2024 Volume 64 Issue 13 Pages 1862-1870
2024 Volume 64 Issue 13 Pages 1862-1870
The influences of SO2 in the recirculation flue gas on the sintering process are studied through simulating the flue gas recirculation with sinter pot test at room temperature. The results show that, with the increasing concentration of SO2, the SO2 is absorbed by CaO, the surface of CaO particles will generate CaSO4 which affects the formation of binding phase, so the sinter yield and particle size will decrease. The residual sulfur in sinter ore will increase obviously when SO2 concentration is higher than 500 ppm, the residual sulfur of middle sinter ore in 2000 ppm is 1.54 times higher than that in 500 ppm. Meanwhile, the SO2 peak concentration in flue gas is enriched, which is conductive for the end-of-pipe treatment of SO2. The RI of sinter ore is increased and softening and melting properties become better, because the consumption of CaO affects the formation of calcium ferrite and increases the reduction rate and melting point of sinter ore, another reason is that the decomposition of CaSO4 leads to an increase in the porosity in the sinter, and the higher porosity makes the sinter expose more surface area to the reduced body, and the RI of the sinter increase. Therefore, adding an appropriate amount of SO2 in the sintering process can improve the quality of the sinter, and if the SO2 content is too high, it will affect the formation of the binder phase and reduce the strength and yield of the sinter.
In recent years, with the rapid development of China’s iron and steel metallurgy industry, the problem of environmental pollution has become increasingly prominent. According to research data,1) pollutant emissions from the iron and steel metallurgy industry account for 16% of the total industrial pollutant emissions in China. There are many types of pollutants produced by the iron and steel metallurgy process, among which SO2 is one of the most important pollutants. In the air SO2 forms gaseous pollutants, which are inextricably linked to environmental pollution problems such as photochemical smog, ozone depletion and PM2.5, posing a serious threat to human health and ecosystems.2) Therefore, the iron and steel metallurgy industry needs to strengthen environmental protection measures while pursuing economic benefits in order to realize sustainable development.
Iron ore powder sintering as the main procedure of iron and steel metallurgy, its SO2 emissions account for 70% of the total emissions of the iron and steel production process.3) At present, sintering flue gas pollutant emission reduction methods are mainly divided into three categories: source emission reduction, process control, and end-of-pipe treatment.4) From the perspective of source emission reduction, Kang Y. et al.5) conducted a flue gas cycle sintering cup experiment with charcoal instead of part of coke to investigate the effect of SO2 content in the circulating flue gas on the concentration of flue gas pollutant emissions, and the results showed that it is more conducive to realize the synergistic control of sintered flue gas by using charcoal to replace 40% of the coke for the circulating flue gas sintering when the concentration of SO2 dosing in the circulating flue gas is 500 ppm. Leonardo Tomas Da Rocha et al.6) studied the effect of adding calcium ferrate to the sintering mix on the quality of sintered ore and SO2 reduction, and found that the low-temperature sintering process with the addition of calcium ferrate to the sintering mix can improve the metallurgical performance of sintered ore and reduce the emission of SO2 at the same time. Seongkyu Cho et al.7,8) studied the SO2 generation during the sintering process of iron ore, and found that the addition of calcined dolomite and iron oxide to the sintering mix had a certain inhibiting effect on the SO2 generation during the sintering process. The main reasons for this phenomenon are that SO2 in the flue gas could be absorbed by the sintering fluxes,9,10) such as burnt lime, lime stone and slaked lime etc. And the SO2 behavior could be described as generated, absorbed and released.11) Li G H et al.12) have studied the SO2 behavior in different sintering zones under the simulated experimental conditions, and found that the SO2 of recirculated flue gas is absorbed by the moisture, slaked lime and molten phases in the sinter bed. From the process control point of view, Yu H et al.13) studied the distribution of sulfur in the sintering process, and found that the sulfur enrichment in the sintering process is mainly in the preheating layer and the drying layer, and the main reaction that affects the residual sulfur is 2CaO+2SO2+O2=2CaSO4, and the equilibrium temperature of the reaction rises with the increase of the concentration of SO2 and O2, which increases the amount of residual sulfur in the sintered ore. From an end-of-pipe perspective, In order to reduce flue gas flow and pollutants emission as well as utilize the sensible heat, the partial recycling process of sinter flue gas is developed such as EOS, LEEP, EPOSINT and so on.14,15,16,17,18,19) Comparing to the conventional sintering process, the compositions of recirculated flue gas is different from the air. In order to achieve the purpose of pollutant emission reduction and at the same time reuse the sintering flue gas, the effects of O2, CO, and CO2 content degree on combustion and heat transfer in the sintering process have been investigated.20,21,22,23,24) However, it was unclear how the sintering process was influenced by the SO2 in recirculation gas. This paper investigates the influence of SO2 in recirculating gas on sintering technical index, sinter metallurgical performance and pollutant emission through sinter cup test, so as to realize SO2 emission reduction while ensuring the quality of sintered ore.
