2024 Volume 64 Issue 6 Pages 928-934
2024 Volume 64 Issue 6 Pages 928-934
The acceleration of the carburizing and melting processes in reduced iron is critical for reducing energy consumption and CO2 emissions produced by the steel industry. The aims of the study were to clarify the carbon dissolution reactions and diffusion processes in molten slag. A combined approach utilizing Raman spectroscopy and XPS was employed to investigate the relationship between carbon-containing species and slag composition in the CaO–SiO2–Al2O3 slag system. The Raman spectra indicated that carbon dissolved in slag in the form of carbides, carbonates, and graphitic structures. The proportion of carbide ions in the slag increased from 64.2% to 74.7%, and the proportion of graphitic structures decreased from 19.7% to 7.4% as the slag’s basicity increased. The carbon dissolution mechanism in slag involved the reaction of carbon with dissolved oxide ions and oxygen-containing anions in the slag. This formed carbon-containing species such as CO32−, C22−, and graphitic structures that diffused in the slag and subsequently underwent carburization and deposition at the iron interface.
The steel industry accounts for approximately 11% of all global CO2 emissions,1) approximately 70% of which are produced by coal-based blast furnace-basic oxygen furnace (BF-BOF) plants.2) Therefore, a low-carbon technology for reducing CO2 emissions during the ironmaking process is necessary to help address global warming. The use of carbonaceous materials during iron ore reduction has attracted attention as a method for decreasing CO2 emissions.3,4) The ironmaking process involves four processes: heating, reduction, carburization, and melting.5) Carbonaceous materials have two important roles in the ironmaking process. One is as a reducing agent for iron ore, which was expected to be an alternative to hydrogen.6) The other is as a carburizing agent for reduced iron to promote iron melting. The carburization of reduced iron may be a critical step in reducing energy consumption, as the incorporation of low-melting carburized iron may lower the temperature of metallurgical processes.7,8) Carburization may also enhance the aggregation-induced coarsening of the metal, which may promote the separation of the slag and the metal.9,10) Rapid initiation of iron melting and molten slag separation can shorten the melting time.
Therefore, enhancing carburization may be a key approach to reduce energy consumption and carbon emissions in the steel industry.11,12) Iron carburization may occur through the molten slag that directly contacts a carbonaceous material, and the molten slag may initiate iron melting by attracting graphite to iron.13) Carburization can occur without direct contact between the reduced iron and carbonaceous material. Carbon species dissolved in silicate melt, including graphitic structures and carbides, are transported in the slag and cause carburization at the metal-slag interface.14,15)
The ironmaking process involves heterogeneous chemical reactions between molten iron and slag. Hence, understanding the carbon-bearing species and their behavior at the iron-slag interface is important for improving energy efficiency. It has been reported that carbon-bearing species and their transfer rate in slag strongly affect reduction and carburization. Chapman reported that carbon dissolution in molten iron was prevented by minerals composed of SiO2 and Al2O3.16) Ohno suggested an iron carburization mechanism during smelting reduction that made carburization possible without direct contact between the reduced iron and carbonaceous material.17) Song reported that carbon dissolved in CaO–SiO2 contained C22− and CO32− species produced by thermodynamic reactions.18) Park investigated the solubility of carbon in CaO–Al2O3(SiO2)–CaF2 slag and proved that carbon dissolution increased with the content of CaO/Al2O3(SiO2) and CaF2.19) Ono reported that reduced iron in the form of C22− during carbon underwent a carburization reaction with reduced iron in slag at 1723 K, and the carburization rate increased with the basicity.20) However, all references only reported carbides based on thermodynamic reactions, while the confirmation of carbon-bearing species and their quantitative distribution in slag have seldom been reported.
The possible mechanisms of the dissolution of carbon-bearing species in slag and their corresponding transfer rates are essential for decreasing carbon consumption and emissions. Information about carbon dissolution in slag is rather limited, and the role of carbon-bearing structures and their diffusion in slag are unclear. The purpose of this study was to estimate the carbon dissolution reaction at the iron/slag interface and clarify dissolved carbon-bearing species, including the intermediate products C22−, CO32−, and graphite in slag and their transfer in molten slag. Carbon-bearing species in slag samples were analyzed by Raman spectroscopy and X-ray photoelectron spectroscopy (XPS). The quantity and transport mechanism of carbon-containing species in slag were determined.
