Abstract
In the current study, the reaction behavior between bottom blowing CO2 and the molten steel at different converter stages was studied by the thermodynamic calculation. At the initial stage, CO2 reacts with [C] before the turning point and then reacts with [C] and [Si] simultaneously after that. CO2 completely reacts with [C] and twice volume of CO generates at the middle stage. The reaction ratio of CO2 and [C] gradually decreases at the final stage due to the ratio of CO2 reacting with [Fe] and the residual unreacted CO2 increases. Then the effect of the bottom blowing gas type and intensity on the [C]·[O] value and T.Fe content in the slag were studied by industrial trials in a 300 t converter. When Ar is blown at the final stage, the CO partial pressure decreases with the increasing of the bottom blowing intensity, while the opposite result is obtained when blowing with CO2. The CO2 bottom blowing into the converter should be switched with Ar, and the blowing time of CO2 should be controlled in the early 12 minutes to avoid increasing the [C]·[O] value and the T.Fe content in the slag. In order to improve the reaction rate of CO2 and [C], it is necessary for the CO2 to be blown during the high-speed decarburization period when the [C] content is between 0.7 mass% and 3.3 mass% and the bottom blowing time is between 5 and 12 minutes.
1. Introduction
The increase of CO2 emissions in the atmosphere is the main reason for the global warming. CO2 emissions from the iron and steel industry is an important proportion of the total carbon emission, especially in the plant with BF-BOF route with 1.7–2.2 t CO2 emissions per ton steel.1,2,3) More and more steelmaking plants are focusing on the reduction of the energy consumption and the CO2 emissions to address the crucial subject of the climate change.4) To achieve carbon neutrality in the steel industry, a large-scale reform is required to improve the steelmaking processes. Research on the resource utilization of CO2 is of great significance to reduce CO2 emissions to cope with the global warming and bring beneficial metallurgical effects.
The application technology of CO2 in the steelmaking has been studied since the 1980s. CO2 is used instead of N2 and Ar for the BOF bottom-blowing5,6,7,8,9,10,11,12,13,14,15,16,17) and for EAF bottom-blowing.18,19) CO2 mixed with O2 is blown from the top of the converter to improve the metallurgical effect20) and reduce the dust generation,21,22,23,24) CO2 replaces Ar for the bottom-blowing to enhance the stirring energy during the LF process25,26,27) and RH process,28,29) and also is used as the protective gas of the molten steel during the continuous casting.30) During the converter process, CO2 is widely used as the bottom blowing gas, which has the following characteristics: I) Bottom blowing CO2 in the converter has excellent denitrification performance since the reaction of CO2 with [C] is a volume increasing reaction.8,9,10,11,12) II) The cooling capacity of CO2 is greater than N2 and Ar because of the endothermic reaction of CO2 with [C], so CO2 can become the cooling gas of bottom blowing in the converter, and even replace the hydrogen carbide to avoid [H] increasing in the molten steel.13,14) III) CO2 is a kind of weak oxidizing gas, which reacts with the molten steel near the bottom blowing tuyere to generate FeO and MnO, resulting in faster corrosion of refractory.8,9,10,15,16,17) IV) As for the effect of bottom blowing CO2 in the converter on the content of [O] in the molten steel and T.Fe content in the slag, some studies8,9) show that the oxygen content of molten steel and the T.Fe content in the slag at the end of converter are significantly increased with the bottom blowing CO2, while other studies10,12) have obtained opposite results.
In summary, researches on CO2 application in the steelmaking mainly focused on the bottom blowing in the converter, which has the advantages of lower cost, excellent denitrification performance, and good cooling capacity for bottom blowing tuyere. However, CO2 accelerates the corrosion of refractory since it is a kind of weak oxidizing gas, and it is controversial about the effect of CO2 on the oxygen content of molten steel and the T.Fe content in the slag. In this paper, the reaction behavior of CO2 bottom blowing in the converter was studied. Firstly, the reaction mechanism of CO2 with main elements in the molten steel was analyzed by the thermodynamic calculation. Then, the industrial trials of the bottom blowing CO2 were carried out in a 300 t converter. The effect of CO2 blowing time and intensity on the [C]·[O] value and T.Fe in the slag was discussed. The effect of bottom blowing CO2 on the decarburization rate was studied by the exhaust gas analysis.
