2023 Volume 63 Issue 6 Pages 981-992
2023 Volume 63 Issue 6 Pages 981-992
The treatment of desulfurization ash (DA) by high-temperature can solve the increasingly environmental risk caused by the accumulation desulfurization ash on the one hand, and realize the reuse of Ca and S on the other. However, the understanding of the high-temperature reduction decomposition process of desulfurization ash is still vague. In this study, a multivariate and multiphase reaction mathematical model of the complex system of desulfurization ash, carbon, and gas is established by using the principle of minimum free energy. The modeling results show that the reductive decomposition of DA has four stages, and the decomposition products are different in each stage. This result confirms that the optimal thermodynamic conditions to obtain only CaO as a decomposed product are a temperature greater than 1400 K and a C/S molar ratio of 0.5. Further, the processes of CaO and CaS production are parallel competitive reactions, but are regulated by different factors at different stages. A micropositive pressure equilibrium reaction crucible was designed for laboratory DA decomposition experiments. The correctness of the calculation result of the minimum free energy mathematical model is proved by the high temperature reductive decomposition experiment. When the temperature and C/S molar ratio are 1500 K and 0.5, the DA decomposition rate can reach 100%. The main reaction product is spherical CaO, the minimum S content is approximately 1.5%, and the desulfurization rate can reach approximately 70%. The present strategy is highly promising for application in industrial DA recycling processes.
Sulfur dioxide is a major component of industrial flue gas emissions and an air pollutant.1) Currently, atmospheric emission standards are increasingly strict worldwide.2) Waste gas from metallurgy and electric power industries must undergo strict desulfurization treatment before it can be discharged into the atmosphere,3,4) so the desulfurization waste produced increases significantly. Desulfurization ash (DA) is a typical desulfurization solid waste from the steel and electric power industries.5) However, the complex chemical composition and spatial structure of desulfurization ash do not allow for large-scale use in construction and other fields.6,7) In addition, compared with natural gypsum, the use of DA for construction purposes is limited due to the fluidity and thixotropy caused by the small particle size; thus, DA cannot replace natural gypsum.8,9,10)
Therefore, the chemical decomposition and recycling of DA to form raw industrial materials is a development with great potential for application. DA is regarded as a polymer of element S, especially in the desulfurization process with calcium-based desulfurizers, the process of decomposing DA will produce sulfur-containing flue gas with high concentration, which can enter the industrial acid production system to produce industrial sulfuric acid. This process will not produce industrial pollutants again. However, control of CaSO4 in the decomposition process and the purity of the decomposition products limit the effectiveness of DA resource recovery.
In addition to the target product CaO, CaS is often formed during the high-temperature decomposition of CaSO4 from DA. The formation of CaS is controlled to ensure the purity of CaO in the decomposition product, which is further put into use as industrial lime. Many scholars have launched a series of discussions on the formation processes and control methods of CaO and CaS. Some scholars have found that the decomposition of CaSO4 into CaO or CaS at high temperature is a parallel competitive process. To study gas reduction, Xiao et al.,11) Zhang et al.12) and Yan et al.13) used experimental data and theoretical models to indicate that CaO is generated by the direct decomposition of CaSO4 in a reducing atmosphere, while CaS and CaO generation are parallel competition reactions. The primary and secondary relations of reactions are mainly affected by the reaction temperature and system reducibility. Furthermore, Robbins et al.14) and S.B. Jagtap et al.15) used the gas-solid reaction model and a large amount of experimental data on decomposition rates to prove that CaO is formed by the direct decomposition of CaSO4 and its gradual removal from the gas products on the surface. The decomposition of CaSO4 to form CaO satisfies both the nucleation regrowth mechanism and the unreacted nucleus model. While studying solid reduction, the essence is still the gas-solid reaction through the gasification of the reducing agent followed by reduction. Jia et al.,16) Niu et al.,17) Xu et al.18) and E.M van der Merwe et al.19) used solid carbon to carry out reductive decomposition, proving that the formation of CaO and CaS in the process of solid carbon reduction decomposition is a parallel competitive reaction and that the reaction follows a typical gas-solid reaction model. Factors such as reaction temperature and reducibility20) determine the direction of the reaction.
