2024 Volume 64 Issue 12 Pages 1813-1819
2024 Volume 64 Issue 12 Pages 1813-1819
The effect of MnS inclusions on the localized corrosion of low-alloy steel in a 0.5 mol/kg (3%) NaCl solution was investigated to propose countermeasures for inhibiting localized corrosion initiated by MnS inclusions. Low alloy steels without MnS inclusions did not corrode in a 0.5 mol/kg (3%) NaCl solution, regardless of the addition of Cu. That is, no matrix improvement effect due to solute Cu was confirmed. Slight corrosion occurred in the Cu containing steel with MnS inclusions; however, the MnS inclusions remained. Cu7.2S4 precipitated on the MnS in contact with a 0.5 mol/kg (3%) NaCl solution. Therefore, Cu7.2S4 precipitates on the MnS inclusions during immersion, which could suppress the localized corrosion initiated by the MnS inclusions because Cu sulfide is not dissolved based on potential-pH diagram. The addition of Cu in a 0.5 mol/kg (3%) NaCl solution does not improve the corrosion resistance of the matrix due to solute Cu but does suppress localized corrosion initiating from MnS by precipitating Cu sulfide on MnS.
Numerous studies have reported localized corrosion initiating from inclusions such as MnS in carbon steels and low alloy steels. For example, grooving corrosion can occur in the welded portion of an electric-resistant welded (ERW) pipe. Grooving corrosion is the selective corrosion of the welded portion in an ERW pipe, and its cross-sectional shape is observed to be V-shaped at the center of the welded portion. This groove corrosion starts with the dissolution of the S-enriched area near MnS, which leads to the dissolution of MnS. Subsequently, the corrosion pits grow and connect with other pits to form grooves. Owing to the formation of the oxygen concentration cell between outside and inside of the corrosion pits and that of the macrocell consisting of the small anode in the welded portion and the large cathode in the base metal, the grooving corrosion dramatically accelerates in the welded portion.1) Reheating an ERW-welded portion effectively suppresses grooving corrosion.2) However, this alone is not a complete countermeasure, and the optimization of alloy components and the addition of a small amount of alloying have also been considered. For example, the addition of alloying elements such as Cu, Ti, Sb, Cr, and Ni has an important effect on grooving corrosion resistance.3,4) ERW steel pipes containing Cu and Ti have been developed based on this concept, and various grooving corrosion resistant steel pipes have been developed and put to practical use.5)
Moreover, when evaluating the resistance of high-strength Oil Country Tubular Goods (OCTGs) to sulfide stress cracking, localized corrosion occurs from inclusions, and cracks initiate localized corrosion.6) In addition, soluble inclusions such as MnS and oxysulfides dissolve and become the initiating point for localized corrosion.6) Furthermore, insoluble inclusions such as Ti–Nb nitrides are thought to dissolve the surrounding steel via galvanic effects.6) To suppress the localized corrosion of these inclusions, fine dispersive Ti–Nb carbonitrides were generated around the oxy-sulfides, thereby suppressing the formation of coarse single carbonitrides. Using this technology, inclusions can be finely dispersed and localized corrosion can be prevented.6,7)
The purpose of this study is to suppress pitting corrosion and local corrosion originating from the soluble sulfide such as MnS. Mizukami et al. has been reported that CuS is deposited to cover MnS by electrolysis using a non-aqueous electrolyte using the SPEED (selective potentiostatic etching by electrolytic dissolution) method.8,9) The SPEED method is a replica method in which a sample is electrolytically polished using a non-aqueous electrolyte, and then the fine inclusions in the steel are collected using a nylon mesh. That is, the sample is electrolytically polished at 30C (Coulomb) in a non-aqueous electrolyte of 970 mol/m3 (10%) acetylacetone, 110 mol/m3 (1%) tetramethylammonium chloride and 2.2×104 mol/m3 (89%) methanol. We tried that it would be possible to apply this phenomenon to suppress the dissolution of MnS to aqueous corrosion. Therefore, this study was focused on the possibility of suppressing local corrosion caused by MnS by covering MnS with Cu sulfide during aqueous corrosion.