2. Experimental Aspects
2.1. Thermodynamic Calculation
Reaction between the CO2 and the molten steel were calculated by the thermodynamic software Factsage7.1. The composition and temperature of the molten steel in three typical stages are given in Table 1. The initial stage, the middle stage, and the final stage represent the molten steel when the [C] is 4.3 mass%, 2.0 mass%, and 0.1 mass%, respectively. CO2 was continuously blown into the molten steel by the step of 0.05 Nm3/t. The molten steel reacted with CO2 in the previous step continues to iterate and then continues to react with CO2 in the next step. A total of 200 steps were calculated and 10 Nm3/t CO2 were blown into the molten steel for each stage.
Table 1. Temperature and elements content (mass, %) of molten steel at different stage.
| Stage | Temperature (°C) | C | Si | Mn | P | S |
|---|
| Initial stage | 1350 | 4.300 | 0.270 | 0.100 | 0.070 | 0.002 |
| Middle stage | 1500 | 2.000 | 0.010 | 0.010 | 0.030 | 0.002 |
| Final stage | 1620 | 0.100 | 0.010 | 0.010 | 0.008 | 0.002 |
Industrial trials were carried out in a 300 t converter. Parameters are listed in Table 2 and Fig. 1, respectively. The top blowing time and intensity of the pure oxygen in the converter were approximately 15 minutes and 3.4 Nm3/(t·min). In order to study the effect of the bottom blowing time and the intensity of CO2 on the metallurgical effect of the converter, 6 groups of trials were designed. As a comparative trial, No. 1 was carried out by the bottom blowing N2/Ar. Trials No. 2 to No. 6 were carried out by the bottom blowing CO2 and Ar at different time. The bottom blowing time of CO2 varied from 3 to 12 minutes respectively and the blowing gas switched to Ar in the rest time in the trials of No. 2 to No. 5. Trial No. 6 was conducted by the bottom blowing CO2 during the whole blowing process. In all trials, the intensity of the bottom blowing gas was fixed at 0.04 Nm3/(t·min) in the early 12 minutes, and then increased to 0.06–0.11 Nm3/(t·min).
Table 2. Industrial trials scheme.
| Trial number | Gas type | Blowing time (min) | Blowing intensity (Nm3/(t·min)) | Amount of Data |
|---|
| Earlier | Later | Earlier | Later | 0 to12 mins | 12 to 15 mins |
|---|
| No. 1 | N2 | Ar | 9 | 6 | 0.04 | 0.060 | 16 |
| 0.04 | 0.080 | 16 |
| No. 2 | CO2 | Ar | 3 | 12 | 0.04 | 0.060 | 28 |
| 0.04 | 0.070 | 12 |
| 0.04 | 0.080 | 18 |
| No. 3 | CO2 | Ar | 6 | 9 | 0.04 | 0.040 | 23 |
| 0.04 | 0.080 | 12 |
| No. 4 | CO2 | Ar | 9 | 6 | 0.04 | 0.060 | 14 |
| 0.04 | 0.070 | 18 |
| 0.04 | 0.080 | 16 |
| No. 5 | CO2 | Ar | 12 | 3 | 0.04 | 0.060 | 41 |
| 0.04 | 0.070 | 10 |
| 0.04 | 0.080 | 16 |
| 0.04 | 0.011 | 16 |
| No. 6 | CO2 | Ar | 15 | 0 | 0.04 | 0.060 | 23 |
| 0.04 | 0.070 | 63 |
| 0.04 | 0.080 | 14 |
| 0.04 | 0.11 | 23 |
3. Results and Discussion
3.1. The Reaction Mechanism between CO2 and the Molten Steel
The thermodynamic software FactSage 7.1 was used to calculate the reaction between the CO2 and the molten steel at different stages, and calculation details are shown in the research method. The content variation of [Si], [Mn] with [C] at the initial stage are shown in Fig. 2(a). The [Mn] content remained unchanged with the decreasing of [C] content. CO2 only reacts with [C] in the molten steel when [C] content is above the turning point of 4.06 mass%. CO2 reacts with [C] and [Si] simultaneously when [C] drops below that value. Therefore, 4.06 mass% [C] and 0.27 mass% [Si] were the turning point at which the Gibbs free energy change of CO2 reacting with [C] and [Si] became the same, which was similar to the study of H. Ono-Nakazato et al.31) The variation of