CaO–SiO2–Al2O3 is the slag system used in ironmaking, steelmaking, and refining. Slag was prepared by pre-melting a mixture of analytical reagent-grade oxide powders (CaO 35–50%, Al2O3 12–25%, and SiO2 40%) at 1673 K in a muffle furnace for 3 h. This procedure was repeated until the slag was in a glassy state. There were three kinds of slag basicity of CaO–SiO2–Al2O3, and the composition of pre-melted slags is shown in Table 1. The slags were melted at a high temperature and then cooled rapidly. The cooled slag was milled into a powder. 99.90% pure iron and 99.90% pure carbon powder were used in this experiment.
| Pre-experimental (wt pct) | Post-experimental (wt pct) | ||||||
|---|---|---|---|---|---|---|---|
| No. | CaO | Al2O3 | SiO2 | CaO | Al2O3 | SiO2 | R(C/S) |
| S1 | 35 | 25 | 40 | 36.0 | 26.2 | 39.8 | 0.88 |
| S2 | 40 | 20 | 40 | 40.6 | 19.7 | 39.7 | 1 |
| S3 | 50 | 10 | 40 | 48.9 | 10.2 | 40.9 | 1.13 |
This experiment consisted of two sub-experiments. The first experiment was carried out to confirm that graphite could diffuse to the surface of reduced iron through CaO–SiO2–Al2O3 slag. Samples were prepared by mixing particles of iron, carbon, and slag with a particle size of 100 μm and pressing them into a cylinder with a 30 mm diameter and 10 mm height. The cylinder was put into an alumina crucible and held at 1723 K (the temperature at which all slag components melted) for 60 min.
In the next experiment, the samples were used to analyze carbon dissolution and diffusion in molten slag. The samples were assembled as depicted in Fig. 1, in which a 1.5 mm layer of pure iron, 20 mm layer of slag, and 2 mm layer of carbon were pressed under a pressure of 3 MPa. An iron plate was laid on the bottom of the alumina crucible and was initially separated from carbon by slag. The carbon sheet was placed on top of the slag to form a carbon/slag two-layer structure. Pressed samples were placed into an alumina crucible with an exterior graphite crucible. The graphite crucible consumed residual oxygen in the furnace in the reaction system. The crucible was placed into a high-temperature tube furnace and held for 2 h at 1723 K. Argon [Ar: 200 cm3/min STP] gas flow was injected during the entire process to prevent carbon oxidation. After the experiment, the slag was quickly cooled to room temperature under an argon atmosphere and then removed and crushed into powder.

The carbon-iron interface was examined with SEM-EDS (Oxford X-MAX20). Analytical conditions for slags were an accelerating voltage of 15 kV and a beam current of 20 nA. The carbon content in slag was determined by a LECO combustion analyzer (CS-300) equipped with a carbon-sulfur analyzer. Raman spectroscopy (LabRAM HR) and XPS (ESCALab) were used to clarify the stable forms of carbon in slag, such as carbonates, carbides, and graphitic structures. The obtained Raman spectra were deconvoluted, and their area was integrated to obtain quantitative information about the amounts of individual structural units within the melt.
The role of molten slag during iron carburization and melting plays a critical role in the ironmaking process, but it is unclear how the molten slag determines the behavior of carbonaceous materials during this process. An experiment was performed to clarify the randomness of carbon diffusion in molten slag with a basicity of 1.13. A mixture of iron powder, carbon, and slag was heated to 1723 K, held for 60 min, and then the crucible was removed from the furnace and cooled rapidly under an argon atmosphere. The mixture of slag, iron particles, and graphite was ground to the micrometer range. Figure 2 shows the morphology of the mixture, in which carbon diffused through the slag and was distributed around iron. A carbon layer with a thickness of about 30 μm was scattered around the iron particles, indicating that forces attracted carbon to the iron interface. The EDS analysis in Figs. 2(a) 2(b) shows that the carbon layer contained 14.89% oxygen and 1.04% calcium, indicating that carbonaceous materials such as calcium carbides and carbonates were present. There was a thin layer of slag distributed between carbon and iron particles, implying that the formation of metal carbides provided a driving force for metal-carbon interactions. Figures 2(c) and 2(d) show that iron and carbon were distributed throughout the scanned area.