Here, the papers regarding the effects of Cu addition on aqueous corrosion were listed as follows. As for atmospheric corrosion and seawater corrosion, it is reported that Cu has the effect of increasing the corrosion resistance of steel materials by facilitating the formation of dense and highly adhesive protective rust.10,11) This Cu effect is solid solution Cu, not Cu sulfide. On the other hand, as for sulfuric acid dew point corrosion, CuS forms on the steel surface and suppresses corrosion.12) In a sour environment, corrosion is suppressed by the addition of Cu, and Cu sulfide is generated in the steel, which provides protection.13,14,15) However, in sulfuric acid dew point corrosion and in sour environment, the corrosion inhibition effect of Cu addition is due to the formation of Cu sulfide on the steel, not on the MnS.
As for the weather resistance and seawater resistance of stainless steel, there is a report that to suppress corrosion in the active state, Cu in the metallic state concentrates at the corrosion interface and suppresses matrix dissolution.16,17,18) Moreover, it has been reported that weather resistance of stainless steel can be improved by adding Cu because MnS and CaS based inclusions was substituted for CuS-based inclusions that are difficult to dissolve.19)
In groove corrosion, S2−, which is generated when the S-enriched region dissolves around MnS inclusions, or when MnS dissolves due to a decrease in pH within a localized corrosion pit, is combined into Cu2+ ions eluted from the steel, eliminating the negative effects of S2− ions.20) This is only an idea and there is no concrete evidence. We are currently investigating the above idea, that is, whether Cu sulfide is precipitated on MnS, using groove-resistant steel pipes.
Therefore, based on the viewpoints of the Mizukami’s paper8,9) and the viewpoint of suppression of MnS dissolution due to above idea of groove corrosion,20) it was investigated in detail how MnS changes due to Cu addition in aqueous solution corrosion.
Furthermore, the composition of Cu sulfide was also investigated. There are various reports regarding the composition of Cu sulfide, such as Cu2S,21,22,23,24,25,26,27) Cu1.8S,28,29,30) Cu1.6S31,32,33,34,35,36,37,38) and CuS.29) The composition of Cu sulfide in aqueous corrosion was also investigated and confirmed at the same time.
In this study, the phenomenon of localized corrosion initiated by MnS inclusions was investigated, and a countermeasure for suppressing the localized corrosion originating from MnS inclusions was proposed. This paper describes the effects of Cu addition on the localized corrosion of low alloy steel in a deaerated 0.5 mol/kg (3%) NaCl solution because there is almost few research in a deaerated NaCl solution. Using in-situ observation, it was investigated how the corrosion originating from MnS changes in Cu added and Cu free steels. The morphology and composition of Cu sulfide precipitated on MnS due to Cu addition was also investigated using the microscope observation.
Table 1 shows the chemical compositions of the tested steels. Two types of steel were used in this study. Steel A was 0.24 mass% C-1.0 mass% Mn steel, and steel B was prepared by adding 0.3% mass Ni and 0.3% mass% Cu to steel A. In practical steel, it is difficult to add Cu alone; therefore, Ni was also added at the same level. Fifty kilograms of the ingots were melted in a vacuum melting furnace and hot-rolled to a thickness of 15 mm in the laboratory. Subsequently, quenching and tempering were performed. After the plates were held at 1473 K (1200°C) for an hour, they were water-cooled. After the plates were held at 923 K (650°C) for an hour, they were air-cooled. During vacuum melting, carbon deoxidation was performed to suppress the oxides as much as possible, and the inclusions in Steels A and B were mainly MnS inclusions. The samples were machined to produce electrochemical test specimens with widths, lengths, and thicknesses of 1.5×10−2, 2.0×10−2, and 3×10−3 m, respectively. The specimens were sequentially polished with #320, #600, #1000, and #1500 emery paper, and polished with 6×10−6 and 1×10−6 m diamond pastes.
| C | S | Mn | Cu | Ni | N | O | |
|---|---|---|---|---|---|---|---|
| A | 0.22 | 0.01 | 0.97 | <0.01 | 0.01 | <0.001 | 0.001 |
| B | 0.24 | 0.01 | 0.97 | 0.29 | 0.31 | <0.001 | 0.001 |
Specimen electrodes with and without MnS inclusions were fabricated. All parts except the electrode surface were coated. The electrode area was 1.5×10−7 m2 or less. A 0.5 mol/kg (3%) NaCl solution was used as the test solution. The initial pH of the solution was ranged from 5.4 to 5.6. The solution was deaerated with nitrogen gas for more than an hour and then poured into a corrosion cell. The test temperature was 298 K (25°C), and the immersion time was 24 hours. During the immersion test, the electrode surface was continuously observed using an optical microscope, and the Open Circuit Potential (OCP) was measured simultaneously. An Ag/AgCl (3 kmol/m3 (3M) KCl) electrode was used as the reference electrode. The difference between the reference electrode and standard hydrogen electrode (SHE) was 0.208 V. In this study, all OCPs were indicated as the hydrogen standard reference electrode (SHE).
