2023 Volume 63 Issue 8 Pages 1274-1280
2023 Volume 63 Issue 8 Pages 1274-1280
With the continued exploitation and utilization of high-grade rare earth ores, it is increasingly important to extract rare earths from separated slag containing low-grade rare earth. The X-ray powder diffraction, scanning electron microscopy, electron probe micro-analyzer and confocal laser scanning microscopy were used to explore the influence of TiO2 and cooling rate on the crystallization of CaO-SiO2-TiO2-10 wt% P2O5-8 wt% Nb2O5-5 wt% CeO2-5 wt% CaF2 slag system. In this study, the britholite was precipitated selectively as the Ce-enriched phase. When TiO2 was added at less than 12 wt%, the britholite was promoted to crystallize meanwhile the Ca2Nb2O7 was suppressed. However, CaTiSiO5 inhibited the growth of britholite when the TiO2 content exceeded 15 wt%. The non-isothermal crystallization kinetics had also been investigated for the TiO2 content and cooling rate varied from 0–18 wt% and 10–40°C/min, respectively. The continuous cooling transformation diagram and the relative crystallinity of the primary crystals were also constructed. Based on the observation and measurement of crystallization process, the modified Avrami model was applied to determine the crystallization mode of britholite with 9 wt% TiO2 addition. It was constant nucleation rate and one-dimensional growth with diffusion controlled. Considering the nucleation and growth of crystals, 20–30°C/min was preferred to be the reasonable parameter during cooling stage.
Although rare earth (RE) is a strategic resource with widespread applications in various fields, the reserves and grades of RE ores are relatively low globally.1,2,3,4) As the high-grade RE ore continues to be developed and exploited, the recoverable grade of the RE ore gradually decreases and the enrichment and extraction of low grade RE slag is of significance. The Bayan Obo deposit is considered as the largest RE-Nb-Fe despoit contains RE reserves of over 57.4 million tonnes, niobium (Nb) reserves of 2.16 million tonnes.5,6) However, the grades of RE and Nb are relatively low. The typical niobium concentrate of Bayan Obo contains 50.7 wt% Fe2O3, 4.4 wt% Nb2O5, 6.7 wt% TiO2 and 2.7 wt% CeO2 (All RE calculated in Ce). Pyrometallurgy should be the common method to separate iron from the RE-bearing slag by reduction and separation.7,8,9) Over the past decades, many researchers have studied the crystallization of the separated slag containing high-grade RE.10,11,12) However, due to the small size of crystals, the rare earth phase is difficult to separate from the separated slag containing low-grade RE. To utilize the resources effectively, it is necessary to study the enrichment from low-grade RE slag.