An experiment was designed to explore the diffusion of carbon through the molten slag to reduced iron at 1723 K for 60 min. Figure 3 shows the morphology of the interface of slag and iron, across which carbon diffused and initiated carburization. The carburization reaction occurred at the interface of iron and carbon, and excess carbon precipitated at the iron interface to form a 25 μm-thick layer. O, Ca, and Si were distributed in the carbon layer according to SEM-EDS analysis. The element distribution of the slag and iron interface was similar to that in Fig. 2, implying the diffusion of oxidized carbon, silicon, and calcium species, such as calcium carbides and carbonates.

The results of the two experiments indicated that carbon dissolved into the molten slag and could diffuse through the molten slag to iron, driven by differences in the carbon concentration. Carbon migrated to the iron interface and underwent further carburization reactions. Previous researchers have shown that carbon is dissolved in molten slag as intermediate soluble carbon-bearing species such as CO32− and C22−.21) To understand the role of carbon in high-temperature slag, it is necessary to determine if carbon exists in various carbon-bearing species and their transport into high-temperature slag.
3.2. Carbon Containing Species in Molten SlagRaman spectroscopy was combined with XPS spectroscopy to characterize carbonaceous species in the slag. Figure 4 shows the Raman spectrum of carbon dissolved in slag with basicities ((%CaO)/(%SiO2)) of 0.8, 1.0, and 1.13. The spectrum in Fig. 4(a) shows that the slag was in the glassy state. The spectrum exhibited a stronger peak at 400–1200 cm−1 whose intensity decreased as the basicity increased. The peaks between 800 cm−1 and 1200 cm−1 corresponded to Si–O stretching vibrations in silica-oxygen tetrahedra. The prominent Si–O–Si stretching bands around 690 cm−1 and the characteristic peaks near 450–550 cm−1 corresponded to Al–O–Al vibrations. The spectrum exhibited a peak at 990 cm−1 due to the characteristic stretching vibration of C22− with a characteristic peak similar to CaC2. The peak at 695 cm−1 corresponded to C–O bending vibrations, but it was located near the Si–O–Si stretching bands and therefore hard to detect. The peak at 1080 cm−1 corresponded to C–O stretching vibrations of CaCO3, but this peak was also hard to detect due to the prominent Si–O stretching bands at 1000 cm−1.