The chemical compositions of iron ore fines and fluxes used in the sintering experiments are shown in Table 1. Iron ore fines include PB, Yandi, Kooly, Carajas, and Sijiaying, fluxes include dolomite, quicklime, and limestone, and the fuel used in the experiments is coke powder whose industrial analysis and main chemical compositions are shown in Table 2.
| Raw materials | TFe | SiO2 | CaO | Al2O3 | MgO | S | P | N | LOI |
|---|---|---|---|---|---|---|---|---|---|
| PB | 62.14 | 3.52 | 0.06 | 2.19 | 0.06 | 0.017 | 0.100 | 0.07 | 4.66 |
| Yandi | 58.20 | 5.35 | 0.07 | 1.37 | 0.09 | 0.009 | 0.040 | 0.20 | 9.69 |
| Kooly | 60.59 | 3.16 | 0.02 | 1.61 | 0.07 | 0.066 | 0.088 | 0.08 | 7.55 |
| Carajas | 64.27 | 2.56 | 0.01 | 1.18 | 0.01 | 0.001 | 0.012 | 0.16 | 2.18 |
| SiJiaYing | 65.56 | 6.58 | 0.24 | 0.58 | 0.37 | 0.021 | 0.014 | 0.08 | −0.39 |
| dolomite | / | 5.98 | 47.74 | 0.88 | 31.86 | 0.054 | / | / | / |
| burntlime | / | 2.58 | 67.89 | 1.58 | 4.13 | 0.270 | / | / | / |
| limestone | / | 5.06 | 46.68 | 1.10 | 1.73 | 0.160 | / | / | / |
| Fuel | Industrial analysis | Main chemical composition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FC | A | V | M | Al2O3 | MgO | MgO | SiO2 | S | N | |
| Coke powder | 86.13 | 12.76 | 1.11 | / | 4.26 | 0.5 | 0.094 | 5.64 | 0.87 | 0.39 |
Taking into account the sintered raw fuel composition, particle size, and sintered mix alkalinity and composition, the sintered mix formulation was developed as shown in Table 3, and the main components were shown in Table 4. After the sintering experiment, the finished sintered ore was analyzed chemically as shown in Table 5.
| Raw materials | Ratios |
|---|---|
| PB | 19.29 |
| Yandi | 15.44 |
| Kooly | 7.72 |
| Carajas | 19.29 |
| SiJiaYing | 15.46 |
| dolomite | 6.77 |
| burntlime | 2.97 |
| limestone | 8.01 |
| Coke powder | 5.05 |
| Return fine (external) | 24.787 |
| Ingredient | TFe | SiO2 | CaO | MgO | Al2O3 | C | S |
|---|---|---|---|---|---|---|---|
| Content/% | 48.18 | 4.43 | 9.32 | 2.51 | 1.49 | 4.35 | 0.062 |
| Sinter | TFe | SiO2 | CaO | MgO | Al2O3 | Basicity |
|---|---|---|---|---|---|---|
| Content/% | 56.6 | 4.8 | 9.6 | 2.0 | 1.8 | 2.0 |
The schematic diagram of sinter pot test for SO2 recirculation simulation is shown as Fig. 1. The operational parameters of sintering pot test are shown as Table 6. The height and diameter of the sinter pot are 600 mm and 300 mm respectively. The recirculation gas is simulated by mixing air with compressed SO2 cylinders. The O2 concentration of the air is 21%, and the N2 is 79%. The SO2 gas distribution concentrations during the experiment were 0 ppm, 200 ppm, 500 ppm, 1000 ppm, and 2000 ppm, and the ventilation flow rate was controlled by a flow meter at 10 L/min. After the sinter mixture is charged and ignited, the hood is placed on the sinter pot, the gap between the hood and sinter pot is sealed with the asbestos pad, then the mixing gas is introduced to the sinter pot through the pipeline and hood. After the sintering process, the flue gas is monitored by the gas analyzer, and discharged after dedust. Finally, the sintering technical indexes and metallurgical performance index of sinter ore are tested.