However, in the previous studies show that it is impossible to avoid the formation of CaS in the one-step reaction process, regardless of the conditions. The decomposition kinetics of CaSO4 shows some differences from the decomposition behavior of the actual reaction process. Therefore, CaS is treated as an intermediate product and becomes a transition reaction in the decomposition process of CaSO4 at high temperature. Zhu et al.21) and Zheng et al.22) studied the high-temperature decomposition of phosphogypsum under different C/S ratios and found that the decomposition of CaSO4 was a typical solid-solid reaction. CaSO4 first decomposes to form CaS and then reacts with CaSO4 to form CaO. However, a gas-solid reaction occurs to a small degree in the reaction. At the same time, Zheng et al.23) used TGA-FTIR to detect the escaping gas species and studied the kinetics of CaSO4 decomposition process, showing that the CaS generation reaction has a lower apparent activation energy than CaO generation, supporting the above-mentioned scholars’ view. Song et al.24) showed that the formation rate of CaO and CaS was negatively correlated in the decomposition process, further proving that CaS was present as a transition product in the decomposition process. Additionally, to exclude the effect of reducing agent on the reaction of CaS generation, Jia et al.25) used different kinds of coal to carry out reductive decomposition, and the decomposition reaction was mainly the CaS formation reaction at 800°C. However, Kamphuis et al.26) found that the rate of solid-solid reaction was much lower than the actual chemical reaction rate, which indicates that a single solid-solid reaction could not explain the whole high-temperature decomposition process, and speculate that there may be a liquid-liquid reaction existing in the reaction process. However, Fan et al.27) used a synchronous analyzer to study the high-temperature melting state of CaS and CaSO4 at 700°C–1300°C and proved that there was no liquid phase between the CaS and CaSO4 phases or CaS, CaSO4 and CaO phases within this temperature range.
Currently, the decomposition temperature of DA is reduced in order to decompose DA effectively. Reducing agents are often used to treat CaSO4, which has a high decomposition temperature in DA, to form CaO and SO2 under low temperature conditions. However, there is substantial disagreement regarding the decomposition process of CaSO4 in desulfurization ash and how to control the formation of CaO and CaS. This study used the thermodynamic principle of minimum free energy to analyze the whole decomposition process of CaSO4 at different temperatures and under different reduction conditions obtain the control conditions for CaO, SO2 and CaS formation. In this study, experimental high-temperature DA decomposition experiments were carried out to verify the calculated thermodynamic conditions and to prepare highly pure CaO samples. This research studied and analyzed the utilization of industrial solid waste resources to achieve a high degree of resource utilization.
Any self-organizing system at equilibrium tends to be in a state of minimal free energy, and the principle of minimum free energy is based on this law. During the entire reductive decomposition of DA, the energy of the system is at its lowest, and the components are in their most stable states when the reaction reaches equilibrium.28) Therefore, the chemical equilibrium of the reduction and decomposition of DA is a mathematical problem of minimization with constraints, and the quantitative and qualitative analysis of the reduction and decomposition process of DA can be calculated by this mathematical model. Due to the possible presence of CaSO4 rich in crystal water in the DA,29) CaSO4·2H2O is used as the initial component in the thermodynamic model.
Based on the reaction parameters of the studied system, the following two points are considered.
1. To simplify the calculation model, the liquid phase and solid solution, which may be present in a small portion of the system, will not be considered.
2. Based on the independent reaction determination conditions, the following 15 independent reactions were considered in the system.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
| (15) |
Substances that may be present at equilibrium: SO2, CO2, CO, C, CaSO4, CaO, CaS, COS, CS, CS2, CaCO3, CaC2, SO3, CaSO3, CaSO4·0.5H2O, CaSO4·2H2O, O2, S, H2, H2O (20 components).
| (16) |
According to the law of conservation of atoms, the above equation has the following constraints:
S.T.
| (17) |
The standard Gibbs free energy of each group element in Eq. (16) is a temperature-dependent function. Based on the Eqs. (18) and (19) the Gibbs free energy is defined with the Gibbs-Helmholtz equation.
| (18) |
| (19) |
Equations (18) and (19) are deformed and then subjected to indefinite integration to obtain the binomial relationship between the standard Gibbs free energy and temperature for each group element, as shown in Eq. (20).
| (20) |
The relevant data from the datasheet literature30) were chosen to calculate the standard molar Gibbs free energy values for group element i.
Where min. G is the minimum value of the total Gibbs free energy G of the system; M is the total number of components in the system; ni is the amount of substance present when component i is at equilibrium (mol); Gi is the molar free energy of component i (J/mol); ai is the activity of component i in the standard state of the pure substance; Ptotal is the total gas pressure in the system, and it is assumed that Ptotal=1; R is the ideal gas constant, and its value is 8.314 J/mol·K; T is the system temperature (K); and
The nonlinear programming problem under the above constraints was calculated through the linGo17.0 software, and the component types of the whole system at different temperatures, the molar ratios and the substance content at equilibrium were analyzed. A numerical error limit below 10−5 is used as the limiting convergence condition in performing the numerical solution process. The optimal reaction conditions for the high-temperature reductive decomposition of DA were further determined.