gas generation with [C] content is shown in Fig. 2(b), the solid line represents the actual gas generated and the dotted line is the extension of the straight line before the turning point. The generated CO gas conforms to the rule in the Fig. 2(a). All the CO2 reacted with [C] to produce double volume of CO before the turning point. After the turning point, CO2 not only reacts with [C] to produce twice the volume of CO, but also reacts with [Si] to produce the same volume of CO, which results in the increase of the CO generation. Figure 2 also indicates that CO2 has been fully reacted. In addition, [Mn] did not react with CO2 since the Gibbs free energy change of the reaction between CO2 with [Mn] was higher than that of CO2 with [C] and [Si] at the initial stage.

The gas generation of CO2 reacting with the molten steel at the middle stage of the converter is shown in Fig. 3. At the middle stage, [Si] and [Mn] in the molten steel have been reacted almost completely to be as low as 0.01 mass%, while the content of [C] is high, so the blowing CO2 completely reacts with [C], and the CO generation increases linearly with the reduction of [C] content.

The calculated results of CO2 reacting with molten steel at the final stage are shown in Fig. 4. The content variation of [O] in the molten steel and (FeO) generation are shown in Fig. 4(a). The [C] content decreases with the CO2 blowing, correspondingly [O] content continues to increase. When the [C] content decreases to approximately 0.035 mass%, the (FeO) generation gradually increases due to the reaction of CO2 and Fe. The variation of CO and CO2 generation with [C] content are shown in Fig. 4(b). CO and CO2 increase with the decreasing of [C] content. The reaction percentage of CO2 at the final stage is shown in Fig. 4(c). The reaction ratio of CO2 with [Fe] is calculated from the (FeO) generation, the reaction ratio of CO2 with [C] is obtained from the total CO generation minus the CO produced by the reaction of CO2 and [Fe], and the ratio of CO2 not participating in the reaction is obtained from the remaining CO2. With the decreasing of [C] content, the reaction ratio of CO2 with [C] gradually decreases. Then part of CO2 reacts with [Fe] to generate the same volume of CO, and another part of CO2 does not participate in the reaction. The FeO generation are attributed to two reasons: (a) FeO was formulated by the reaction between [Fe] and CO2 as expressed in Eq. (1). (b) CO2 is decomposed into CO(g) and [O] as expressed in Eq. (2), then [O] reacts with [Fe] to form FeO as expressed in Eq. (3).
|
C
O
2
(g)+Fe(l)=(FeO)+CO(g)
| (1) |

Summarizing the above results of the thermodynamic calculation, the reaction mechanism between the CO2 and the molten steel during the converter smelting process is shown in Fig. 5. The reaction behavior of the bottom blowing CO2 is analyzed according to the typical three stage of the converter. (a) Initial stage: the temperature of molten steel is low as 1350°C, and the contents of [C], [Si], and [Mn] are relatively high. CO2 reacts with [C] before the turning point and reacts with [C] and [Si] simultaneously after that. (b) Middle stage: the temperature increases to 1500°C, [Si] and [Mn] are almost completely oxidized, but the [C] content is still high. This stage is high-speed decarburization period. CO2 reacts with [C] to generate twice volume of CO. (c) Final stage: the temperature increases to 1620°C, and the [C] content decreases to about 0.1 mass%. Firstly CO2 reacts with [C] to generate CO. Then part of CO2 begins to react with [Fe] and the other part of CO2 does not react with the molten steel.