2.3. Inclusions AnalysisAfter the immersion test, MnS inclusions were observed using optical microscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and scanning transmission electron microscopy (STEM). The compositions of the inclusions were analyzed using energy-dispersive X-ray spectroscopy (EDS) combined with SEM and STEM. Before STEM observation, a cross-section of the MnS inclusions was processed using a Focused Ion Beam (FIB), and the cross-section sample was removed and then observed using STEM-EDS. The crystal structure and stoichiometric ratio of the inclusions were analyzed to identify the inclusions. As previously mentioned, it was confirmed that the major inclusion in Steels A and B was MnS inclusions.
Figure 1 shows the optical micrographs of the electrode surface without MnS for Steels A and B after immersion in a 0.5 mol/kg (3%) NaCl solution for 24 hours. On the electrode surface, where MnS did not exist, there was no visible corrosion in Steels A and B. Immersion tests were conducted numerous times for Steels A and B without MnS, and the same phenomenon was observed. Figure 2 shows the time dependence of OCP for Steels A and B. The time dependence of the OCP was almost the same for Steels A and B, with no significant change. Furthermore, the OCPs of Steels A and B are nobler than the hydrogen redox potential. Hydrogen red/ox potential at pH 5.5 are calculated as Reversible Hydrogen Electrode (RHE). That is, in the 0.5 mol/kg (3%) NaCl environment, when MnS was not present, there was almost no corrosion, regardless of whether Cu or Ni was added.


Figure 3 shows the changes in the electrode surface on which MnS was present in Steel A, which was continuously photographed using an optical microscope. Fifteen minutes after the start of immersion, the area around the MnS began to corrode and the matrix around the MnS turned brown. After 3 hours, localized corrosion occurred at the MnS interface, and after 12 hours, rust covered the entire surface. The same phenomenon was observed for Steel A when the experiment was repeated several times. In other words, corrosion started around MnS approximately 15 minutes after the start of immersion. After a few hours, localized corrosion started from MnS, and then corrosion progressed until the entire surface was covered with rust.
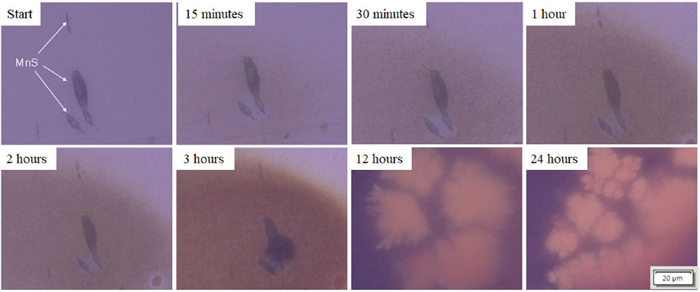
Figure 4 shows continuous optical micrographs of the specimen electrode surface with the MnS of Steel B during immersion. In Steel B, there was no change in the MnS until 3 hours after the start of immersion, and the matrix changed slightly after 12 hours. Figure 5 shows optical micrographs of the electrode surfaces of steel A before and after 24 hours of immersion. In Steel A, the matrix was severely corroded, and the entire surface was covered with rust, whereas in Steel B, the matrix was slightly corroded but no rust was formed and MnS inclusions remained. Figure 6 shows the time dependence of the OCP for Steels A and B when immersed in a 0.5 mol/kg (3%) NaCl solution for 24 hours. The OCP of Steel A was −0.20 V (SHE) immediately after immersion and gradually moved to the less noble side, reaching −0.42 V (SHE) after 24 hours. In contrast, the OCP of Steel B remained almost the same from −0.22 to −0.26 V (SHE). The OCP of Steel B was more noble than its hydrogen redox potential at pH 5.5 and was 0.18 V more noble than that of Steel A.