The DA used was prepared by a steel company in China. Homogeneous samples were obtained by thorough stirring in a stirrer. The sample was sieved through a 200-mesh screen, dried in a blast drying oven at 105°C for 3 hours, removed and placed in a desiccator for later use. As the desulfurization process in sintered flue gas has a low desulfurization efficiency, the amount of desulfurization agent used in the desulfurization process is increased to meet the flue gas emission targets, which will result in a low S content in the desulfurization ash. A high frequency infrared carbon and sulfur meter was used to detect the S content in the desulfurization ash. Compared to other desulfurization ashes, the S level of the sample only has 4.92%. The elemental composition and phase composition of the sample were analyzed by X-ray powder diffraction (XRD) and atomic fluorescence spectroscopy (XRF), respectively. The components of the DA are listed in Table 1.
| Composition | CaO | SO3 | MgO | F | Fe2O3 | SiO2 | Na2O | K2O | Al2O3 |
|---|---|---|---|---|---|---|---|---|---|
| Content % | 74.13 | 12.29 | 5.09 | 3.19 | 0.30 | 0.47 | 0.77 | 0.27 | 0.34 |
DA is a high-calcium and low-sulfur solid waste whose main components are calcium-containing compounds and Si, Na, K, Al, and Fe. According to the XRD pattern and SEM micrograph analysis of the DA (Fig. 1), it is mainly composed of three calcium-containing phases, Ca(OH)2, CaCO3 and CaSO4. S in DA is present only in the form of CaSO4. The content of S in DA was determined by infrared carbon and sulfur analysis, and the content of CaSO4 was 20.91%. Raw DA is a complex system composed of a variety of compounds, and the composition consists mainly of agglomerates formed by flakes, strips, and numerous irregular bodies of particulate matter. This structural characteristic is an important reason why DA cannot be directly applied for construction materials.
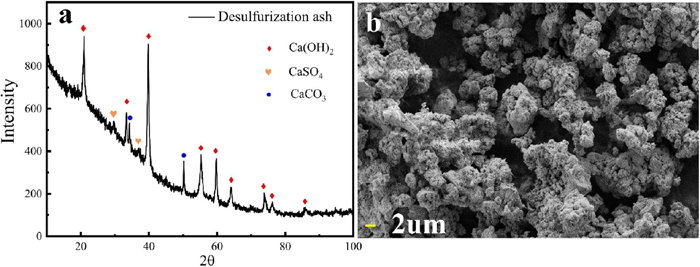
Composition and micromorphological analysis of DA (a. XRD pattern, b. SEM micrograph of the DA). (Online version in color.)
The equipment methods used in this research were as follows: a high temperature reactor; atomic fluorescence spectroscopy (XRF); an X-ray diffractometer; a scanning electron microscope (SEM, FEI Quanta 250) equipped with an energy dispersive spectrometer (EDS); and a high frequency infrared carbon-sulfur analyzer.
3.3. Transient High-temperature Reduction ExperimentsA micropositive pressure nested reduction crucible, as shown in Fig. 2, was designed to prevent carbon from being oxidized by air while heating during the carbon-based reduction process. An internal corundum crucible was used for the high-temperature reduction reaction, and an outer graphite crucible was used to isolate the outside air; lightweight corundum spheres controlled the internal gas exhaust and isolated the sample from outside air. Samples with different C/S molar ratios were placed in the micropositive pressure nested reduction crucible. When the temperature was increased to the specified temperature, the device was placed in a high-temperature reaction furnace, and heated there for 30 min, and then removed for air cooling. Finally, the cooled samples were collected for further analysis to determine five evaluation indicators: the residual S content, CaSO4 content, CaS content, decomposition rate and desulfurization rate. In the experiments, the CaSO4 and CaS contents were determined by the iodometric-weight method, and the total S content was determined by means of a high-frequency infrared carbon sulfur meter. The decomposition rate and desulfurization rate are calculated according to reaction Eqs. (21) and (22), respectively.
| (21) |
| (22) |

A photograph and schematic of the device. (Online version in color.)
During the minimum free energy simulation calculation, the number of substances in each component was studied when the decomposition of DA reached equilibrium under temperature conditions of 400 K–1500 K and a C/S molar ratio of 0.1–2.5. Any compound consisting of elements introduced by the initial group in the reaction system may be present in the reaction equilibrium system. Therefore, the 20 most likely components composed of the five elements C, H, O, Ca, and S are considered to be present in the reaction system. The specific results are shown in Fig. 3.

Variation in components of the reaction field at equilibrium. (Online version in color.)
Although 20 components may be present in the reaction system, 8 components, CaSO4, CaO, CaS, COS, CO2, SO2, CO2, and CO, are the main components present at various temperatures, and the other 12 components do not appear or are present in extremely low quantities throughout the reaction, so they will not be discussed here.