Compared with the inert gas, CO2 reacts with elements in the molten steel. Some studies indicate that bottom blowing CO2 increases the content of [O] in the molten steel and T.Fe content in the slag at the end of the converter.8,9) However, other studies hold that the stirring energy of the molten bath is enhanced due to the reaction of CO2 with [C], then the better kinetic conditions make the [O] and T.Fe content decrease.10,12) The [C]·[O] value is the key index to evaluate the oxidation of molten steel at the end-point state. The analysis of [C]·[O] value avoid the influence of the variation of the [C] content. In this paper, [C]·[O] value and T.Fe content in the slag are used as indicators to measure the oxidizability of molten steel and slag. The decarburization reaction, the standard Gibbs free energy change, the equilibrium constant, and the [C]·[O] value are shown as follows:
|
Δ
G
θ
=-22 000-38.34T
| (5) |
|
K=
P
CO
[C]⋅[O]⋅
f
C
⋅
f
O
| (6) |
|
[C]⋅[O]=
P
CO
EXP(
-Δ
G
θ
RT
)
⋅
f
C
⋅
f
O
| (7) |
where Δ
Gθ and
K represent the standard Gibbs free energy change and equilibrium constant of reaction (4), respectively.
T represents the temperature.
fC and
fO represent the activity coefficients for carbon and oxygen, respectively, R is a gas constant. [
C] and [
O] are the mass fraction of carbon and oxygen, respectively.
PCO is partial pressure of CO.
The effect of the gas type and the bottom blowing intensity on the [C]·[O] value are shown in Fig. 6. The box plots from the top to bottom are the maximum, upper quartile, median, lower quartile, and minimum values. The circle symbol is the average value. In the industrial trials of No. 1 to No. 5, Ar was blown at the final stage of 12–15 minutes. Trial No. 6 was conducted by the bottom blowing CO2 during the whole process. The temperature was 1630–1670°C and the tapping [C] was from 0.03 mass% to 0.05 mass%. The results of No. 1 to No. 5 show the same trend that the [C]·[O] value decreased with the increasing of the bottom blowing intensity. The volume of Ar in the molten steel increases with the increasing of bottom-blown Ar intensity, and correspondingly the CO volume decreases, resulting in a decrease in CO partial pressure, which leads to a decrease in the [C]·[O] value. Interestingly, trial No. 6 shows the opposite trend that the [C]·[O] value increased with the decreasing of the blowing intensity at the final stage. The reasons were attributed to two aspects: (a) CO partial pressure is increased by the reactions of CO2 with [C], resulting in the[C]·[O] value increasing; (b) FeO is generated by the reaction of CO2 and [Fe] when the [C] content is low at the final stage, then FeO is decomposed into [Fe] and [O]. Therefore, the [C]·[O] value is mainly determined by the gas type and the bottom blowing intensity at the final stage. When the bottom blowing gas is inert gas at the final stage, [C]·[O] decreases with the increasing of the bottom blowing intensity for the inert gas reduces the partial pressure of CO. However, when the bottom blowing gas at the final stage is CO2 gas, the [C]·[O] value increases with the increasing of the bottom blowing intensity for the reaction of CO2 with the molten steel increases the CO partial pressure, in addition, the oxidation properties of CO2 increase the [O] content of the molten steel.