Nowadays, britholite is considered as the applicable phase to enrich RE from low-grade rare earth slag for its high conductivity and chemical stability. It is the potential materials of the solid oxide fuel cells and immobilization of high-level radioactive waste.13,14,15) Elwert et al. reported that the strong affinity of RE to phosphate structures to form a kind of silico-phosphate in the crystallization process, but the molecular formula was not identified.16) The possible phase was further detected as britholite which evolved from Ca5(PO4)3F in the CaO–SiO2–CaF2–P2O5–Ce2O3 system by Lan et al.17) The phase equilibrium relations of related slag system in specific temperature were studied by Lan and Qiu respectively.18,19) However, the available thermodynamics and kinetics data of britholite is still limited.20,21,22) As an important component of low-grade RE slag, the influence of TiO2 on the nucleation and growth of RE enriched phase could not be ignored. Meanwhile, the cooling rate is also the essential parameter which affects the growth rate in the crystallization process.
To explore the influence of TiO2 and cooling rate on the crystallization behavior of low-grade RE slag, the phase and composition of crystals containing RE were identified by X-ray powder diffraction (XRD) and electron probe micro-analyzer (EPMA). The equivalent crystal size and quantity were analyzed by scanning electron microscopy-energy dispersive spectrometer (SEM-EDS). The confocal laser scanning microscopy (CLSM) was also used to study the crystallization process of crystals containing RE. This study provides a theoretical basis for the recovery of rare earth from low-grade RE slag system containing niobium and titanium.
The composition of the low-grade RE slag with various TiO2 additions (Table 1) was designed primarily with reference to the separated slag of the niobium concentrate of Bayan Obo. The experimental materials were analytical reagents with a purity of 99.9 wt%. After dried, weighed and mixed, the mixtures (particle size < 74 μm) were placed in molybdenum crucibles and heated at the constant temperature zone of vertical tube furnace at 1550°C for 30 min. An argon atmosphere was supplied throughout the experiment to avoid oxidation. In order to ensure the accuracy of synthesized slag, the glassy slags were obtained by quenching in water. Part of glassy slags were ground and then analyzed by X-ray fluorescence spectrometer (Thermo Scientific, ARL 3600, America). The relative error between the chemical compositions of the designed and the real slags was under 2%. After that, the glassy slags were reheated to 1550°C for 30 min and cooled to 1100°C at the cooling rate of 10°C/min with an argon atmosphere. Each sample was analyzed by XRD (Bruker, D8 ADVANCE, Germany), SEM-EDS (Zeiss, SUPRA 55, Germany), and EPMA (Shimadzu, 1720H, Japan).
| No. | CaO | SiO2 | Nb2O5 | TiO2 | CeO2 | CaF2 | P2O5 |
|---|---|---|---|---|---|---|---|
| 1 | 36.0 | 36.0 | 8.0 | 0 | 5.0 | 5.0 | 10.0 |
| 2 | 33.0 | 33.0 | 8.0 | 6.0 | 5.0 | 5.0 | 10.0 |
| 3 | 31.5 | 31.5 | 8.0 | 9.0 | 5.0 | 5.0 | 10.0 |
| 4 | 30.0 | 30.0 | 8.0 | 12.0 | 5.0 | 5.0 | 10.0 |
| 5 | 28.5 | 28.5 | 8.0 | 15.0 | 5.0 | 5.0 | 10.0 |
| 6 | 27.0 | 27.0 | 8.0 | 18.0 | 5.0 | 5.0 | 10.0 |
The non-isothermal crystallization kinetics of crystals was observed by CLSM (Yonekura, VL2000DX, Japan). The sample was placed in a platinum crucible respectively, then put on an alumina holder equipped with a B-type thermocouple in the heating zone. After filled with argon, the sample was heated to 1550°C and cooled to 1100°C at the different cooling rates varied from 10–40°C/min. The heating schedule of the experiment is shown in Fig. 1.