| Parameters | Value |
|---|---|
| Bed height | 600 mm |
| Bed diameter | 300 mm |
| Ignition temperature | 1100°C |
| Ignition time | 90 s |
| Ignition suction | 6000 Pa |
| Sintering suction | 14000 Pa |
| Water content | 7.0% |
| Sinter basicity | 2.0 |
Sinter metallurgical performance indexes mainly include reduction index (RI), low temperature reduction differentiation index (RDI), softening and melt drop properties.
1) reduction index (RI)
| (1) |
Where Q0 (g) for the sample mass; Q1 (g) for the reduction before the start of the sample mass; Q2 (g) for the reduction of the sample mass; w1 (%) for the reduction of the sample before the FeO content; w2 (%) for the reduction of the sample before the full iron content; 0.11 for the oxidation of FeO to the corresponding amount of oxygen required Fe2O3 conversion coefficient; 0.430 for the oxidation of TFe all the way to the oxygen required by the conversion factor of Fe2O3. The reduction degree experiment is mainly to evaluate the reduction behavior of different iron-containing charges in the medium temperature zone of the blast furnace body, which is used to measure the difficulty of removing oxygen from the sintered ore.
2) low temperature reduction differentiation index (RDI)
The low temperature reduction differentiation rate is mainly a parameter to simulate the degree of fragmentation pulverization produced during the reduction of the charge located in the upper part of the blast furnace body (low-temperature zone).
| (2) |
| (3) |
| (4) |
Where: Q+3.15 mm (g) is the mass of the sample with a particle size greater than 3.15 mm after the drum, Q+6.3 mm (g) is the mass of the sample with a particle size greater than 6.3 mm after the drum, Q−0.5 mm (g) is the mass of the sample with a particle size less than 0.5 mm after the drum, and Qtotal (g) is the total mass of the sample before the drum.
3) softening and melt drop properties
The softening and melting droplet experiments mainly simulate the high-temperature reduction process of iron ore in the blast furnace in order to detect the effects of the operating conditions of the blast furnace, the formation of the soft-melting zone, and the location of its formation.
T10% is the softening start temperature (temperature when the layer shrinkage reaches 10%), T40% is the softening end temperature (temperature when the layer shrinkage reaches 40%), ΔT1 is the softening temperature interval, ΔT1=T40% − T10%, Ts is the melting start temperature (the temperature that sample differential pressure reaches 490 pa), ΔHS is the shrinkage value of the height at the beginning of the melt, Td is the drop start temperature (temperature when the first drop falls), ΔHd is the height at the beginning of the drop of the contraction value, ΔT2 is the temperature interval of the molten drop, ΔT2=Td −Ts, ΔH is the thickness of the molten drop band, ΔH=ΔHd −ΔHS.
2.4. Testing of Sintering Technical IndexesSintering production indicators mainly include vertical sintering velocity, productivity, yield and tumbling index.
1) vertical sintering velocity
| (5) |
Where U (mm/min) is the vertical sintering velocity, h (mm) is the height of the sintered material layer, and t (min) is the time taken to reach the sintering end point. The vertical sintering velocity is closely related to the permeability of the sintered material layer, the sintering thermal state, the negative pressure of the extracted air and the fuel characteristics.
2) productivity
| (6) |
Where ηa (t·m−2·h−1) is the productivity, Qa (t/h) is the sinter output per hour, and F (m2) is the effective air extraction area of the sinter.
3) yield
| (7) |
Where ηnp (%) is the yield, Q (kg) is the total mass of finished products larger than 5 mm, and Qa (kg) is the quality of sintered ore after burning. With the yield relative to the indicator is the return rate, the sum of the two is 1. The yield is one of the important indicators in the production of sintered ore, improve the yield can greatly improve the production efficiency.
4) tumbling index
| (8) |
where TI (%) is the tumbling index, m0 (kg) is the mass of the specimen entering the drum, and m1 (kg) is the mass of the specimen larger than 6.3 mm after the tumbling test.