The partial pressure of CO is calculated by Eq. (8), and the effect of the bottom blowing intensity on the partial pressure of CO is shown in Fig. 7. Under the condition of bottom blowing Ar, the CO partial pressure decreases from 0.70 atm to about 0.65 atm with the increasing of the bottom blowing intensity. With the bottom blowing CO2 at the final stage, the CO partial pressure increases from 0.70 atm to 0.76 atm with the increasing of the bottom blowing intensity.
|
P
CO
=[C]⋅[O]⋅EXP(
-Δ
G
θ
RT
)
⋅
f
C
⋅
f
O
| (8) |

The effect of the bottom blowing intensity on the T.Fe content in the slag is shown in Fig. 8. Due to the interaction between [O] in the molten steel and the T.Fe in the slag, the effect rule of the bottom blowing gas and the intensity on the T.Fe content and [C]·[O] value was similar. The results from No. 1 to No. 5 show the same trend that the T.Fe content decreased with the increasing of the bottom blowing intensity. That was mainly due to the decreased [O] content at the final stage with increasing of the bottom blowing intensity. Similarly, trial No. 6 shows the opposite trend that the T.Fe content in the slag gradually increased with the increasing of the bottom blowing intensity. The [O] content in the molten steel increased when CO2 was blown at the final stage, and FeO was generated by the reaction of CO2 with [Fe]. Therefore, T.Fe content in the slag increased with the increasing of the bottom blowing intensity of CO2 at the final stage.

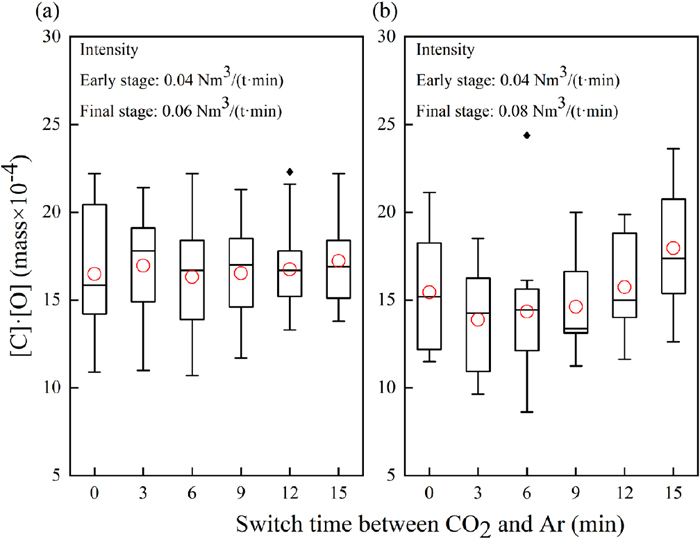
Under the blowing intensity at the final stage of 0.06 Nm3/(t·min), the effect of CO2 blowing time on [C]·[O] value is shown in Fig. 9(a). Compared to the CO2 blowing time from 0 min to 12 min, [C]·[O] value slightly increased when the CO2 blowing time increased to 15 min. When the blowing intensity at the final stage was 0.08 Nm3/(t·min), the effect of CO2 blowing time on [C]·[O] value is shown in Fig. 9(b). Compared to the CO2 blowing time from 0 min to 12 min, [C]·[O] value significantly increased when the CO2 blowing time increased to 15 min.
Similarly to variation pattern of [C]·[O] value, the effect of CO2 blowing time on the T.Fe content in the slag are shown in Fig. 10. With the bottom blowing intensity at the final stage of 0.06 Nm3/(t·min), compared to the CO2 blowing time from 3 min to 12 min, there was a slight increase as for T.Fe content (maximum value and average value) when the CO2 blowing time increased to 15 min. With the bottom blowing intensity at the final stage of 0.08 Nm3/(t·min), compared to the CO2 blowing time from 0 min to 12 min, T.Fe content significantly increased from 17.5 mass% to 20.5 mass% when the CO2 blowing time increased to 15 min.