The heating schedule of the non-isothermal experiment. (Online version in color.)
The XRD patterns of the samples with 0 wt%, 6 wt%, 12 wt%, 18 wt% TiO2 addition are shown in Fig. 2. As there is no standard XRD pattern of britholite, fluorapatite (Ca5(PO4)3F, COD ref. code: 96-900-1879) is selected to represent the britholite. Owing to the similarity in ionic radii and oxygen coordination, there is an incomplete occupancy relationship between Ca2+ and Ce3+, (SiO4)4− and (PO4)3−. The composition of the britholite is quantitatively identified by EDS and EPMA later. Without TiO2, the wollastonite (CaSiO3, COD ref. code: 96-900-2180), quartz (SiO2, COD ref. code: 96-901-2601) and pyrochlore (Ca2Nb2O6F, COD ref. code: 96-900-4070) are obtained. When the TiO2 addition increases from 6 wt% to 12 wt%, quartz and pyrochlore disappears in the slag, the simple phases are appropriate to separate. The titanite (CaTiSiO5, COD ref. code: 96-900-0513) replaces the wollastonite to be the dominant phase in the slag with 18 wt% TiO2.

XRD patterns of the samples with various TiO2 addition. (Online version in color.)
According to the back-scattered electron (BSE) images and the corresponding results of the spot energy disperse spectrum (EDS) results of samples which are shown in Fig. 3 and Table 2, TiO2 should affect the enrichment of crystals containing Ce. Consistent with the XRD results, the wollastonite (phase C) is the dominate phase, and the quartz (phase B) and pyrochlore (phase D) are randomly distributed around the britholite (phase A) when the slag is without TiO2 (Fig. 3(a)). Only the wollastonite (phase C) and britholite (phase A) are detected in the samples with the TiO2 addition increases from 6 to 12 wt% (Figs. 3(b)–3(d)). Nb and Ti are concentrated in wollastonite (phase C). It can be concluded that the TiO2 will inhibit pyrochlore (phase D) precipitation, which should be contribute to beneficiation. When the TiO2 addition is above 15 wt%, the growth of britholite (phase A) is hindered by the presence of titanite (phase E) (Figs. 3(e)–3(f)). Though the tiny britholite crystals containing more Ce than larger crystals, it is difficult to recover tiny crystals from the low-grade RE slag.

BSE images of the samples with various TiO2 addition. (a) 0 wt% TiO2, (b) 6 wt% TiO2, (c) 9 wt% TiO2, (d) 12 wt% TiO2, (e) 15 wt% TiO2, (f) 18 wt% TiO2. (Online version in color.)
| TiO2 | Phase | O | F | Si | P | Ca | Ti | Nb | Ce |
|---|---|---|---|---|---|---|---|---|---|
| 0 wt% | A | 50.6 | 4.63 | 2.59 | 14.62 | 25.45 | 0 | 0 | 2.11 |
| B | 60.74 | 0.11 | 38.02 | 0.91 | 0.21 | 0 | 0 | 0.01 | |
| C | 56.31 | 0 | 22.23 | 0.01 | 21.43 | 0 | 0 | 0.03 | |
| D | 49.34 | 8.03 | 0.90 | 1.06 | 17.70 | 0 | 22.5 | 0.47 | |
| 6 wt% | A | 50.83 | 1.65 | 1.94 | 14.17 | 29.5 | 0.04 | 0.04 | 1.82 |
| C | 56.46 | 1.85 | 19.26 | 0.44 | 16.97 | 2.73 | 1.66 | 0.61 | |
| 9 wt% | A | 42.33 | 3.5 | 2.02 | 17.21 | 33.16 | 0.15 | 0 | 1.63 |
| C | 57.41 | 0.54 | 16.6 | 0.44 | 17.77 | 4.46 | 2.01 | 0.78 | |
| 12 wt% | A | 50.79 | 4.05 | 1.75 | 15.22 | 26.72 | 0.05 | 0 | 1.43 |
| C | 58.35 | 1.24 | 17.37 | 0.36 | 14.86 | 5.42 | 1.73 | 0.67 | |
| 15 wt% | A | 51.57 | 4.17 | 1.71 | 14.93 | 26.27 | 0.06 | 0 | 1.29 |
| C | 56.19 | 0.31 | 17.4 | 0.42 | 18.11 | 4.74 | 2.51 | 0.33 | |
| E | 58.35 | 0 | 10.93 | 0.22 | 11.54 | 15.28 | 1.87 | 1.81 | |
| 18 wt% | A | 42.09 | 5.92 | 1.32 | 17.94 | 31.55 | 0.11 | 0 | 1.06 |
| A1 | 52.95 | 3.66 | 8.35 | 7.81 | 18.99 | 0.46 | 0 | 7.78 | |
| C | 54.03 | 5.6 | 17.56 | 0.19 | 17.05 | 1.89 | 2.71 | 0.96 | |
| E | 58.59 | 0.09 | 14.43 | 0.2 | 13 | 11.81 | 1.73 | 0.15 |
As shown in Fig. 3, the crystals of britholite are randomly distributed in the slag. The number density, average area of single crystal and area fraction of britholite are quantified to evaluate the effect of TiO2 on the enrichment of RE. Three random shots (1124 μm×768 μm) of each sample are taken by BSE, then the britholite is identified and measured by software. Figure 4 shows the result which is obtained as the average of the measurements from the three images of each sample. The area fraction of britholite increases when the TiO2 addition is under 12 wt%, then drops quickly with the TiO2 addition increase. When TiO2 is added at 9 wt%, the values of the average area of single crystal and the area fraction of britholite are the highest. The titanite replaces the wollastonite to be the main phase when the TiO2 addition is more than 12 wt%. As the initial crystalline temperatures of britholite and titanite are similar, crystals should grow competitively. The area fraction of britholite and average area of single crystal deceases rapidly. Further evidence should be shown in Section 3.3.