The whole reaction equilibrium process is divided into four temperature regions by three temperature nodes of 700 K, 1200 K and 1400 K. When the reaction reaches equilibrium, CaSO4 is removed from the waters of crystallization in the form of dihydrate compounds at the beginning of the reaction and remains in the gas phase throughout the reaction.
In the low-temperature region (400 K<T≤700 K), according to reaction (5), CaCO3 and CO2 form.31) As the C content increases, the contents of CaCO3 and CO2 also increase. CO forms according to reaction (4), and the gas of CO reacts with the S formed from COS by reaction (8).32) However, reaction (5) becomes limited as the temperature continues to increase. The production of CaCO3 and COS gradually decreases until the temperature reaches 700 K, at which point it stops completely. At this moment, reaction (7) becomes the dominant reaction and produces CaS and CO2. Zeng confirmed that the ignition point temperature of COS is lower than 600 K,33) and the decomposition of COS occurs as the temperature increases.
In the same system, the binomial relationship curve between the standard Gibbs free energy and temperature determines the priority of the reaction. Therefore, the binomial relationship between the standard Gibbs free energy and temperature of the different reactions of CaSO4 and C in the high-temperature reduction and decomposition process was calculated by the indefinite integral method, and the priority of CaSO4 decomposition under different conditions was determined. The specific results are shown in Fig. 4.

The standard Gibbs free energy of different reactions during high-temperature reduction and decomposition. (Online version in color.)
In the same closed system, the standard Gibbs free energy of the reductive decomposition of CaSO4 to CaO and SO2 is greater than 0 when the temperature is lower than 1100 K. The reaction is thermodynamically unfavorable. The curves of reaction (6) and reaction (7) intersect at 1400 K. Therefore, reaction (7) proceeds more easily than reaction (6) when the temperature is below 1400 K, while the opposite is true when it is above 1400 K.
In the low-medium temperature region (700 K≤T<1100 K), CaS is obtained through the reduction of CaSO4 by C. Regardless of how strong or weak the reduction is in the reaction system, CaO and SO2 do not form in this reaction zone because the standard Gibbs free energy of reaction (6) is greater than zero in this temperature region and reaction (6) will be suppressed.
In the medium-high temperature region (1100 K≤T<1400 K), the reaction temperature zone is still dominated by reaction (7). However, the amount of C required for the complete reduction of CaSO4 decreases with increasing temperature. The main reason is that reaction (6) is promoted by increasing temperature, which leads to the formation of small amounts of CaO and SO2 in the reaction equilibrium group element.
With increasing C content in the reaction, reaction (6) occurs to a progressively greater extent that leads to the CaO and SO2 contents gradually increasing, but the content of CaS is not affected by the formation of SO2 and CaO. These two reactions are parallel competition reactions, but the CaS formation reaction is the dominant reaction in the process.
In the high temperature zone (1400 K≤T<1500 K), the production of CaO and SO2 increases and then decreases with increasing temperature, and there are peaks. Reaction (6) is the dominant reaction because the standard Gibbs free energy versus temperature curve for reaction (6) in the high temperature region is below that of reaction (7). The amount of C required for the complete reductive decomposition of CaSO4 is at the lowest value, and the amount of CaO and SO2 formed reaches its maximum value when the C/S molar ratio is 0.5. This result is similar to that of the thermodynamic software calculation employed by Yan, which suggests that the CaO content increases substantially at 1100°C (1373 K). However, the amount of C required for the complete reduction of CaSO4 is not lower, and the excess carbon leads to lower CaO and SO2 contents when the temperature rises to 1500 K. Thus, the reaction is influenced by the amount of C in the system.
4.2. The Effect of the C Content on the Mathematical ResultsIn the low-temperature and low-medium temperature regions, CaSO4 decreases with increasing C content in the high-temperature reduction process. Conversely, the reductive decomposition products of CaSO4, such as CaCO3, COS and CaS, increase with increasing C content. However, the variation in the high-medium temperature and high temperature regions is more complex. In the high-medium temperature region, CaO and SO2 appear in the reaction system due to reaction (6), and the presence of the C content required for the peak content of CaO, SO2. With increasing temperature, the peak C content decreases continuously because excessive C causes the reaction system to become strongly reducing, which inhibits CaO and SO2 generation.