The sinter metallurgical performance is shown in Figs. 2 and 3. As can be seen from Fig. 2, the reduction index RI shows an increasing trend with the increase of SO2 gas distribution concentration. The reason is that with the increase of SO2 concentration in the sintering process, more CaO reacts with SO2, resulting in the decrease of reaction between CaO and Fe2O3, and the increase of Fe2O3 content in the sintered ore, which leads to the increase of reduction index RI. Therefore, an appropriate increase in the concentration of SO2 gas distribution in the sintering process is favorable to improve the reduction index RI of sintered ore. Compared with the reduction degree index RI reduction pulverization index RDI change is not obvious, the analysis suggests that in the experimentally designed research scheme, although the proportion of calcium ferrite-based bonding phase in sintered ore shows a negative correlation trend with the concentration of SO2 fluxed in, the designed scheme, the content of calcium ferrite-based bonding phase can ensure the quality of ore formation in sintered ore, and therefore the trend of its RDI change is not significant.


As can be seen in Fig. 3, as SO2 concentration increases, Td and T10% do not change much, T40%, Ts, and ΔHS rise, and ΔHd decreases, resulting in ΔT1 becoming larger and ΔH and ΔT2 becoming smaller. The above indicates that adding SO2 during the sintering process is beneficial for improving the high-temperature melting performance of the sinter. This is mainly because increasing the concentration of SO2 will reduce the content of calcium ferrite in the sinter, resulting in a decrease in the amount of sinter slag and thus improving the permeability of the melting zone. Therefore, the SO2 content in the intake gas has the advantage of sinter softening and melting performance. An appropriate concentration of SO2 is not only beneficial for improving the reduction performance of the sinter but also for improving its high-temperature melting performance.
3.2. MicrostructureThe analytical results of X-ray diffraction are shown in Fig. 4. From the figure, it can be seen that the sintered mineral phase mainly includes Fe2O3, Fe3O4, calcium ferrate, SiO2, etc. The microstructure of sintered ore measured by SEM is shown in Fig. 5. When the SO2 concentration is 0 ppm, the sintered ore is interwoven structure, and Fe2O3 is distributed in a skeletonized crystalline structure near the holes of the sintered ore, and when the SO2 concentration is 2000 ppm, the microstructure of the sintered ore is a melt-eroded structure, and the flocculated Fe2O3 is distributed around the calcium ferrate.


In order to have a clearer understanding of the effect of SO2 gas distribution concentration on the change of sintered mineral phases, the results of each phase were analyzed semi-quantitatively by XPS method and the results are shown in Table 7. Compared with SO2 gas distribution concentration of 0 ppm, when the SO2 gas distribution concentration was 2000 ppm, the content of Fe2O3, Ca2Fe2O5, calcium sulfate and calcium silicate increased, and the content of Fe3O4, CaFe2O4 content decreased. As shown in Table 8, Fe2O3 and Ca2Fe2O5 are high melting point substances and CF is low melting point substance, so when the content of Fe2O3 and Ca2Fe2O5 in the sintered ore rises and the content of CF decreases, the high melting point substance in the sintered environment increases and the low melting point substance decreases. This phenomenon leads to a rise in T40%, ΔT1, and Ts, and a rise in Ts leads to an increase in ΔHS. With the increase of temperature and the reduction of Fe2O3 and calcium ferrite, the early slag is easy to be formed in the melting zone. Due to the high reduction index RI of the sintered ore, the initial slag contains more FeO, which makes the melting point and viscosity of the early slag decrease, and it is easy to drip, so ΔHd decreases.
| Phase | Fe2O3 | Fe3O4 | Ca2SiO4 | CaSiO3 | Ca2Fe2O5 | CaFe2O4 | CaSO4 |
|---|---|---|---|---|---|---|---|
| 0 ppm | 22.4 | 16.5 | 8.8 | 7.9 | 11.5 | 24.7 | 0.6 |
| 2000 ppm | 30.9 | 12.3 | 8.9 | 9.7 | 15.9 | 18.3 | 0.8 |
| Phase | Fe2O3 | Fe3O4 | Ca2Fe2O5 | CaFe2O4 |
|---|---|---|---|---|
| Melting Point, °C | 1536 | 1590 | 1436 | 1216 |
| Reduction Rate, % | 49.9 | 26.7 | 28.5 | 40.1 |
The influence of SO2 dosing concentration on sintering technical indexes is shown in Fig. 6. With the increase of SO2 dosing concentration, the vertical sintering speed first becomes larger and then smaller, and the sintering productivity is also affected by the vertical sintering speed, which shows the trend of first increasing and then decreasing. The reason is that the vertical sintering speed is mainly affected by the permeability of the sinter bed, and the permeability of the sinter bed is affected by the SO2 concentration in the combustion environment, and an appropriate increase in the concentration of SO2 gas distribution will reduce the content of calcium ferrite in the sintered ore, leading to a decrease in the amount of sintered slag, which improves the penetration rate of the melting zone and accelerates the vertical sintering speed. When the SO2 concentration in the combustion environment is too high, ΔT1 rises, the permeability of the sintered bed deteriorates, and the vertical sintering speed decreases.