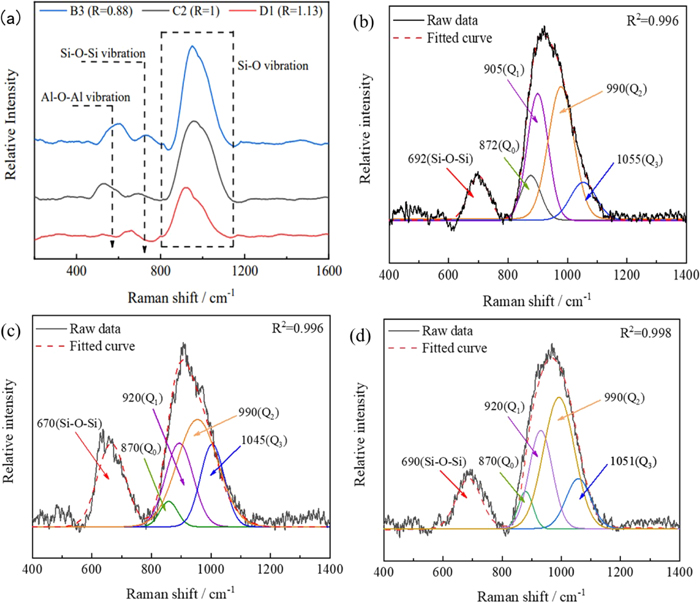
In the spectra of slag with basicities of 0.88, 1.0, and 1.13, there were four Si–O stretching vibrations in the ranges of 850–880 cm−1, 900–920 cm−1, 950–1000 cm−1, and 1050–1100 cm−1, according to the Gaussian simulations shown in Figs. 4(b)–4(d). The peaks representing Si–O–Si vibrations became weaker as the slag basicity increased, indicating that the slag network depolymerized to simpler structural units. The complexity of the slag decreased, and the degree of aggregation decreased, which enabled the effective free oxygen ion content to increase, which may have promoted carbon dissolution reactions in the slag. The Raman peaks between 1045 cm−1 and 1055 cm−1 shifted as the basicity changed. Carbon became soluble in silicate slag due to the direct substitution of C4+ for Si4+ in the Si–O–Si tetrahedra and the formation of C–O–Si bonds by introducing carbon into the silicate network.22)
The Raman spectra revealed that Si–O–Si bending vibrations decreased at a higher basicity. This correlated well with the higher free oxygen ions (O2−) in the slag system and thus greater carbon dissolution. This mechanism also implies that the carbon solubility in various calcium silicate slags was similar and that the C–O bonds were dependent on oxygen. Carbon was an effective reducing agent at processing temperatures and could react with the oxide ion and oxyanion impurities dissolved in the molten slag.
The solubility of carbon increased upon increasing the basicity. The solubility of carbon was 0.0278% and increased to 0.08% as the basicity increased from 0.88 to 1.13, as determined using a carbon-sulfur analyzer. Carbon dissolved in slag and formed carbonaceous structures, including graphite, CaC2, and CaCO3 in slag with a basicity of 1.13.
3.3. Carbonaceous Species Distribution in SlagThe slag basicity strongly influenced the carbon dissolution reaction and carbon speciation in molten slag. Figure 5 shows the quantities of carbon species in slag with a basicity of R = 0.88, R = 1, and R = 1.33. Carbon species produced XPS peaks at 270–300 eV. The peaks in Fig. 5(a) with binding energies at 289.5 eV, 284.7 eV, and 280.1 eV indicated that carbon existed in slag as calcium carbonate, graphitic structures, and calcium carbide, respectively.23) The Raman spectra showed that carbon dissolved in molten slag with different basicity and formed graphitic structures, C22− and CO32−, as shown in Figs. 5(b)–5(d).

The proportion of carbonaceous species curves was fitted by a Gaussian function (the mathematical calculation method involved averaging the fluctuating curve and summing the area). The proportion of carbonaceous species was obtained by dividing the total carbon content with each peak area fraction, as shown in Fig. 6. The proportion of carbide ions was higher than that of carbonates and graphite in slag. The carbide ion proportion was 74.74% in slag with a basicity of 1.13, which was 4.1% and 10.32% higher than in slag with a basicity of 1.0 and 0.88, respectively. The proportion of carbonate ions was 17.86% in slag with a basicity of 1.13, which was 2.79% and 2% higher than in slag with a basicity of 1.0 and 0.88, respectively. The proportion of graphite was 7.4% in slag with a basicity of 1.13, which was 6.89% and 12.31% lower than that in slag with a basicity of 1.0 and 0.88, respectively. As the basicity of the slag increased, the proportion of carbide ions gradually increased, while the proportion of graphitic carbon decreased.
