2O3–MgO is lower than the steel matrix, while the thermal expansion coefficient of CaS is higher than the steel matrix.129) Thus, it is suggested to promote the transformation of inclusions from the CaO–Al2O3–MgO to Al2O3–MgO–CaS, avoiding the formation of cracks between the steel matrix and inclusions.

The evolution of inclusions from a liquid CaO–Al2O3 in the molten steel to the Al2O3–CaS in the steel Al-killed Ca-treated steel during the solidification process at 1515°C and the heat treatment process at 1000°C were in-situ observed using the high-temperature confocal scanning laser microscopy, as shown in Fig. 20.130) The change of inclusion shape was observed due to the reaction between the spherical CaO–Al2O3 inclusion and steel, resulting in the formation of an irregular dense CaS layer around the inclusions. The transformation of inclusions from CaO–Al2O3–MgO to CaS–Al2O3–MgO in the solid steel at the heat treatment temperature was in-situ observed using the confocal laser scanning microscopy. It is found the rate-limiting step is the mass transfer of elements between the reaction interface and the steel matrix at the heating temperature of the solid steel. With the increase of the heating temperature from 1000°C to 1400°C, the transformation fraction of inclusions increases due to the higher mass transfer of elements at a higher temperature.

Distributions of Al2O3, MgO, CaO, and CaS in inclusions in the thickness direction of the Al-killed Ca-treated steel continuous casting slab are detected in Fig. 21.132) The distribution of Al2O3 and MgO contents of inclusions in the slab is relatively uniform, indicating that the transformation of Al2O3 and MgO in inclusions is little influenced by the cooling rate of the molten steel during solidification and cooling processes. The transformation rate of inclusions on the slab surface is higher than that of the slab center. Thus, the CaO content of inclusions is higher on the surface of slab, while the CaS content of inclusions inside the slab is relatively higher. The higher CaS content inside the slab is related to the change of inclusions from CaO to CaS with the decrease of temperature. Besides, the centerline segregation of sulfur also has an influence on the distribution of CaS content, which needs more investigation to quantify the transformation of inclusions. After heat treatment and rolling processes, the distribution of Al2O3, MgO, CaO, and CaS in inclusions in the thickness direction of the rolled plate is uniform. Ren et al. developed a kinetic model coupling the heat transfer in the slab, the mass transfer in steel, and thermodynamic calculation on the inclusion transformation to predict the evolution of inclusions in continuous casting slab, as shown in Fig. 22.132) The model can be used to adjust the composition distribution of inclusions and optimize operation parameters during the continuous casting process.


The calcium treatment is one of the most effective methods to modify oxide inclusions to avoid the nozzle clogging during the continuous casting process. The excessive or insufficient calcium treatment can hardly modify inclusions to liquid or partially liquid ones, which even causes the formation of large inclusions. During the production process, the optimal calcium content in steel for various grades and various heats is different, while many steel plants still conducts the empirical way of the calcium treatment. Thus, it is meaningful to further investigate the precise calcium treatment during the refining process. First, the calcium yield is necessary to be stably improved to achieve the accurate calcium content in the molten steel. It is necessary to optimize the calcium wire structure and operation parameters of calcium treatment. Besides, it is necessary to develop the thermodynamic and kinetic model of calcium treatment to reasonably modify inclusions to target ones.133) The thermodynamic data of the calcium in steel are still debatable, which is very important to predict the inclusion composition during the calcium treatment. It is suggested to introduce the big data technology to optimize the calcium treatment process. Moreover, the modification target of inclusions for various steel grades is different. More investigations are necessary to determine the target inclusion in various steel grades by calcium treatment. It is noted that the modification effect of oxide inclusions by the calcium treatment will be weakened with the development of preventing nozzle clogging technology in the future.
The calcium treatment can effectively modify sulfide inclusions in the steel. It is of great importance to investigate the transformation, precipitation, and deformation mechanism of sulfide inclusions in the steel. Firstly, the CaS is formed in the molten steel and precipitated in the solid steel. The formation of sulfides in steel is also influenced by the element segregation. The thermodynamic, kinetic, solidification, and segregation coupling calculation model is useful to predict the composition, size, number, and distribution of sulfide inclusions in the steel. Secondly, the influence mechanism of calcium treatment on the precipitation of MnS along the grain boundary and inside the grain is still unclear. The calcium content and cooling parameters needs to be optimized to avoid the formation of large size sulfides. Thirdly, it is recommended to quantitatively investigate the deformation ability of MnS–CaS at various temperatures. The composition target of MnS–CaS inclusions are very important to improve the performance of sulfur-containing steels.
(1) Full solid Al2O3-rich and CaO-rich inclusions at the steelmaking temperature results in the nozzle clogging, while full liquid 12CaO·7Al2O3 and 3CaO·Al2O3 inclusions at the steelmaking temperature lead to remained large inclusions due to the low removal rate of liquid inclusions. Thus, the partial liquid inclusion is suggested to avoid the nozzle clogging and the formation of large inclusions in the Al-killed Ca-treated steel.
(2) Influence factors of the calcium yield during the calcium treatment are summarized to steadily increase calcium yield, including steel composition, temperature, feeding speed, etc. The neural network technology is introduced to effectively predict the calcium yield and optimize operation parameters during the calcium treatment process with complex influence factors. An empirical equation is fitted to predict the [Ca] content in steel based on T.Ca, T.O, T.S, and [Al] in steel.
(3) During the calcium treatment process, the added Ca firstly modifies Al2O3 inclusions to liquid CaO–Al2O3 for T.Ca<T.O, while the composition of liquid CaO–Al2O3 is little influenced by the T.Ca/T.O ratio for T.Ca>T.O. Meanwhile, the CaS/(CaO+Al2O3) in inclusions increases with a higher (T.Ca-T.O/T.S) ratio in steel with T.Ca>T.O.
(4) The calcium treatment retards the precipitation of linear sulfides along the grain boundary of the steel matrix and lowers the deformability of sulfides during the rolling process due to the higher hardness of CaS. The calcium treatment also effectively modifies MnS inclusions in the low sulfur steel. The ACR index of T.Ca, T.S, and T.O is used to evaluate the modification of MnS by the calcium treatment. The length of sulfides increases with a higher T.Ca content, while it decreases with a lower T.O and T.S.
The authors are grateful for support from the National Nature Science Foundation of China (Grant No. U22A20171, No. 51874032), the High Steel Center (HSC) at North China University of Technology, Yanshan University and University of Science and Technology Beijing, China.