From the Fig. 6, it can be seen that the sintered ore yield decreases with the increase of SO2 concentration, and it is analyzed that both SO2 and Fe2O3 are easy to react with CaO in high temperature environment, so SO2 in the circulating flue gas is easily absorbed by CaO in the sintering flux, and CaSO4 is generated on the surface of CaO particles, which is difficult to decompose at high temperature, so that the generation of calcium ferrite bonding phase decreases and the sintered ore The bonding force is reduced and fragile, thus affecting the sintered ore yield.
In order to further verify the effect of SO2 content on the strength of sintered ore during the sintering process, the sintered ore was dropped and sieved through sieves with different apertures, and the particle size distribution of the sintered ore was determined according to the content of sintered ore on sieves with different apertures as shown in Fig. 7. It can be seen from the figure that the SO2 content has a certain effect on the particle size distribution of the sintered ore, and the main particle size ranges from 25 to 40 mm when the SO2 content is 0 ppm and 5–10 mm and 10–16 mm with the increase of SO2 content. With the increase of SO2 content, the main particle size range is concentrated in 5–10 mm, 10–16 mm, analyzing the reason: The CaO contained in raw materials has certain effect on sulphur fixation. With the increase of SO2 dosing, more CaO participates in the sulfur fixation reaction (2CaO + 2SO2 + O2 = 2CaSO4), which reduces SO2 emission, while the sintered ore has less bonded phase, which reduces the room-temperature strength of the sintered ore,26) and therefore the sintered ore is more likely to be split into smaller sizes when falling through the sieve, so that in practice, we can control the SO2 concentration to improve the sintered ore quality.

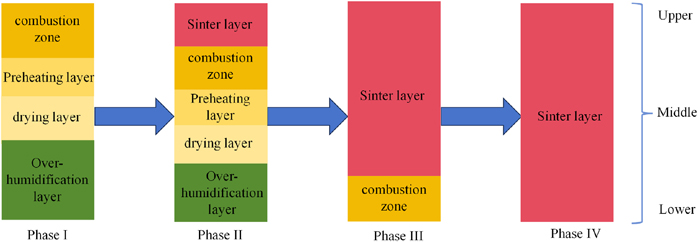
According to the different temperature levels and physico-chemical changes of the sintering process, the sintered material layer is classified into four states, as shown in Fig. 8. The fourth stage is the sintered ore layer stage, in which, after the sintering process is finished and the sintered ore is gradually cooled down, samples are taken from the upper, middle and lower layers of the sintered ore to determine the sulphur content, and the overall sulphur content of the sintered ore is roughly calculated from the sulphur content of each layer of the sintered ore.
As can be seen from Fig. 9, for the middle layer sintered ore, the SO2 dosing concentration of 2000 ppm corresponds to a residual sulfur amount that is 1.54 times that of 500 ppm. As can be seen from Fig. 10, when the SO2 dosing concentration is lower than 200 ppm, the residual sulfur amount in the sintered ore does not change significantly. When the SO2 dosing concentration is higher than 500 ppm, the amount of residual sulfur increases with the increase of SO2 dosing concentration. The reason was analyzed as follows: with the increase of SO2 dosing concentration, there was more and more SO2 in the reaction environment, and a large amount of SO2 would react with CaO to generate CaSO4,14) and according to the results of thermogravimetric-differential thermal analysis (TGA-DSC) of CaSO4,27) below 1200°C, CaSO4 has a very high thermal stability and is not easy to be decomposed, so the undecomposed CaSO4 is easy to stay in the sintered ore. The residual sulfur of sintered ore rises.


As can be seen from Fig. 11, the input of sulfur mainly comes from SO2 in the distribution gas and sintering mixture, and the output of sulfur mainly comes from the amount of residual sulfur in the sintered ore, SO2 in the sintered flue gas, sintered ore return, dust, etc. The sulfur content of the sintered ore and sintered flue gas increases with the increase of SO2 concentration. As can be seen from Fig. 12, with the increase of SO2 gas distribution concentration, sintered ore residual sulfur and sintered flue gas sulfur content on the whole show a rising trend. When the concentration of SO2 gas distribution is more than 500 ppm, the sulfur content in the sintered flue gas rises rapidly. The reason is that with the increase of SO2 concentration in the sintered flue gas, part of SO2 will react with CaO to form CaSO4, and the other part of SO2 that is not involved in the reaction will be discharged with other sintered flue gas. It can be observed from Figs. 2 and 3 that the reduction index RI and the softening and melting characteristics of the sintered ore show an improvement trend with the increase of SO2 distribution gas concentration. In summary, a moderate SO2 gas distribution concentration can improve the quality of sintered ore while controlling the concentration of SO2 emission in the flue gas.