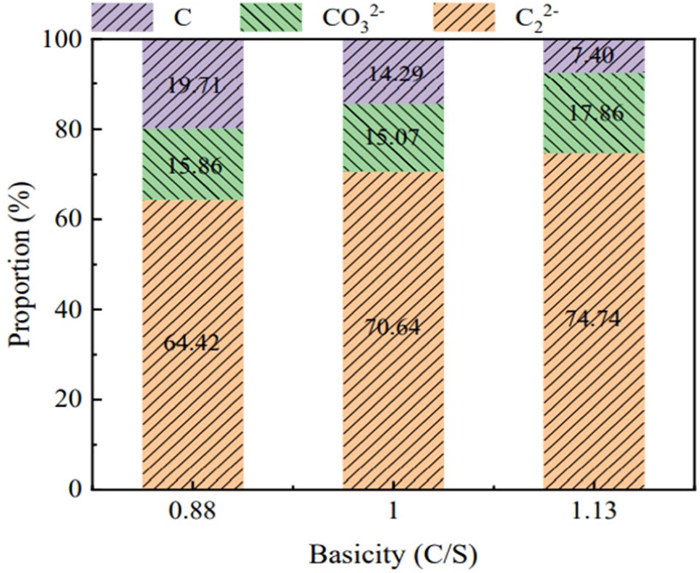
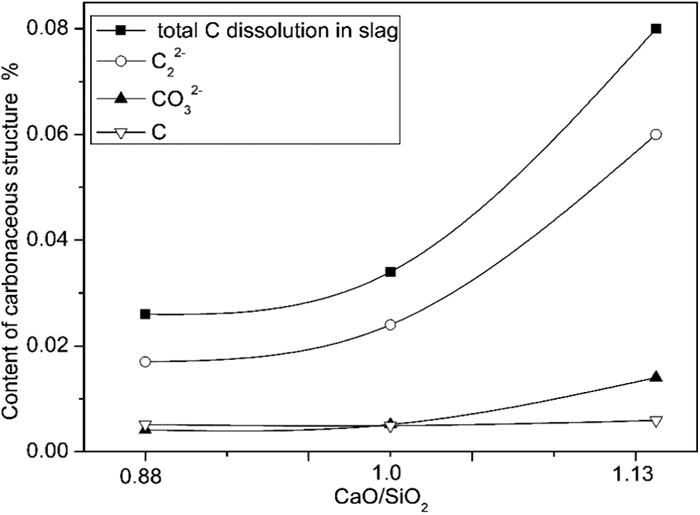
The carbide was dominant but increased slightly, and the carbonate content increased relatively, which implied that carbonate became the more stable species at a higher basicity (oxygen ion content increased). The stable form of carbon changed from graphite to carbides and carbonates upon increasing the oxygen ion content in slag. The total carbon content was 0.026% in slag with a basicity of 0.88, as measured using a carbon-sulfur analyzer. The carbon content increased to 0.08% as the basicity increased to 1.13. The contents of carbonate, carbide ions, and graphite in slag were estimated by the area fraction of each carbon species peak. Figure 7 exhibits the solubility of carbon species in different slags as a function of the basic oxide content. The carbide ion content was 0.017%, 0.024%, and 0.06% in slag with a basicity of 0.88, 1.0, and 1.13, respectively. The content of carbonate ions increased from 0.0041% to 0.014% when the basicity increased from 0.88 to 1.13, while the content of graphite was 0.0051%, 0.0049%, and 0.0059% in slag with a basicity of 0.88, 1.0, and 1.33, respectively. The dissolution of carbon in the slag and the corresponding carbide ions content also increased as the slag basicity increased because the content of effective bridging oxygen ions decreased. The content of free oxygen ions increased, which reduced the complexity of the slag.
The metallurgical behavior of carbon can be described as: Carbon dissolved in the slag and then reacted with dissolved free oxygen, oxide ions, and oxyanions in slag to form C22−, CO32−, and graphite. The carbon-containing species diffused in the slag and underwent carburization or excess carbon deposited on the iron interface.
Carbon dissolution in the slag as carbonaceous species may have included carbides, and the dissolved carbides could be reduced on metal surfaces and deposited as carbon. Carbide was more stable in the high-basicity slag, which was the main dissolved carbon species. The dissolution mechanism of carbon in slag could be expressed by the following equation:24) 2C(s) + O2−(slag) → C22−(slag) + (O). The carbide capacity in the slags increased with the basicity. The driving force for this process was the carbide-forming reactions that occurred on graphite surfaces.