From the above results, in the absence of MnS, neither steel A without Ni and Cu nor steel B with Ni and Cu corroded in a 0.5 mol/kg (3%) NaCl solution. However, in the presence of MnS, it was confirmed that the entire surface was corroded and covered with rust in Steel A, although only slight corrosion occurred and MnS remained in Steel B. Therefore, the MnS inclusions in Steel B after 24 hours of immersion were investigated in detail.
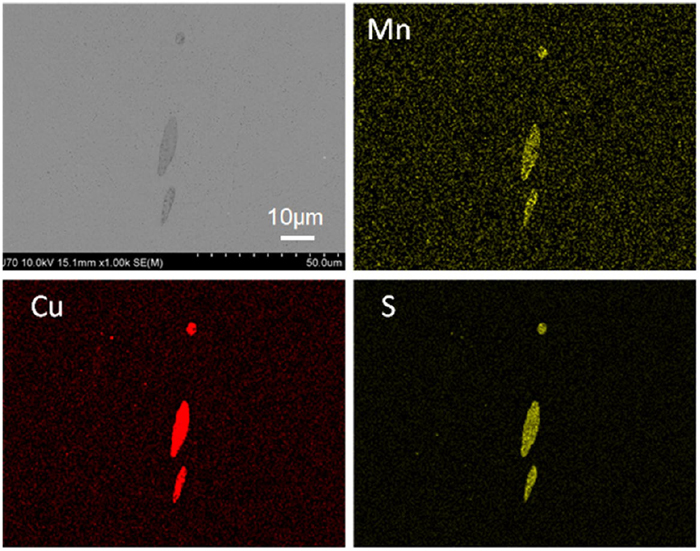
3.2. Inclusion Analysis after Immersion TestFigure 7 shows the results of the SEM-EDS analysis of the change in the MnS composition after the immersion test of Steel B. A cross-section of MnS was fabricated using FIB and analyzed using SEM-EDS, as shown in Fig. 8. As shown in Figs. 7 and 8, Cu sulfide precipitated on MnS. As shown in Fig. 8, Cu sulfide formed on MnS in contact with the 3% NaCl solution. In contrast, no Cu sulfide formed at the MnS interface when in contact with the matrix. That is, Cu sulfide did not precipitate around MnS before immersion. Furthermore, as shown in Fig. 8, Cu sulfide precipitated only on MnS and not on the matrix. Figure 9 shows a STEM-EDS cross-sectional analysis of MnS after immersion. The enriched Cu and S overlapped on the MnS, indicating the precipitation of Cu sulfide. Furthermore, it was revealed that some Fe and Mn were present in the Cu sulfide. Notably, no NiS was precipitated. The composition of Cu sulfide was investigated using TEM. Figures 10 and 11 show the TEM images and diffraction patterns of Cu sulfide, respectively. From analysis of the diffraction pattern, it was identified as Cu7.2S4 with an fcc structure. The composition ratio of Cu to S was 1.8 and was consistent with the previous studies in which it ranges from 1.6 to 2.0.21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38) Further studies are required to investigate the composition of X in CuXS.




From Figs. 1 and 2, the OCPs of the electrodes without MnS in both Cu added and Cu free steels existed on the more noble side than the hydrogen redox potential, and these electrodes were not corroded. Therefore, these electrodes are considered to be in a passive state. Similarly, as can be seen from Figs. 5 and 6, the OCP of the electrode with MnS in Cu added steel exists on the more noble side than the hydrogen redox potential, and this electrode was slightly corroded. Figure 12 shows the anode polarization curve after 24 hours immersion in Steel B which is Cu added steel. The anodic polarization curve exhibiting a passive state is shown in Cu added steel after 24 hours immersion. Therefore, the anode reaction is dominated by the reaction of the formation of an iron hydroxide, and the cathode reaction at this time is considered to be an oxygen reduction reaction due to a very small amount of residual dissolved oxygen. On the other hand, the OCP of the electrode with MnS in Cu free steel exists on the less noble side than the hydrogen redox potential, and this electrode is general corroded. In this case, the anode reaction is dominated by the iron dissolution reaction, and the cathode reaction is considered to be a hydrogen reduction reaction.