3.3. Effect of Bottom Blowing CO2 on the Decarburization Rate
The role of CO2 in the converter process mainly depends on the reaction between CO2 and [C] in the molten steel. Therefore, it is necessary to analyze the effects of the bottom blowing inert gas and the bottom blowing CO2 on the decarburization rate. Figure 11 shows the variation of exhaust gas under the inert gas mode (No. 1) and CO2 mode (No. 6). CO was generated by the primary combustion of [C]. CO2 was derived from secondary combustion of CO gas with the oxygen in the converter and the air inhaled from the gap between the furnace and the exhaust gas hood. The sum of CO and CO2 represent the consumption of carbon in the molten steel. (CO+CO2) continues to increase to maximum value at 4 to 5 min which was estimated as the first critical point, shown as ICP in the Fig. 11. After the first critical point ICP, (CO+CO2) was stable about 80 volume% for a period of time. (CO+CO2) decreased sharply at about 12 to 13 min, which was estimated as the second critical point, shown as IICP in the Fig. 11.

Exhaust gas analysis was used to calculate the dynamic [C] content and decarburization rate. According to the mass balance of carbon elements in the molten steel, the decarburization rate is obtained from the variation of the exhaust gas in the converter. No. 1 and No. 6 are compared as pure inert gas and pure CO2 bottom mode. The Eqs. (9), (10), and (11) are used to calculate the [C] content in the converter process by the mass balance of carbon.
|
C
0
=
W
hot metal
⋅
[C]
hot metal
0
+
W
scrap
⋅
C
scrap
0
| (9) |
|
Δ
C
t
=
∫
0→t
[
k⋅
12 000
22.4
⋅
V
waste gas
⋅(
CO
%
waste gas
+C
O
2
%
waste gas
)
]×dt
| (10) |
|
[C]
t
=
(
C
0
-Δ
C
t
)
/
(
W
hot metal
+
W
scrap
)
| (11) |
where
C0 is the total carbon mass brought by the hot metal and the scrap.
Whot metal and
Wscrap represent the weight of hot metal and scrap, respectively. [
C]0
hot metal and
C0
scrap are the carbon content of hot metal and scrap, respectively.
t is the blowing time. Δ
Ct is the consumption of carbon at the blowing time t.
k is the correction coefficient. [
C]
t is the carbon content of the molten steel at the blowing time
t.
Figure 12 shows the relationship between the decarburization rate and the carbon content under two modes. The solid line and dashed line represent the decarburization rate of N2–Ar blowing mode and CO2 blowing mode, respectively. The first stage was mainly for the oxidation reaction of [Si] and [Mn]. The decarburization rate increased to 0.37 mass%[C]/min with the [C] content decreasing from 4.3 mass% to ICP of 3.3 mass%. The second stage was the high-speed decarburization period with the [C] content decreasing from ICP to IICP of 0.7 mass%, and the decarburization rate was maintained constantly 0.35 to 0.4 mass%[C]/min. The third stage was the final stage of decarburization, carbon mass transfer became the limiting link at the final stage, and the decarburization rate decreased from 0.37 mass%[C]/min to 0 mass%[C]/min. Therefore, it was mutually confirmed with the thermodynamic calculation results in the previous section. Comparing the decarburization rate of the N2–Ar blowing mode and CO2 blowing mode, the variation of decarburization rate of these two modes was the same. The values of decarbonization rate at the first critical point (ICP), the second critical point (IICP), and the peak decarbonization period are quite close. CO2 has little effect on the decarburization rate of molten steel, for the flow of CO2 (800 Nm3/h) accounts for less than 1 volume% of the total flue gas flow (240000 Nm3/h). In order to improve the reaction rate of CO2 and [C] in the molten steel, CO2 gas is blown into the molten steel during the high-speed decarburization. It is necessary for the CO2 to be blown during the high-speed decarburization period when the [C] content is between 0.7 mass% and 3.3 mass% and the time is between 5 and 12 minutes.