When the reaction temperature is 1400 K, with an increasing C/S molar ratio, the main products of high-temperature reductive decomposition are CaO and SO2, and when the C/S molar ratio is 0.5, CaSO4 completely decomposes to form CaO and SO2. The continuous increase in the molar ratio of C/S in the system is conducive to reaction (7) and the formation of CaS. The S in CaSO4 is captured by CaS, reducing the formation of CaO and SO2. Reaction (7) overtakes reaction (6) as the main reaction due to this process until the formation of CaS in the high-temperature reductive decomposition is complete. When the reaction temperature is too high (1500 K), the excess C oxidizes to form a small amount of CO, and a gas-solid reaction occurs to a small extent during pyrolysis in the reaction system. This result was demonstrated by Zheng D using Fourier infrared spectroscopy for the detection of CO.
4.3. Simulation of Ca- and S-containing Compounds to Establish Reaction Dominance ZonesTo further analyze the evolution of Ca and S during the high-temperature reductive decomposition of CaSO4, reaction dominance zones were established for the simulation of the main compounds in the reaction process. In the reaction process, the various reaction regions composed of different reaction temperatures and different C/S molar ratios are collectively referred to as the reaction field. The area where each group element is present is the dominant area. A graph of the dominance of the five most prevalent compounds in the reaction field is shown in Fig. 5.

Diagram of the dominance of the five compounds in the reaction field (a. Diagram of CaSO4, b. Diagram of CaS, c. Diagram of CaO, d. Diagram of SO2, e. Diagram of COS, f. Equilibrium phase diagram calculated by fact sage). (Online version in color.)
CaSO4, as the initial compound, is present throughout the reaction field, but it appears primarily in the reaction zone with a C/S molar ratio below 2 at low-medium temperatures (<1100 K), and the residual amount decreases continuously with increasing temperature and C content. However, the CaSO4 content shows anomalies in the high-temperature region (>1400 K). When the temperature is 1400 K and the C/S molar ratio is 0.5, the CaSO4 content is 0 in the system. The dominant region of CaS is generally distributed in the low-temperature high reduction region. However, the presence of COS in the temperature interval of 400 K–700 K leads to a low CaS content in this reaction region. The map has approximate mirror symmetry between the reaction dominance regions of CaSO4 and CaS. This result indicates that CaS easily forms as a reduced decomposition product of CaSO4 throughout the reaction process. However, this mirror symmetry is destroyed by COS in the low-temperature region.
The dominant regions of CaO and SO2 are similar throughout the reaction field. They increase and then decrease with increasing C content, and they peak at C/S=0.5 when the temperature is greater than 1100 K. A C/S molar ratio that is too high or too low results in CaS production and the presence of residual undecomposed CaSO4. Only when the temperature reaches above 1400 K and the C/S molar ratio is controlled at 0.5 is single CaO and SO2 produced, but such reaction conditions are difficult to achieve either in the laboratory or in industry. The dominant zone where COS is present is in the temperature interval of 400 K–700 K, and its generation is suppressed as the temperature increases.
In the Phase diagram module of Fact Sage 7.3 software, the Ca–O–S–C system was selected, the temperature range was 1200–1500°C, the state and composition of each reactant product was measured in units and the total P=1.01325×105 Pa, etc. The molar ratio n(C)/n(CaSO4)=0–2.5 was used as the horizontal coordinate and the temperature was used as the vertical coordinate. The dominant zone diagram (also called state diagram) of the C-reduction CaSO4 reaction was calculated as shown in Fig. 5(f). In contrast to Fig. 5(f), CaO will be formed by CaSO4 decomposition when the reaction temperature is above 1395 K and the C/CaSO4 molar ratio is around 0.5. Decreasing the C/CaSO4 molar ratio will result in insufficient CaSO4 decomposition, while too high C/CaSO4 molar ratio will lead to the formation of CaS as a by-product. When the temperature is below 1395 K, both CaO and CaS coexist at this time, and the C content required for the complete decomposition of CaSO4 gradually increases as the temperature gradually decreases until the C/CaSO4 molar ratio is about 2. The results of the above thermodynamic software calculations are like those of the thermodynamic model calculations, which corroborate the accuracy of the calculations in this study.
4.4. High-temperature Reductive Decomposition ExperimentThe accuracy of the simulation results of the thermodynamic mathematical model was verified by high-temperature reductive decomposition tests over the whole reaction field. The evaluation indexes of residual S content, CaSO4 content, CaS content, decomposition rate and desulfurization rate were analyzed and explained for each temperature range.
4.4.1. The Low-temperature and the Medium-low Temperature RegionsIn the above analysis, the low-temperature and medium-low temperature zones are mainly dominated by CaS generation and the formation of CaCO3 and COS due to the occurrence of reactions (4), (5), (8). The variation patterns of the five evaluation indicators after the high-temperature reduction of DA in the medium-low temperature region are shown in Fig. 6.

Changes in various evaluation indexes in the medium-low temperature area (a. CaS content change, b. CaSO4 content change, c. decomposition rate change, d. S content change, e. Desulfurization rate change). (Online version in color.)