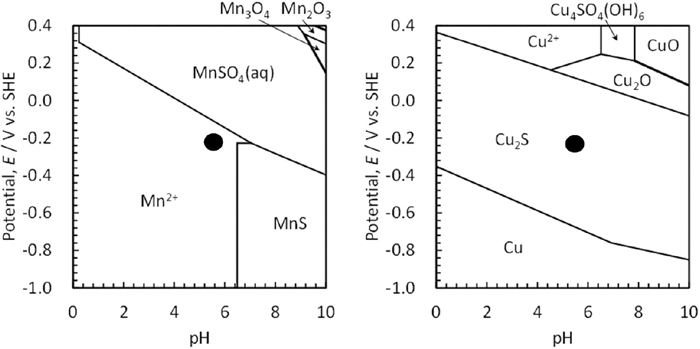
This study revealed that Cu7.2S4 precipitated on MnS in a 0.5 mol/kg (3%) NaCl solution at pH 5.5 and had the potential to suppress localized corrosion from MnS inclusions. In other words, localized corrosion initiated by the MnS inclusions was observed in the Ni and Cu free steels. However, in the Ni and Cu added steel, Cu sulfide precipitated on MnS. Notably, no NiS was generated. Therefore, the reason why Cu sulfide precipitates on MnS at pH 5.5 was considered. The solubility product (Ksp) of MnS is 3×10−14 (mol/L)2, and that of Cu sulfide is 8×10−37 (mol/L)2, which is much smaller than that of MnS, and Cu sulfide is easy to precipitates on MnS.8,9) As indicated in Fig. 6, a large difference of the OCP between Cu added steel and Cu free steel begins to occur after 5 hours immersion, assuming that Cu sulfide begins to precipitate on MnS. Then, the difference of the OCP between them is almost constant after 20 hours immersion, assuming that Cu sulfide covers the MnS at this point in Cu added steel. Therefore, MnS covered by Cu sulfide is suppressed to dissolve from MnS. Figure 12 shows the potential-pH diagrams of Mn–S–H2O and Cu–S–H2O. This diagram was calculated as 298 K (25°C). To precipitate Cu sulfide on MnS, MnS must be dissolved, S ions must be present in the solution, and Cu ions dissolved from the matrix must be present in the solution. From the potential-pH diagram of Mn–S–H2O in Fig. 12, the pH must be 6.5 or lower so that MnS can be dissolved. In contrast, a region in which Cu sulfide is indicated as Cu2S in this diagram is stable and exists at all pH values on the potential-pH diagram. However, Cu ions must exist in the solution in order that Cu sulfide precipitates. The OCP of Cu added steel in a 0.5 mol/kg (3%) NaCl solution of pH 5.5 existed in the range from −0.22 to −0.26 V (SHE) in Fig. 6. In this environment, MnS was dissolved, and Cu sulfide was stable as indicated in Fig. 12. As shown in Fig. 4, the matrix of Cu added steel was slightly corroded, sufficient Cu ions and S ions were present in the solution, and it is thought that Cu sulfide on MnS is easy to precipitate.
In contrast, in an acidic environment with a pH of 4.5 or less, corrosion in the matrix is severe, and even if Cu sulfide precipitates on MnS, it is considered to be difficult to maintain for Cu sulfide. In fact, it is confirmed that matrix is easily corroded at pH of 4.5 for many times. The optimal conditions for precipitating Cu sulfide on MnS will be investigated in the future. Finally, in the absence of MnS in a 0.5 mol/kg (3%) NaCl environment at pH 5.5, the matrix did not corrode, regardless of whether Ni or Cu was added. Therefore, it is believed that the soluble effects of Cu and Ni in improving the corrosion resistance do not contribute to this environment.
Localized corrosion initiated by MnS inclusions was investigated in detail, and countermeasures for suppressing localized corrosion starting from MnS inclusions were proposed. The main results are as follows:
(1) In the absence of MnS, no corrosion occurred in 0.5 mol/kg (3%) NaCl regardless of the presence or absence of Cu. In contrast, in the presence of MnS, localized corrosion initiated from MnS, and corrosion progressed to the entire surface of the Cu free steel. However, in the Cu containing steel, slight corrosion occurred, and MnS inclusions remained. The OCP of the Cu containing steel was more noble than its hydrogen redox potential. Moreover, the OCP of the Cu containing steel was more noble than that of the Cu free steel.
(2) In the Cu containing steel, Cu7.2S4 precipitated on the MnS that was in contact with the solution after the immersion test. In other words, Cu7.2S4 precipitates on the MnS inclusions during immersion, thereby suppressing localized corrosion from the MnS inclusions because Cu7.2S4 precipitated on the MnS does not dissolve.