The SO2 concentration in flue gas is shown as Fig. 13. The SO2 emission profile is similar at different SO2 concentration in inlet gas, and the peak concentration of SO2 in flue gas is improved with increasing SO2 concentration in inlet gas. When the SO2 concentration in inlet gas vary from 0 ppm to 2000 ppm, the peak concentration of SO2 in waste gas increase from 1156 ppm to 2859 ppm. The reason is that the production of CaSO4 is increased with the increasing SO2, when the combustion layer reaches to the bottom, more SO2 enters into the flue gas because of the decomposition of CaSO4 in combustion layer. It indicates that the SO2 concentration in flue gas could be enriched through the flue gas recirculation, which is beneficial for the end-of-pipe treatment of SO2.

As shown in Fig. 14, the sinter mixture is mixed by iron ore fines, fluxes and coke breeze, the SO2 flows with the inlet gas. Before the combustion of coke breeze, the SO2 react with CaO generating CaSO4 on the surface, meanwhile, the solid-phase reaction is occurred between the iron ore fines and fluxes generating low melting point materials, such as calcium ferrite. After the combustion, the temperature rise to 1300–1400°C, the products of solid-phase reaction is melting and liquid phase is generated, meanwhile the CaSO4 begins to decompose. The melting point of CaSO4 and FexO is high, so the undecomposed CaSO4 and unreacted FexO is distributed in the liquid phase. Moreover, many pores are exist in liuid phase because of the SO2 release from the decomposition of CaSO4, as well as the cooling shrinkage.

It suggests that the higher the SO2 concentration in inlet gas, the more CaO is consumed, which result in the reduction of calcium ferrite and liquid content, and increase of FexO. Therefore, the adhesion of binding phase become weaken, and the sinter ore is fragile in the shatter test, which result in that the yield is low and the grain size is small. In addition, more CaSO4 is generated, so the SO2 peak concentration is higher after the decomposition of CaSO4, which is shown as Fig. 13, and the decomposition of CaSO4 also increase the porosity of sinter ore which is considerd to be another reason that increase the RI and ΔHS The undecomposed CaSO4 stay in sinter ore as residual sulfur and rise the content, which is shown as Fig. 10.
Influences of the SO2 content in inlet gas on sintering process are researched at room temperature, to provide technical guidance for the waste gas recirculation technology. The following conclusions were obtained:
(1) With increasing SO2 concentration, the sinter yield and particle size is decreased because the SO2 is absorbed by CaO and CaSO4 is generated on the surface of CaO particles, which affects the formation of binding phase, Therefore, adding the right amount of sulfur dioxide to the sintering process can help improve the quality of the sinter, and if the amount of sulfur dioxide is excessive, it will reduce the quality of the sinter.
(2) The residual sulfur in sinter ore increases obviously when SO2 concentration is higher than 500 ppm, the residual sulfur of middle sinter in 2000 ppm is 1.54 times higher than that in 500 ppm, this is mainly because with the increase of SO2 concentration in the reaction environment, the CaO produced by the decomposition of CaSO4 will undergo a secondary reaction with excess SO2, resulting in incomplete decomposition of CaSO4 and an increase in residual sulfur content in the sinter.
(3) The SO2 emission profiles are similar at different SO2 concentration in inlet gas, while the SO2 peak concentration in flue gas is increased with the increasing SO2, this is beneficial for the end-of-pipe treatment of SO2.
(4) The consumption of CaO reduces the amount of calcium ferrate produced and leads to an increase in the Fe2O3 content, melting point and reduction rate during sintering. In addition, the decomposition of CaSO4 could increases the porosity in sinter ore. Therefore, the RI is increased and softening and melting properties become better with increasing SO2 concentration.
This work was financially supported by the Foudation and project: China Baowu Low Carbon Metallurgy Innovation Foudation (No. BWLCF2002122); Science and Technology Research Project of Higher Education Institutions in Hebei Province (No. ZD2020116).