Effect of TiO2 addition on the crystallization of britholite. (Online version in color.)
The composition of britholite of each sample with different TiO2 addition is also detected by EPMA. Figure 5(a) shows TiO2 has little effect on crystal composition, however, the composition of the britholite varies greatly in different sizes. When TiO2 content is 18 wt%, the content of Ca and P elements in small crystals (18 s) decreases and the content of Si and Ce increases compared with large crystals (18). Figure 5(b) shows the mole ratio of (Ca+Ce):(Si+P) is held as 5:3, and the mole ratio of Si : Ce is held as 1:1. According to the law of conservation of charge, when (PO4)3− occupies the position of (SiO4)4−, Ce3+ should be replaced by Ca2+ at the same time. The TiO2 addition and the size of crystals have little impact on the mole ratio of britholite. It can be concluded that the molecular formula of britholite is Ca5−xCex[(SiO4)x(PO4)3−x]F while the x depends on the size of crystals. According to previous research,17) (PO4)3− should promote the nucleation and improve the initial crystalline temperature of britholite. As the concentration of (PO4)3− in molten slag decreases when the britholite and titanite begin growing, the crystalline temperature of britholite decreases. Late nucleation and limited growth space are responsible for the formation of small crystals.

Effect of TiO2 addition on the composition of britholite. (Online version in color.)
To explore the effects on TiO2 and cooling rate on the crystallization process, non-isothermal experiments were observed by CLSM. The initial crystalline temperatures of different conditions are shown in Fig. 6. Clearly, for the same slag, the initial crystalline temperature decreases when the cooling rate increases. When the TiO2 addition is less than 12 wt%, the initial crystalline temperature increases with the increase of TiO2 addition. However, the initial crystalline temperature of molten slag with 18 wt% TiO2 is lower than that with 12 wt% TiO2. According to the CaO–SiO2–TiO2 ternary phase diagram, the crystalline temperature of titanite (1382°C) is close to that of britholite, titanite will disturb the crystallization of britholite. Further quenching experiments were carried out in slag with different cooling rate when the initial crystalline temperature was lower than 10°C. XRD analysis of the quenched sample shows the crystals of titanite and britholite coexist when the TiO2 addition is 18 wt%.

The continuous-cooling transformation diagram of molten slag. (Online version in color.)
To describe the crystallization process under non-isothermal conditions, Jeziorny proposed the modified Avrami equation.23,24) The logarithmic expression is:
| (1) |
Where α and β represents the relative crystallinity and the cooling rate, respectively. Zc is the dimensionless parameter which is only determined by the crystals, t represents the crystallization time, n is the crystallization mechanism coefficient, which is the sum of spatial dimension and temporal dimension. The temporal dimension is 1 or 0, which represents the nucleation model is homogeneous or heterogeneous. Due to the time dependence of the crystals during the initial nucleation stage and the possibility of simultaneous homogeneous and heterogeneous nucleation, the coefficient n is not an integer. The relationship between the coefficient n and crystallization mechanism is shown in Table 3.25,26)
| Diffusion | Interface reaction | |
|---|---|---|
| Constant nucleation rate | ||
| 3-dimensional growth | 2.5 | 4 |
| 2-dimensional growth | 2 | 3 |
| 1-dimensional growth | 1.5 | 2 |
| Constant number of nuclei | ||
| 3-dimensional growth | 1.5 | 3 |
| 2-dimensional growth | 1 | 2 |
| 1-dimensional growth | 0.5 | 1 |
Furthermore, the half-crystallization time t1/2 can be calculated by the initial crystalline temperature T0, half-crystallization temperature T1/2 and the cooling rate β.
| (2) |
According to Fig. 4, the values of the average area of single crystal and the area fraction of britholite are the highest when the TiO2 is added at 9 wt%. In this study, slag with 9 wt% TiO2 is chosen to analyze the non-isothermal crystallization kinetics at different cooling rate. Due to the opaque nature of the molten slag, the britholite floating on the surface of the slag can be observed and recorded by CLSM. Figures 7(a)–7(d) shows the overall crystallization process of britholite during the cooling stage. Define the relative crystallinity as the ratio of the crystallization area at given temperatures to the final crystallization area, the effect of cooling rate on the relative crystallinity has been calculated as shown in Fig. 8. The crystallization time and the temperature range of crystallization increases with the increase of cooling rate. The relatively low temperature increases the viscosity and impedes ion movement in the slag. Further analysis is also carried out that the mechanism coefficient n, dimensionless parameter Zc and half-crystallization time t1/2 are calculated subsequently by equations and Fig. 9.