The minimum free energy model is based on the reaction equilibrium, and the reaction temperature, reaction time, and reducibility all affect the reaction process. The production of CaO and SO2 is inhibited by temperature, and CaS is formed by the decomposition of CaSO4 in the low- to medium-temperature region. CaSO4 continuously decomposes to form CaS as the reducing atmosphere in the system increases, as shown in Figs. 6(a) and 6(b). However, lower reaction temperatures require longer reaction equilibrium times. Therefore, the decomposition is not sufficient at temperatures below 1000 K. The deduction of the minimum free energy principle calculation that the decomposition rate and the CaS content reach a maximum when the C/S molar ratio reaches 2 is also verified by the above experimental results. Furthermore, the S that should have been released is once again immobilized in CaS. For this reason, as shown in d and e in Fig. 6, the desulfurization rate is maintained at a low level, and the S content of the sample is close to the initial S content. Only a small fraction of S is released due to the inhomogeneity and instability of the reaction field. The above results show that CaSO4 decomposition will form CaS between decomposition at temperatures below 1100 K and there is no CaO and SO2 generation.
4.4.2. The Medium-high Temperature RegionIn the medium- to high-temperature region, CaS is no longer the only solid product of CaSO4 decomposition, and the desulfurization rate as well as the residual S content in the system change due to the generation of CaO and SO2.
The change curve of each evaluation index in the medium-high temperature region is shown in Fig. 7. The rate of change of the indicator is characterized by the slope of the line tangent to the curve. In the medium-high temperature region, the decomposition rate of CaSO4 and the production rate of CaS increase with increasing temperature and the reduction of the reaction system, as shown in Figs. 7(a), 7(b) and 7(c). When the high-temperature reduction reaction gradually reaches equilibrium, the decomposition rate of DA is close to 100%. However, the plots from d and e show that the residual S content increases with increasing carbon content until it is close to the initial S content. According to the results of the minimum free energy model calculations, the CaO and SO2 generation reactions are initiated in the medium-high temperature region. And the mathematical model calculations show that the process of CaO production is not affected by CaS when the reaction reaches equilibrium. CaO is not formed by further reaction of CaS. Clearly it is in parallel competition with the CaS generation reaction process. And this process is mainly regulated by the temperature. The enhancement of reducibility will only increase the process of CaS production. With the gradual increase in the reducing conditions, CaS production continues to increase, though more S is not released, and the desulfurization rate is close to 0% at a C/S molar ratio of 2.

Changes in various evaluation indexes in the medium- and high-temperature zones (a. CaS content change, b. CaSO4 content change, c. decomposition rate change, d. S content change, e. desulfurization rate change). (Online version in color.)
Reaction (6) is the dominant reaction in the reaction system in the high temperature zone, where the temperature is higher than 1400 K, and CaO and SO2 generation are maximized.
The change curve of each evaluation index in the high temperature zone is shown in Fig. 8. The increase in temperature causes a greater rate of decomposition of CaSO4 in this temperature interval, and the amount of C required for complete decomposition is reduced to the lowest value. According to the results of the model calculations, CaS is not formed in the high-temperature region of the reaction system when the C/S molar ratio is below 0.5. Therefore, as shown in Fig. 8(a), the rate of CaS production under slightly reducing (C/S < 0.5) conditions is smaller, and the content of CaS is lower. It is worth noting that at a temperature of 1400 K and a C/S molar ratio of 0.5, the decomposition rate shown in Fig. 8(c) is only 60% because the reaction is limited by time and does not reach equilibrium. However, when the temperature is 1500 K, as the C/S molar ratio increases, the S content of DA and desulfurization rate increase, and the maximum is reached when the C/S molar ratio is 0.5. The main reaction, reaction (6), occurs in the system under these conditions, the S is released from the DA at its maximum, and the desulfurization rate is nearly 70%. This variation is also consistent with the minimum free energy principle, which holds that the release of S is most complete at a C/S molar ratio of 0.5. Further CaS generation reactions gradually progress with increasing reduction, which causes the lowering of S release, and the desulfurization rate gradually decreases. CaSO4 completely decomposes to form CaS, and the desulfurization rate is close to 0% when the C/S molar ratio is 2. The above results suggest an important conclusion. In the high temperature region, the formation of CaO and CaS is still a parallel competitive reaction process, but the process is regulated by the strength of reduction. This means that in low-reducing conditions there is a single generation of CaO, while in high-reducing conditions there will be a parallel generation of CaS.