Carbide ions were the dominant species, but carbonate ions and graphite were also present. The carbide content increased and carbonate also increased as the basicity increased, which implied that carbonates were the more stable species upon increasing the oxygen ion content in slag. This suggests that the stable form of carbon changed from graphite to carbonate upon increasing the oxygen ion content.25)
Carbonaceous species dissolved in the slag interacted with bridged oxygen (O0) in the melt slag, demonstrating that CO32− was generated by reactions involving oxide ions. The interaction between oxides and carbon generated CO32− according to follow equation: C(S) + (O2−) + (O) → (CO32−). The mechanism for the continuous transport of carbon to metallic surfaces through the melt slag was determined by morphology observations. The formation of reduced C by carbonates occurred under reducing conditions. In addition, graphite can be considered as an intermediate carbonaceous species. Graphite was formed at the iron interface under reducing conditions.26,27)
The microstructure of the CaO–SiO2–Al2O3 slag system was dominated by silicates. The slag was a polymer melt that contained large metal-oxygen complexes containing metals or metallic cations. The complexes in slag typically included (SiO44−), (Si2O76−), (AlO54−), (AlO75−), and other metalloid-oxygen complexes.28,29) Carbon also substituted Si or Al of these complexes and formed carbonaceous species that allowed carbon to diffuse through slag and precipitate at the interface with iron.30) These results further verified the presence of elements such as C, O, Ca, and Si at the slag-iron interface in Fig. 3.
The XPS spectra show that carbide was stable at a high oxygen ion content in slag and carbonates formed at bridging oxygen atoms. These phenomena could be explained by the carbide and carbonate generation reaction, in which carbon in the slag was expected to increase upon decreasing the free oxygen content as carbides under a fixed composition and temperature. The carbon in slag were dependent on the oxygen ion activity, which increases with a higher ratio of of CaO/SiO2.
The rate of carbon transport depended on the dissolved carbon content in slag, which was affected by the intermediate carbon species. A low oxide content decreased carbon transport and vice versa. The carbon-slag interface was considered as the initial position (0 mm). The carbon capacity at distances from the carbon-slag interface of 5 mm, 10 mm, 15 mm, and 20 mm are shown in Fig. 8. The carbon capacity at 5 mm was 0.025% and 0.067% in slag with a basicity of 0.88 and 1.13, respectively, and the carbon capacity was 0.0071% and 0.052% at 20 mm in slag with a basicity of 0.88 and 1.13, respectively. This meant that the carbon diffusion rate increased as the basicity increased. The increase in the carbon capacity corresponded to a higher carbide solubility in slag due to the depolymerization of the silicate network by oxygen ions as the basicity of the slag increased. Thus, carbon solubility in the silicate melt was strongly correlated with slag basicity.

Here, we investigated the influence of slag composition on the carbon-containing species and carbon capacity in a CaO–SiO2–Al2O3 ternary slag system at 1723 K. The conclusions were as follows:
(1) The composition of the slag was independent of carbon-containing species in the slag. Stable carbonaceous structures in the slag samples with different basicity included carbonate ions (CO32−), graphitic carbon (C), and carbides (C22−), all of which could diffuse through the slag to the iron interface to undergo carburization and precipitation.
(2) The proportion of carbon-containing species in the slag varied with the slag basicity. The proportion of carbide ions in the slag increased from 64.2% to 74.7%, and the proportion of graphitic carbon decreased from 19.7% to 7.4% when the slag basicity increased from 0.88 to 1.13.
(3) The dissolution mechanism of carbon in the slag was as follows:
(4) The XPS spectra proved that carbon dissolved in ironmaking slag existed as carbides under reducing conditions. The rate of carbon transport depended on the content of dissolved carbon in slag, which was influenced by the intermediate carbon-containing species.
This work was supported by the National Natural Science Foundation of China under Grant [No: 51974185; 52022054], the State Key Laboratory of Advanced Special Steel, and the Science and Technology Commission of Shanghai Municipality [No. 21DZ1208900].
The authors declare no conflicts of interest.