The crystallization process of britholite (Cooling rate=30°C/min).
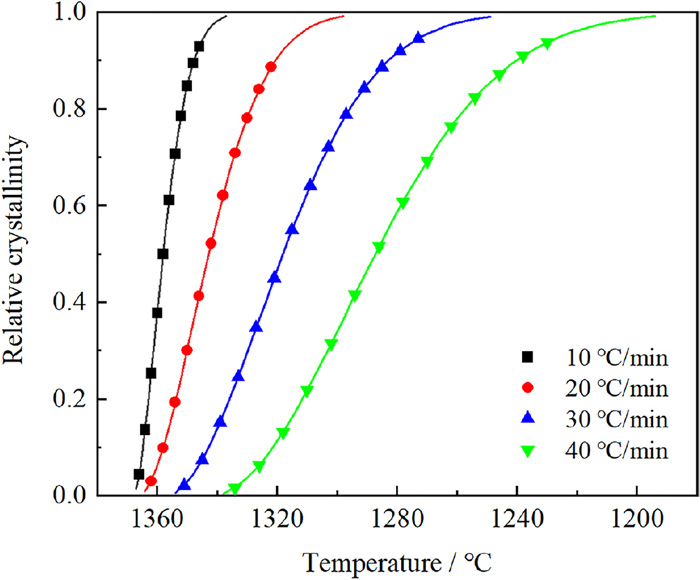
Effect of cooling rate on the relative crystallinity of britholite. (Online version in color.)

Effect of cooling rate on the crystallization kinetics of britholite. (Online version in color.)
Table 4 shows the parameters of crystallization process of britholite. All the values of n are between 1.5 and 2. It indicates that the crystallization mechanism is constant nucleation rate with one-dimensional growth and it is controlled by diffusion. The parameter Zc decrease slightly while the half-crystallization time t1/2 increases with the increase of cooling rate. It also indicates that the crystallization process is hindered by the lower temperature and higher viscosity as the cooling rate increases.
| Cooling rate (°C/min) | n | Zc | t1/2 (min) |
|---|---|---|---|
| 10 | 1.65 | 0.97 | 0.99 |
| 20 | 1.74 | 0.94 | 1.16 |
| 30 | 1.82 | 0.92 | 1.29 |
| 40 | 1.90 | 0.91 | 1.37 |
The Friedman equation27,28) is regard as a suitable method to calculate the effective activation energy, especially for the cooling process. It expressed as:
| (3) |
| Cooling rate (°C/min) | EX (kJ/mol) |
|---|---|
| 10 | 372.36 |
| 20 | 331.42 |
| 30 | 318.74 |
| 40 | 311.33 |
According to the classical nucleation theory, the slag can be regarded as a supersaturated solution.29,30) The nucleation rate J of crystals in the molten slag can be expressed by Eq. (4):
| (4) |
Where k is the pre-exponential constant, ΔGcrit and ED are the nucleation energy barrier and the barrier to diffusion across the phase boundary respectively. R and T stand for the gas constant and temperature, respectively. Assuming that the crystal nucleus is spherical, the Gibbs free energy of the critical radius ΔGcrit can be shown as Eq. (5):
| (5) |
Where σ is the slag-crystal surface energy per unit area, M and ρ describe the molecular mass and the density of crystal. S is the supersaturation which is negatively correlated with temperature T. Substituting Eq. (5) into Eq. (4), the nucleation rate J is:
| (6) |
When the degree of supercooling is relatively low, the barrier to diffusion is very small and the term exp[−ED/(RT)] should be neglected. However, when the degree of supercooling is large, the diffusion barrier should not be neglected due to the increasing viscosity of the melt, while the effect of the nucleation energy barrier gradually diminishes. The nucleation rate J was measured at the early stage of nucleation when the crystals should be distinguished.
Given that diffusion is the limiting step, a higher nucleation rate implies that more nuclei are formed simultaneously. Competitive growth of crystals results in tiny crystals, so the nucleation rate needs to be controlled. Since the cooling rate affects the initial crystalline temperature, which is related to the degree of undercooling and supersaturation, so the nucleation rate J is also affected. The effect of cooling rate on the final crystallization of britholite is shown in Fig. 10. The number of crystals and the average area of single crystal at 1150°C with different cooling rate are identified and measured, as shown in Fig. 11.

