2023 Volume 63 Issue 12 Pages 1951-1956
2023 Volume 63 Issue 12 Pages 1951-1956
Self-healing function in the ceramic-based composites is one of unique characteristics to improve the strength reliability by oxidation of dispersoids. Metallic iron particles are one of base metal and easy to oxidize at air atmosphere. The objective of this study is to investigate the crack disappearance behavior of Fe-dispersed alumina composite ceramics by high-temperature oxidation.
Surface cracks were introduced on Fe/Al2O3 samples. The samples were heat treated at 700–900°C for 1–24 h in air. Crack lengths were measured before/after heat treatment and the crack disappearance rates were calculated. The introduced cracks disappear by the formation of Fe2O3. The formed oxides appear to have a spider-web shape. The mesh diameter of spider-web is approximately 1–2 µm, which corresponds to the Al2O3 grain size of sintered body. The crack disappearance rate increases with increasing heat treatment temperature and time. From the temperature dependence of the crack disappearance rate, the apparent activation energy for the crack disappearance is found to be Q = 160 kJ/mol. The value of activation energy in this study is lower than the values of volume diffusion of Fe ions through Al2O3. It implies that grain boundary diffusion of Fe ions contributes to the crack disappearance.
The mechanical strength of structural ceramics is strongly influenced by defects introduced during the manufacturing process. In particular, surface cracks dominate the mechanical strength of the entire structural ceramics due to stress concentration. It is economically difficult to detect all surface cracks by non-destructive testing. Therefore, it is important for structural ceramics to minimize the risk of strength degradation due to surface cracks. Methods to improve the strength reliability of structural ceramics have been proposed, such as increasing the resistance to crack propagation by fiber reinforcement1,2) and controlling the particle orientation.3) These methods improve mechanical reliability by introducing strengthening mechanism too much higher than the designing strength. Furthermore, the self-healing effect has been proposed4) as the method to maintain the designing strength with high mechanical reliability.
The self-healing function of ceramic-based composites has been investigated by Ando’s group.4,5,6) When cracks are initiated on the material at high-temperature, the non-oxide dispersoids are oxidized. The formed oxide fills the cracks and recoveries mechanical strength of the ceramic composite. SiC has been widely studied as a dispersoids for self-healing.4,5,6,7,8) Other dispersoids such as TiSi29) and TiC10) have also been investigated. These dispersoids are non-metallic compounds. The self-healing function using carbide is mainly obtained at over 1000°C for few hours in air, depending on the particle size. Self-healing function have also been reported in Al2O3 composites containing metallic Ni particles (Ni/Al2O3).11,12,13) Ni/Al2O3 is known as a nanocomposite14) with has high mechanical strength and fracture toughness. Other metallic dispersoids such as NiAl15) and Co16) have been reported. It has been reported that Ni/Al2O3 recovers its strength after oxidation at 1000°C for 1 h.10) However, the oxidation temperature is not sufficiently low for application such as ceramic brake, cutting chip and so forth. In addition, Ni and Co particles are relatively noble metal and expensive.
In this study, author group has focused on metallic iron particles. Metallic iron is one of base metal and is more easily oxidized than metallic Ni and Co by high-temperature environment. Fe2O3 is formed at lower temperatures and shorter times than NiAl2O4 formed by reaction of Ni with Al2O3. In previous study,17) metallic iron particles dispersed into mullite matrix composite (Fe/mullite) has been prepared and heat treated at high temperature. As the results, hematite is formed by heat treatment at 900°C for 1 h in air and it shows strength recovery. In order to achieve even lower temperatures for the self-healing effect using metallic iron particles, it is important to investigate the crack disappearance process. For the analysis of the oxidation process, it is expected to apply common structural ceramics rather than the mullite matrix. Therefore, the objective of this study is to investigate the crack disappearance behavior of Fe-dispersed alumina composite ceramics (Fe/Al2O3) by high-temperature oxidation.
