2025 Volume 65 Issue 2 Pages 323-325
2025 Volume 65 Issue 2 Pages 323-325
Wettability between molten iron and non-metallic inclusions is an important factor, as it influences the behavior of non-metallic inclusions during secondary metallurgy. In the present study, the wettability of CaS against molten iron was investigated using the sessile drop method at 1873 K. The CaS substrate was fabricated using spark plasma sintering, achieving a high relative density of 98%. The measured contact angle of CaS against molten iron was found to be 118 degrees. This finding indicates that the wettability of CaS is poor, which contrasts with the previous report. This result will contribute to further understanding regarding the inclusions’ behavior during secondary refining processes.
In a production process for high-cleanliness steels, impurities such as sulfur and phosphorus are removed from metal using CaO-containing slag. Hydrogen, nitrogen, oxygen, and other gas components are removed by adding elements with high affinities or through degassing operation. In these processes, non-metallic inclusions (hereafter, referred to as “inclusion”), such as oxides and sulfides, are generated. Some inclusions remain in metal and cause surface or inner defects. Promoting the aggregation and coalescence of inclusions facilitates coarsening of inclusions, contributing to an efficient floatation and separation from melt. Generally, the in-metal behavior of inclusions can be understood with the viewpoint of their interfacial properties against molten metal.
Along with various oxides, CaS is known to affect the inclusion’s behavior in molten steel, therefore, it also is recognized as the target to control during steelmaking processes. For example, Ca treatment is widely applied as a countermeasure for nozzle clogging by transforming solid inclusions into liquid CaO–Al2O3 inclusions. During this transformation of inclusions, transient CaS phase forms before the formation of liquid CaO–Al2O3 phase.1) Also, as for inclusion formation behavior in steel refining processes, CaS phase can be formed by reactions between existing inclusions and sulfur in molten steel.2) These facts signify that CaS phase influences the inclusion behavior in molten steel during secondary metallurgy.
Regarding the interfacial properties between CaS and molten iron, the contact angle between CaS and molten iron was reported as 87 degrees by Staronka and Gotas,3) in other words, CaS was reported to show high-wettability with molten iron. Ever since this has been reported, the wettability of CaS against molten iron has not been studied or reported. However, Yoshikawa et al.4) reported that CaO doesn’t wet with Al–S containing molten steel, and its wettability becomes poor by forming CaS phases. This report implied that the CaS wettability is poorer than that of CaO. Thus, there is no consistent understanding regarding wetting properties of CaS, causing a difficulty for discussion regarding the behavior of CaS inclusions in molten steel.
The aim of this study is to evaluate the wettability of CaS with molten iron by sessile drop method for a precise understanding of the interfacial properties of CaS.
Table 1 shows chemical compositions of iron samples employed in this study . The chemical compositions of iron sample in the previous study are also listed in the same table.3) The sample was washed with dilute hydrochloric acid before the experiment to remove the oxide layer on the iron surface. A CaS substrate (ϕ30 mm×t2 mm) was fabricated from CaS powder (Kojundo Chemical Laboratory Co. Ltd., ≧99%) by Spark Plasma Sintering (SPS), whose conditions were 1573 K, 60 MPa, and 10 min. The relative density of the CaS substrate was about 98% and the remaining 2% is closed pore, which were measured using Archimedes method with referencing the theoretical density of CaS.
| C | Si | Mn | P | S | Cr | Al | T.O | N | |
|---|---|---|---|---|---|---|---|---|---|
| (mass%) | (mass%) | (mass%) | (mass%) | (mass%) | (mass%) | (mass%) | (ppm) | (ppm) | |
| This work | 0.001 | 0.03 | 0.01 | 0.002 | 0.0007 | 0.02 | 0.001 | 338 | 45 |
| Staronka and Gotas3) | 0.013 | 0.005 | 0.06 | 0.008 | 0.01 | – | 0.004 | – | – |
Figure 1 shows a result of powder XRD analysis for the sintered CaS substrate, and it was confirmed that the substrate was composed of a single phase of CaS. The analysis conditions are as follows: an XRDynamic 500 (Anton Paar, Austria) equipped with a Co–Ka radiation source and the Pixos 2000 energy dispersive detector were employed with the operating condition of 40 kV and 40 mA for X-ray generation. PXRD profiles were recorded in the 2-theta range from 20 to 40 degrees with a step size of 0.005 degrees and a counting time of 60 sec. per increment. Phase identification was performed on the collected PXRD profiles using the ICDD database (version PDF-4+ 2023).

The CaS substrate was polished using SiC waterproof abrasive paper (#1200, and #2000) and vibratory polisher (Buehler, VibroMet2) with 1 μm diamond paste. Thereafter, they were washed by ultrasonic cleaner in acetone bath. Figure 2 shows the appearance of the polished substrate.