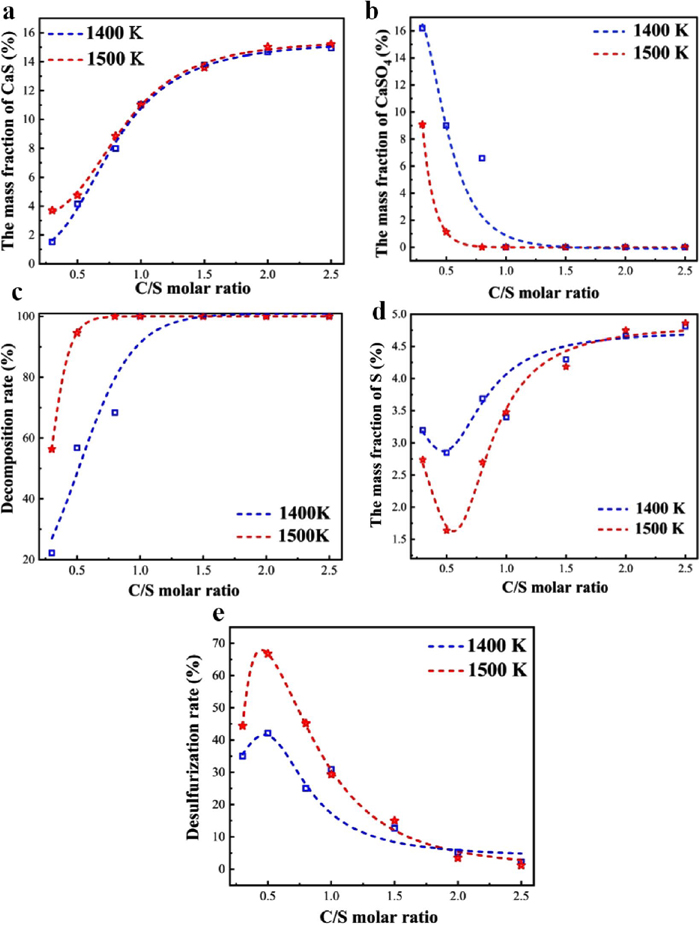
Changes in various evaluation indexes in the high-temperature zone (a. CaS content change, b. CaSO4 content change, c. decomposition rate change, d. S content change, e desulfurization rate change). (Online version in color.)
Through the simulated calculation of the free energy minimization and laboratory experiments, it was found that the desulfurization rate reaches approximately 70%, the decomposition rate of CaSO4 is 98%, and the minimum content of S is 1.6% when the reaction temperature, C/S molar ratio, and reaction time are 1500 K, 0.5, and 30 min, respectively. To study the actual industrial use under these conditions, the microscopic morphology of the decomposition sample was characterized.
The microscopic morphology of the samples after the decomposition of DA at 1400 K and 1500 K with different C/S molar ratios is presented in Fig. 9. The elemental analysis of the specific morphology was performed through EDS, and the EDS data are shown in Table 2. After the high-temperature reductive decomposition of DA, the sample morphology mainly shows spherical bodies of different sizes with a minimal number of lumps. When the C/S molar ratio is 0.5, the amount of bulk CaS in the decomposed sample decreases significantly with increasing temperature, and spherical CaO with a diameter of 2–7 μm is be the primary component of DA. However, the lumpy CaS content increases with increasing C content, which also verifies the variation pattern of CaO and CaS in the above discussion. When the temperature is 1500 K and the C/S molar ratio is 2, some CaO forms due to the decomposition of Ca(OH)2 and CaCO3 in the DA, but the particle size is smaller than that at other temperatures. Furthermore, due to the reductive decomposition of CaSO4 to form CaS, the sample morphology shows a three-dimensional porous structure with CaO spheroids connected and wrapped around each other with less individual independence. This result is similar to the CaS morphology observed in the study by Li C.34)

SEM micrograph of the decomposition products in the high-temperature zone (a. 1400 K–C/S=0.5, b. 1500 K–C/S=0.5, c. 1400 K–C/S=1, d. 1500 K –C/S=2). (Online version in color.)