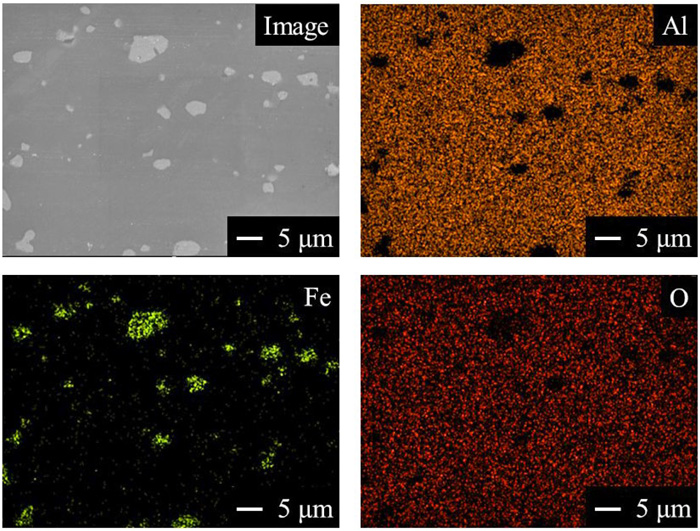
As starting materials, α-Al2O3 powder (Taimei Chemicals Co., Ltd., TM-DAR, Purity: 99.99%, average particle size: 0.14 μm) was mixed with metallic iron powder (High Purity Chemical Laboratory Co., Ltd., Purity: 99%, particle size: 3–5 μm) at the ratio of 5 vol% Fe and dry ball milled for 12 hours. The powder mixture was pressed at 94 MPa to form green bodies with diameter of 22 mm and height of 5 mm. The green bodies were set in horizontal electric furnace with inner diameter with 42 mm and sintered at 1400°C for 6 h in a stream of Ar (purity: 99.9999%) gas with 1 L/min (12 mm/s). The surface of the sintered specimen was polished to a mirror finish using diamond particles and lubricants. Figure 1 shows SEM image and EDS mappings of original sample surface. Two different contrasts are observed in the sample. The ternary phase diagram of Fe–Al–O systems at 1400°C shows that Fe and Al2O3 do not form another compounds.18) From EDX mapping, the white contrast regions correspond the metallic Fe particles and the black contrasts are Al2O3 matrix. The particle sizes of the metallic Fe are almost same with the starting particles. Most of metallic Fe particles is uniformly dispersed into Al2O3 matrix.
Three Vickers indentations were placed on the sample surface to obtain 12 surface cracks. The indentation load was 9.8 N and the loading time was 10 s. Figures 2(a) and 2(b) show the SEM image of the specimen surface after crack introduction and crack propagation path. The lines as shown in Fig. 2(b) show crack propagation path. The cracks were initiated from the four corners of the Vickers indentation. The crack lengths were approximately 25 μm measured by SEM with magnification up to 5000 times. The cracks propagate from the edge of Vickers indentation. Figure 3 shows SEM image of fractured sample. The fractured sample was prepared by introducing crack using Vickers indentation followed by impact loading with hammer. The sample surface with the Vickers indentation is observed by SEM. Based on the initial grain size, it is considered that the bright contrast particles in Fig. 1 correspond to the metallic iron particles. The grain size of Al2O3 is approximately 1–2 μm. In monolithic Al2O3, grain growth would have been more advanced.19) Therefore, grain growth was relatively suppressed in Fe/Al2O3.


The samples were then heat-treated at 700–900°C for 1–48 h in air. The heating rate was 600 K/h. Surface cracks were observed by SEM after heat treatment, and crack lengths were measured. The crack disappearance rate, defined as ΔC, was calculated using the crack lengths before and after heat treatment as shown in Eq. (1)20)
| (1) |
where C1, C2, ... C12 are the surface crack length of the three indentations before heat-treatment, and C′1, C′2, ... C′12 are the values after heat-treatment. Phase identification of samples were performed by X-ray diffraction (XRD).
Figure 4 shows the XRD pattern of the sample surface after heat treatment. The XRD patterns of all samples show the peaks of Hematite. On the other hand, the peaks of FeAl2O4 are not identified because of heat treatment in air. Although Fe peaks are also identified in all samples, it is considered that these peaks are identified from beneath oxide layer and Al2O3 matrix.

Figure 5 shows SEM images of the sample surfaces after heat treatment. Oxide formation is preferentially observed at the cracks. Introduced surface cracks make the exposed surface. The volume of Fe2O3 formed on the exposed surface is much larger than vacancies around crack tip, indicating the oxides are overflowing from the cracks.17) Other spherical oxides are also observed on the sample surface. Whisker-like oxides are also observed on the spherical oxides. Whisker-like oxides have been observed in previous studies.17) Whisker-like oxides are reported to form when the oxidation of magnetite to hematite is mainly due to diffusion of oxygen ions rather than oxidation through the gas phase. Introduced cracks are completely disappeared on the sample heat treated at 900°C for 1 h. On the other hands, introduced cracks partly remain on samples after heat treatment at 800°C, 3 h and 700°C, 12 h. In a previous study using Ni/Al2O3,11) the crack was filled with the formed oxide, leaving only 1 μm pores. The strength recovery of Ni/Al2O3 and Fe/Mullite17) were also obtained when the oxide overflowed from the specimen surface.

Although crack disappears by the formation of Fe2O3 as show in Figs. 4 and 5, the Fe2O3 itself has weaker mechanical strength than Al2O3 matrix. Actually, the self-healing ceramics reported previous studies show the mechanical strength of the formed oxide is smaller than that of the ceramic matrix.5) It implies that the reduction of stress intensity at the crack tip is a factor in the recovery of strength. In addition, the XRD results as shown in Fig. 4 represents that the peaks of Fe2O3 are slightly shifted to the high angle side. This may indicate that Fe2O3 makes solid solution with Al2O3 matrix based on the Fe2O3–Al2O3 pseudo phase diagram.22) The phenomena imply the Fe2O3/Al2O3 interface is considered to have strong adhesion even at low temperatures. Therefore, strength recovery by crack disappearance is expected to obtain in Fe/Al2O3.