Figure 3 shows a schematic illustration of the ultra-high-temperature wetting furnace.5) The substrate was set horizontally on the sample stage, and an alumina crucible filled with iron sample (about 2 g) was located above the substrate.
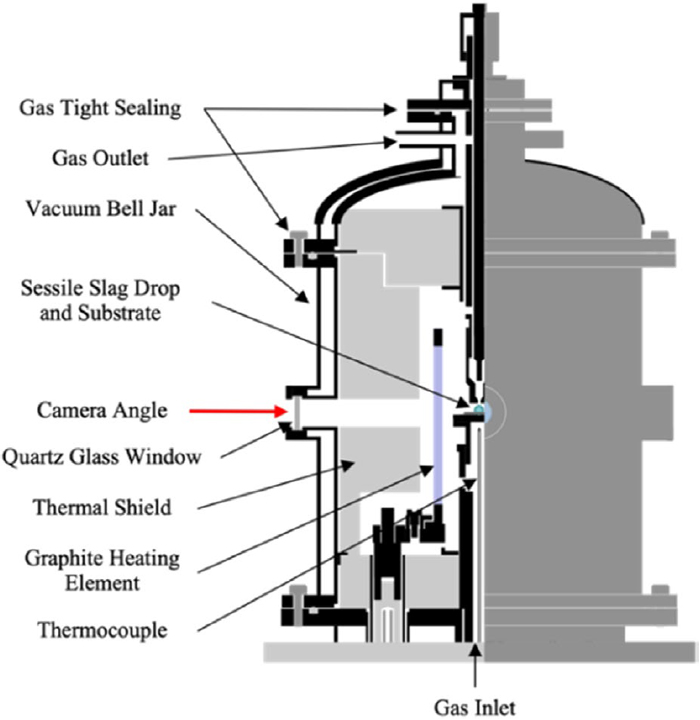
The furnace was evacuated from the ambient atmosphere to 50 Pa and filled with Ar gas up to 1 atm. This procedure was repeated three times. Ar gas was passed through the silica gel and P2O5 reagent to remove H2O in the gas prior to the inlet to the furnace. The oxygen partial pressure in the Ar gas was measured as 10−22 Pa using a ZrO2 oxygen sensor (STLab Co. Ltd., SiOC-200C) in another experiment with the same conditions. The furnace was heated to 1873 K at 15 K/min with an Ar gas flow rate of 0.2 L/min. Figure 4 shows an illustration of molten iron before and after dropping onto the substrate. The iron was pushed out from the crucible using an alumina pushing stick, and then the melt dropped onto the substrate. A digital camera was used to capture images of the iron droplet, and the contact angle was measured using the curve-fitting method with the obtained images.

Figure 5 shows a molten iron droplet just after dropping. It seemed that the molten iron was not oxidized throughout the experiments as its surface kept reflective (the dark areas on the droplet surface are reflected image of the inner furnace window). Apparently, the contact angle was above 90 degrees. In other words, CaS was found not to wet against molten iron. The contact angle was determined as 118 degrees using the curve-fitting method.

As confirmed thus far, the wettability of CaS against molten iron found to be “poor”, despite it being believed to be good in the previous study. For comparison, both the experimental conditions of this study and those of Staronka and Gotas are listed in Table 2. The measurement method for the contact angle of CaS was the same. On the other hand, the experimental conditions, such as the substrate fabrication method, atmosphere, and temperature, were different. In the previous study, the CaS substrate was fabricated by compacting the powder in a hydraulic press and then sintering it for 8 hours under Ar atmosphere. It can be pointed out that the CaS powder could have reacted with some elements in the atmosphere during the long sintering time, which might have resulted in a composition change of CaS. In the present study, the CaS substrate was fabricated by SPS method, therefore, the sintering had been completed in a very short time within a closed atmosphere. Additionally, the oxygen partial pressure was confirmed to be controlled at a low level of 10−22 Pa. These experimental conditions prevented CaS from changing its composition, as confirmed by the XRD pattern shown in Fig. 1. Also, the reflective surface of molten iron was observed throughout the experiments as shown in Fig. 5, indicating that the oxidation of molten iron during the experiments has been suppressed. Thus, the experimental conditions for measuring the CaS contact angle in this study seem to be quite appropriate, which can support the validity of the obtained finding that the wettability of CaS against molten iron is poor.
| Staronka and Gotas3) | This work | |
|---|---|---|
| Contact angle measurement method | Sessile drop method | Sessile drop method |
| Substrate fabrication method | Hydraulic press, SiC tube furnace (300 kg/cm2, 1200°C, 8 h) | SPS (60 MPa, 1573 K, 10 min) |
| Density of substrate | No data | 98% (relative density) |
| Atmosphere | Ar (10−8 vol%O2, 20 L/min) | Ar (PO2= 10−22 Pa, 0.2 L/min) |
| Temperature | Melting point of metal | 1600°C |
So far, a lot of research has already pointed out the significance of the interfacial property, namely contact angle, of particles (inclusions) against liquid bath (iron melt) in terms of their behaviors, such as penetration, flotation and removal. As for the removability of particles from liquid, the common conclusion out of previous studies was that the 90 degrees of contact angle is the critical threshold whether particles can be removed smoothly or not.6,7,8,9) From this viewpoint, the result obtained in the present study is quite intriguing since the wettability of CaS has been found to be “poor (> 90 degrees)” contrary to the previous result. This finding will contribute to further understanding regarding the behavior of inclusions in molten iron during steel refining processes.
The wettability of CaS against molten iron was evaluated by the sessile drop method at 1873 K. The contact angle between CaS and molten iron was 118 degrees. The wettability of CaS phase against molten iron found to be poor in contrast to the result obtained in previous research. This finding contributes to further understanding regarding the inclusions’ behavior during secondary refining processes.