| Spectrum | O | Mg | S | Ca | |
|---|---|---|---|---|---|
| a | Spectrum 1 | 16.46 | 1.20 | 0.75 | 80.37 |
| Spectrum 2 | 10.73 | 0.83 | 35.50 | 52.94 | |
| Spectrum 3 | 37.12 | 0.50 | – | 58.28 | |
| Spectrum 4 | 12.35 | 1.41 | 34.97 | 51.26 | |
| Spectrum 5 | 48.37 | 5.86 | 0.68 | 41.44 | |
| b | Spectrum 1 | 38.52 | 0.91 | – | 54.94 |
| Spectrum 2 | 48.50 | – | – | 51.50 | |
| Spectrum 3 | 38.08 | – | – | 61.92 | |
| c | Spectrum 1 | – | – | 42.03 | 57.97 |
| Spectrum 2 | – | – | 40.15 | 59.85 | |
| Spectrum 3 | 38.37 | – | – | 56.87 | |
| d | Spectrum 1 | 9.30 | – | 36.96 | 53.74 |
| Spectrum 2 | – | 2.42 | 12.15 | 85.43 | |
| Spectrum 3 | 35.49 | 2.38 | 1.90 | 60.23 |
The XRD patterns of the sample after the high-temperature decomposition of DA at 1500 K and C/S molar ratios of 0.5 and 2 are shown in Fig. 10. When the desulfurization ash decomposes at 1500 K with a C/S molar ratio of 0.5, it is mainly dominated by CaO, and the S-containing phase is mainly in the form of CaS. When the C/S molar ratio reaches 2, CaO is formed by the decomposition of Ca(OH)2 and CaCO3, but the intensity of the diffraction peak of CaS is strengthened, which indicates that the content of CaS is greater than that when the C/S molar ratio is 0.5. This result also proves that when the C/S molar ratio is 2 in the above experiments, CaSO4 decomposes at high temperature to form mainly CaS.

XRD pattern of the decomposition products at different C/S ratios when the reaction time and reaction temperature were 30 min and 1500 K, respectively. (Online version in color.)
The unreacted core model was applied to the reduction and decomposition of CaSO4 in DA. The specific decomposition mechanism is shown in Fig. 11. The carbon in the coal is oxidized to form the CO gas phase, which enters the reaction interface by external diffusion and undergoes interfacial chemical reactions to form a solid product layer. The gaseous products are released through the solid product layer to the outside of the reaction system by internal diffusion. The types of solid product layers and gaseous products are determined by the different reaction temperatures. As the reaction temperature increases, the sequence of change in the product layer is CaCO3–CaS–(CaO+CaS)–CaO; the change in the gas phase product is COS–CO2–(CO2+SO2)–SO2. However, the reaction proceeds in the direction of CaS and CO2 when reduction is enhanced.

Decomposition mechanism of CaSO4. (Online version in color.)
The decomposition of CaSO4 in desulfurization ash to form CaO involves the reaction process of multiphase complex system, and the mathematical model of decomposition is constructed by using the principle of minimum free energy, which can better simulate the trend of CaSO4 decomposition in different stages. The results of the model calculations are consistent with the previous experimental results which showing that CaO can be formed more efficiently and CaS formation can be avoided under high temperature and low reducibility conditions. The mathematical model shows that the decomposition mechanism of CaSO4 is specifically divided into four stages, and the accuracy of the model calculation is verified using the laboratory test results.
There are four reaction stages in the decomposition of DA throughout the reaction field. The formation of CaCO3, COS and CaS mainly occurs in the low-temperature region of 400 K<T≤700 K. Due to temperature limitations, only the formation of CaS occurs in the low-medium temperature region of 700 K≤T<1100 K. The desulfurization efficiency of the samples was gradually enhanced in the medium-high temperature region of 1100 K≤T<1400 K, where CaO and SO2 were generated in the system. At the same time, the production process of CaO and SO2 did not change the slope of the curve of CaS content change, and the two belong to the parallel competition relationship. When the reaction temperature was in the high-temperature region of 1400 K≤T<1500 K, the formation of CaS could be strictly suppressed in the system. When the temperature was 1400 K and the C/S molar ratio was 0.5, CaSO4 completely decomposed to form CaO and SO2.
The data of high temperature reaction test showed that the decomposition process of desulfurization ash had the characteristics of obvious four stages, which proved the accuracy of mathematical model calculation. It is worth noting that the processes of CaO and CaS production are in a parallel competitive reaction relationship, but in the low and medium low temperature regions, only CaS is produced. Competitive relationships in the mid- and high-temperature regions are regulated by temperature and reductive strength, respectively.
Furthermore, the decomposition rate of CaSO4 was close to 100%, and the desulfurization rate reached a maximum (70%) when the temperature was 1227°C and the C/S molar ratio was 0.5 the subsequent experimental study. The samples were mainly composed of spheroidal CaO, which contained less massive CaS, as analyzed by SEM and XRD. The sample of DA decomposed under these conditions formed a raw industrial material in which CaO was the main component and the sulfur content was low, which meets actual industrial production needs and realizes the recycling of solid waste resources.
This work was supported by the National Science Foundation of China (grant number 51704021), Fundamental Research Funds for the Central Universities (grant number FRF-TP-20-004A3, FRF-TP-19-030A2, FRF-TP-16-079A1), Key research and development projects of Sichuan Province(021YFG0114) and the Joint Funds of the National Natural Science Foundation of China (U1560203).