Effect of cooling rate on the final crystallization of britholite (1150°C).

Effect of cooling rate on the number density of crystals and average area of per crystal (1150°C). (Online version in color.)
As the cooling rate increases, the number density of crystals first decreases and then increases, with the trend of the average area of per crystal being opposite to that of the number density. Figure 6 mentioned that the initial crystalline temperature decreases with increasing cooling rate. When the cooling rate gradually increases (10–30°C/min), the effect of decreasing the initial crystalline temperature is greater than that of increasing the supersaturation, the nucleation rate should decrease, and the formed crystal nuclei should grow sufficiently. When the cooling rate is more than 30°C/min, the influence of supersaturation is dominant, it promotes the increase of nucleation rate. Due to the diffusion limitation and competitive growth, tiny crystals are distributed in the slag. Compare with the results of non-isothermal experiments, the cooling rate in the range of 20–30°C/min is preferred. Fewer crystals and larger single crystals provide superior conditions for further separation.
To facilitate the understanding of the origin of the microscopic crystalline morphology, Fig. 12 shows a schematic diagram of the effect of the cooling rate on nucleation and growth for the same composition of slag. During the nucleation stage, the number of nucleons first decreases and then increases with the cooling rate. However, during the growth stage, the crystallization mechanism coefficient n increases with the cooling rate and the crystal changes from a needle shape to a slab shape, but it remains one-dimensional in general.

Schematic diagram of the effect of cooling rate on nucleation and growth.
Influence of TiO2 and cooling rate on the crystallization behavior of low-grade RE slag were investigated in the present work. The main conclusions are summarized as follows:
(1) The appropriate amount (<15 wt%) of TiO2 should inhibit the pyrochlore precipitation, which should be contribute to beneficiation. When the TiO2 addition is above 15 wt%, the growth of britholite is hindered by the presence of titanite.
(2) The composition of britholite is not fixed due to the isomorphism. The general molecular formula is Ca5−xCex[(SiO4)x(PO4)3−x]F, and TiO2 should not affect the composition of britholite.
(3) The initial crystalline temperature of britholite decreases with decreasing TiO2 addition or increasing cooling rate. When the TiO2 content in the slag is less than 15 wt%, the crystallization process of britholite is homogeneous and one-dimensional nucleation, which is controlled by diffusion conditions. Considering larger average volume of single crystal should be preferred for separation, the recommended range of cooling rate is 20–30°C/min.
The authors gratefully acknowledge financial support by Major Projects of Inner Mongolia Natural Science Foundation (2018ZD07), Inner Mongolia Natural Science Foundation (2020BS05016), Open Project for Key Basic Research of the Inner Mongolia Autonomous Region (20140201), Open Project for Major Basic Research of Inner Mongolia (0406091701) and Scientific Research Project of Inner Mongolia (ZDZX2018032).