4. Conclusions
In the current study, the reaction mechanism of CO2 with molten steel was studied by the thermodynamic software, the effect of CO2 bottom blowing time and the blowing intensity on the end-point state were studied by industrial trials in a 300 t converter. The main conclusions are as follows:
(1) The reaction behavior of CO2 after the bottom blowing into the molten steel is mainly divided into three stages: (a) Initial stage: CO2 reacts with [C] before the turning point, and reacts the same with [C] and [Si] after that. (b) Middle stage: CO2 completely reacts with [C] to produce twice the volume of CO. (c) Final stage: with the decreasing of [C] content, CO2 begins to react with [Fe], and the ratio of unreacted CO2 gradually increases.
(2) The [C]·[O] value is mainly affected by the CO partial pressure at the final stage. CO partial pressure decreases from 0.70 atm to 0.65 atm with the increasing of bottom blowing intensity when Ar is bottom blown at the final stage, and the [C]·[O] value and the T.Fe content in the slag both decrease correspondingly. CO partial pressure increases from 0.70 atm to 0.76 atm with the increasing of bottom blowing intensity when CO2 is bottom blown at the final stage, the [C]·[O] value and the T.Fe content in the slag both increase correspondingly.
(3) CO2 bottom blowing has little effect on the decarburization rate. In order to improve the reaction rate of CO2 and the [C] in molten steel, it is necessary for the CO2 to be blown during the high-speed decarburization period when the [C] content is between 0.7 mass% and 3.3 mass% and the time is between 5 and 12 minutes.
Acknowledgments
The authors deeply appreciate Shougang Group Co., Ltd. for permission to publish this paper.
References
- 1) K. Han, C. K. Ahn and M. S. Lee: Int. J. Greenhouse Gas Contr., 27 (2014), 239. https://doi.org/10.1016/j.ijggc.2014.05.014
- 2) P. Cavaliere: Clean Ironmaking and Steelmaking Processes: Efficient Technologies for Greenhouse Emissions Abatement, Springer International Publishing, Cham, (2019), 1. https://doi.org/10.1007/978-3-030-21209-4
- 3) D. Gielen: Energy Convers. Manage., 44 (2003), 1027. https://doi.org/10.1016/S0196-8904(02)00111-5
- 4) C. C. Xu and D.-q. Cang: J. Iron Steel Res. Int., 17 (2010), 1. https://doi.org/10.1016/S1006-706X(10)60064-7
- 5) K. Dong and X. Wang: Metals, 9 (2019), 273. https://doi.org/10.3390/met9030273
- 6) Y. Zhou, G. Wei and R. Zhu: J. CO2 Util., 60 (2022), 102016. https://doi.org/10.1016/j.jcou.2022.102016
- 7) W. Wu, R. Zhu, Z. Li, C. Wang and G. Wei: Ironmaking Steelmaking, 48 (2021), 852. https://doi.org/10.1080/03019233.2021.1874623
- 8) A. S. Normanton, D. M. Bryce, E. Craggs and K. McGregor: Steel Times, 216 (1988), 503.
- 9) R. J. Selines: Metal. Int., 2 (1989), 133.
- 10) G. Jin: Steelmaking, 3 (1985), 96 (in Chinese).
- 11) B. Hao, G. Jin, J. Gao and E. Ma: Angang Technol., 4 (1983), 12 (in Chinese).
- 12) G. Wei, Q. Zhu, S. Hu, R. Zhu and C. Feng: Steelmaking, 37 (2021), 8 (in Chinese).