Figure 6 shows SEM image of the sample surface far from the cracks after heat treatment at 900°C for 1 h. The formed oxide phases appear to have a spider-web shape. The mesh diameter of spider-web is approximately 1–2 μm, which corresponds to the Al2O3 grain size after sintering. Therefore, it is supposed that spider-web shaped oxides are formed along the Al2O3 grain boundaries. It is considered that the model in which Fe ions diffuse in the Al2O3 grain boundaries toward the sample surface. Figure 7 shows the schematic diagram of cross-sectional views of sample during high-temperature oxidation. First, the metallic iron particles on the sample surface including the crack surface are oxidized and spherical Fe2O3 is formed. While, the spider-web shaped Fe2O3 fill the cracks, leading to the crack disappearance. On the other hand, oxygen anion should be also diffused to inside of sample and metallic Fe particle is internally oxidized. Since diffusion of Fe ions in Fe2O323) is faster than that in Al2O3,24,25) it is considered that grain boundary diffusion of Fe ions in Al2O3 matrix is rate controlling for crack disappearance. The growth rate of oxide generally follows parabolic manner. If the crack width is assumed 2xc, the time until for complete crack disappearance corresponds to the time for the oxide layer to grow to xc.
| (2) |
Where, kp is parabolic rate constant and tc is heat treatment time. From Arrhenius equation,
| (3) |
Where, T is absolute temperature, A is frequency factor, Q is activation energy and R is gas constant. Substituting Eq. (3) into Eq. (2) yields the following Eq. (4)20)
| (4) |
Therefore, it is expected to obtain the apparent activation energy for the crack disappearance by evaluating the heat treatment conditions under complete crack disappearance Fig. 8 shows the crack disappearance rate for each heat treatment condition. X-axis is the reciprocal temperature and Y-axis is the log of the inverse of the heat treatment time. For all experimental conditions, the crack disappearance rates are evaluated and classified into three types. The crack disappearance rate increases with increasing heat treatment temperature and time. The complete crack disappearance of Fe/Al2O3 occurs approximately 200°C lower than that of the previous study using metallic Ni as dispersoids.11) It is reported that Fe/mullite shows the highest strength recovery was obtained at 900°C for the 1 h. Fe/Al2O3 is also expected to contribute strength recovery by the crack disappearance. From Eq. (4) and the slope of the approximate straight line of Fig. 8, the activation energy is calculated as Q = 160 kJ/mol. Table 1 lists the reported activation energies for the diffusion of Fe ions in Al2O3. The volume diffusion of Fe ions through Al2O324,25,26) is higher than the activation energy obtained in this study. Activation energies for diffusion of Fe ions along Al2O3 grain boundaries are not found as far as the author have searched literatures. In volume diffusion, the concentration of cation vacancies in the grain increases with increasing temperature, which significantly affects the increase in diffusion coefficient. On the other hands, the width of grain boundaries is approximately few nm, indicating less sensitivity to temperature.27) Further, it is reported that activation energy of volume diffusion of Ni ions in Al2O3 matrix is higher than its grain boundary diffusion.28) Therefore, it is considered that the activation energy for diffusion of Fe ions passing through Al2O3 grain boundaries is smaller than that of volume diffusion of Fe ions.



This study indicates that grain boundary diffusion of Fe ions contributes to the crack disappearance. Usage of Al2O3 particles with finer grain size is expected to promote transfer of Fe ions at the Al2O3 grain boundary. It is also effective to select the matrix having large grain boundary diffusion of Fe ions, e.g., MgAl2O4.29) It is expected that self-healing ceramics using metallic iron particles can be applied at lower temperatures and faster time by incorporating the recommendations.
Al2O3 matrix with dispersed metallic iron particles are prepared and its crack disappearance behavior are investigated. The influence of heat treatment temperature and time on the crack disappearance rate are measured, and the following findings are obtained.
(1) The introduced cracks disappear by the formation of Fe2O3. Fe2O3 is preferentially formed at the cracks. The volume of oxide formed on the exposed surface is much larger than vacancies around crack tip, indicating the oxides are overflowing from the cracks.
(2) The crack disappearance rate increases with increasing heat treatment temperature and time. From the temperature dependence of the crack disappearance rate, the apparent activation energy for the crack disappearance is found to be Q = 160 kJ/mol.
(3) Usage of Al2O3 particles with finer grain size is expected to promote transfer of Fe ions at the Al2O3 grain boundary. It is also effective to select the matrix having large grain boundary diffusion of iron. It is expected that self-healing ceramics using metallic iron particles can be applied at lower temperatures and faster time by incorporating the recommendations.