- 13) Z. Ibaraki, M. Okashima, Y. Ueda, M. Kanemoto and K. Arima: Tetsu-to-Hagané, 71 (1985), S175 (in Japanese). https://doi.org/10.2355/tetsutohagane1955.71.4_S151
- 14) H. Iso, H. Jiyono, M. Honda, K. Arima, M. Kanemoto and Y. Ueda: Tetsu-to-Hagané, 69 (1983), S1012 (in Japanese). https://doi.org/10.2355/tetsutohagane1955.69.12_S963
- 15) M. Sato, K. Ichihara and T. Okada: Tetsu-to-Hagané, 70 (1984), S945 (in Japanese). https://doi.org/10.2355/tetsutohagane1955.70.12_S923
- 16) T. Takahashi, F. Kitani, Y. Nimura, H. Hasegawa, Y. Shiratani and N. Hiraga: Tetsu-to-Hagané, 68 (1982), S995 (in Japanese). https://doi.org/10.2355/tetsutohagane1955.68.11_S965
- 17) H. Kobayashi, Y. Nimura, S. Kuriyama, Y. Shiratani, M. Hanmyo and Y. Miyawaki: Tetsu-to-Hagané, 70 (1984), S253 (in Japanese). https://doi.org/10.2355/tetsutohagane1955.70.4_S251
- 18) G. Wei, R. Zhu, Y. Wang, X. Wu and K. Dong: J. Iron Steel Res. Int., 26 (2019), 909. https://doi.org/10.1007/s42243-018-0163-7
- 19) B. Tian, Y. Zhou, G. Wei, R. Zhu, W. Wu and Z. Yang: Ceram. Int., 48 (2022), 36936. https://doi.org/10.1016/j.ceramint.2022.08.260
- 20) W. Dong, A. Xu, H. Li, S. Guan, C. Ji, N. Hao and X. Deng: Metall. Mater. Trans. B, 53 (2022), 3575. https://doi.org/10.1007/s11663-022-02621-3
- 21) Y. Zhou, R. Zhu and G. Wei: Energy Rep., 8 (2022), 7274. https://doi.org/10.1016/j.egyr.2022.05.234
- 22) M. Lv, R. Zhu, X. Wei, H. Wang and X. Bi: Steel Res. Int., 83 (2012), 11. https://doi.org/10.1002/srin.201100166
- 23) C. Yi, R. Zhu, B. Chen, C. Wang and J. Ke: ISIJ Int., 49 (2009), 1694. https://doi.org/10.2355/isijinternational.49.1694
- 24) R. Zhu, X. Bi, M. Lv, R. Liu and X. Bao: Adv. Mater. Res., 284–286 (2011), 1216. https://doi.org/10.4028/www.scientific.net/AMR.284-286.1216
- 25) W. H. Wu, R. Zhu, H. Wang, C. Wang and G. Wei: Ironmaking Steelmaking, 49 (2022), 147. https://doi.org/10.1080/03019233.2021.1972738
- 26) Y. Gu, H. Wang, R. Zhu, J. Wang, M. Lv and H. Wang: Steel Res. Int., 85 (2014), 589. https://doi.org/10.1002/srin.201300106
- 27) K. Dong, R. Zhu, R. Liu, H. Wang and C. Zhou: J. Univ. Sci. Technol. Beijing, 36 (2014), 226 (in Chinese). https://doi.org/10.13374/j.issn1001-053x.2014.s1.042
- 28) G. Chen, J. Yang, L. Li, M. Zhang and S. He: J. CO2 Util., 50 (2021), 101586. https://doi.org/10.1016/j.jcou.2021.101586
- 29) B. Han, G. Wei, R. Zhu, W. Wu, J. Jiang, C. Feng, J. Dong, S. Hu and R. Liu: J. CO2 Util., 34 (2019), 53. https://doi.org/10.1016/j.jcou.2019.05.038
- 30) Q. Li, H. Wang, R. Zhu, D. Shou, R. Liu and Y. Gu: Contin. Cast., 40 (2015), 5 (in Chinese). https://doi.org/10.13228/j.boyuan.issn1005-4006.20140053
- 31) H. Ono-Nakazato, Y. Morita, K. Tamura, T. Usui and K. Marukawa: ISIJ Int., 41 (2001), S61. https://doi.org/10.2355/isijinternational.41.Suppl_S61
 https://orcid.org/0000-0002-6397-5572
https://orcid.org/0000-0002-6397-5572












