Abstract
Shinkaia crosnieri, a galatheid crab, has ectosymbiotic bacteria on
its ventral setae, and forms very dense crowds in hydrothermally active
regions and seep areas. They feed on the symbiotic bacteria and do not chase
other animals for predation. To study how they move and behave in jostling
crowds, we developed a vital staining to mark their individuals and trace
them by using a camera on a remotely operated vehicle (ROV). Among the
various dyes examined, Coomasie Brilliant Blue R250 (CBB) stained the
galatheid crab the darkest, and its color lasted for more than 5 months in
the laboratory at 4–5°C. The ventral setae were strongly
stained, while the dorsal shell was weakly stained. The stained galatheid
crab survived for more than 8 months. For the in situ staining of
S. crosnieri at the Iheya North hydrothermal field in the Okinawa
Trough, Japan, we applied a dye solution mixture (20 L) containing CBB and
Acid Blue 161 to the galatheid crab population through a funnel
equipped on the ROV Hyper-Dolphin. After staining for approximately
5 minutes, more than 18 individuals of S. crosnieri were dyed blue.
They were disturbed by the staining process but seemed to be unharmed. The
dyed galatheid crabs were identified by the ROV one and two days post
staining. They seemed to remain at the place where they were stained.
The present vital-staining marking method may present a new way to analyze
the behavior and changing habitable range of deep-sea animals like
S. crosnieri, and may give us a deeper insight into how these animals
behave in a very dense population and explore newer habitats.
1. Introduction
In deep-sea ecosystems at hydrothermal vents and seeps, high-density animal
communities are often found (Van Dover, 2000). Two dominant animal groups in
the communities, the tubeworms and the giant deep-sea Calyptogena clams, are known to have intracellular symbionts, which provide
chemosynthetic products to the host animals (Van Dover, 2000). The galatheid
crab Shinkaia crosnieri, which has been originally described in the
Edison Seamount near Papua New Guinea, forms very dense
populations, jostling each other in crowds at deep-sea hydrothermal vent
fields and methane seep sites in the Okinawa Trough and South China Sea
(Chan et al., 2000; Baba and Williams, 1998; Tokeshi, 2011; Tsuchida et al.,
2003; Yang et al., 2016). Although it has been observed that S. crosnieri feeds on dead shrimp or some other meat such as tuna flesh under
cultivation in aquaria (Kitajima et al., 2012), no predatory behavior has
been observed during in situ observations. S. crosnieri
harbors ectosymbiotic bacterial communities including thioautotrophs and
methane-oxidizing bacteria on their ventral setae (Tsuchida et al., 2011;
Watsuji et al., 2010; Watsuji et al., 2012), and feeds on the bacteria by
combing and harvesting them with its third maxllipeds (Tsuchida et al., 2011;
Watsuji et al., 2015). S. crosnieri does not feed on other animals;
it does not seem to move around to chase other prey animals in the habitat.
It typically does not move unless disturbed, but it has recently been
reported to migrate from large colonies to areas where new hydrothermal
vents had been artificially created on the seafloor (Nakajima et al., 2015).
While it appears to be able to traverse large distances over the time scale
of months, information regarding its local motion is not available. It is
not clear how far S. crosnieri could travel and communicate with
other individuals of the species in high-density populations in their
natural habitats. However, individuals of S. crosnieri are
difficult to distinguish in a crowd and need to be marked to overcome this
problem.
Marking methods using catch and release processes are powerful tools to
analyze how animals move in the natural habitats (Haddaway et al., 2011).
However, this strategy seems to be unsuitable for monitoring the in situ behavior of deep-sea animals because of the stresses caused by
environmental changes, including temperature and pressure shifts during the
procedures. Therefore, to analyze their behavior and movements on the
deep-sea floor near hydrothermal vents, we aimed to develop a simple,
inexpensive, and easily practicable method to mark them in situ without causing them noticeable stress. Crystal Violet has been used to
stain the ectosymbiotic bacteria of live S. crosnieri, and to prove
the digestion of the symbionts in the digestive tracts of galatheid crabs
(Watsuji et al., 2015). The carapace of crustaceans (cuticle) contains
calcified chitin (Nagasawa, 2012). Uncalcified chitin has been reported to
be stained with Acid Blue dyes for analyzing the growth of the tubeworm
sheaths in deep-sea environments (Bergquist et al., 2000, 2002). In the
present study, we compared the efficiency of dyes for vital staining in a
laboratory experiment, and developed a new in situ staining method
for deep-sea crustaceans. This is the first report about marking S. crosnieri populations and tracking them in the natural habitat using an ROV
(Remotely Operated Vehicle) in a deep-sea vent field. Vital staining for
studying the behaviors of deep-sea animals which form very dense jostling
crowds is discussed.
2. Materials and Methods
To identify a suitable dye for the vital staining, we used a live S. crosnieri, which had been captured from a hydrothermal field at the Iheya
North in the Okinawa Trough, Japan during dive #1618 on 29 January, 2014 (27°47.45'N, 126°53.81'E, at a depth of 988 m) using the remotely operated vehicle (ROV) Hyper-Dolphin during the R/V Kaiyo cruise (KY14-01), and cultured in 100 L of artificial sea water (ASW, pH7.6; (3.5% Rohto Marine [Rei-Sea Ltd., Tokyo, Japan] in tap-water)) at 4–5°C with methane (final concentration = 34 μM; Watsuji et al., 2017) in a tank (length × width × height = 80 × 45 × 45 cm). One small individual, with a carapace approximately 2 cm in width, was
selected and used for staining with a series of various dyes. We examined
Acid Blue 161 (AB161; Sigma-Aldrich, 0.5% in ASW, once for 4 minutes and
the other time, for one hour), Rose Bengal (Wako Pure Chemicals, 0.5% in
ASW, 13 minutes), Alizarin Red-S (Wako Pure Chemicals, 1.66 mg/L in ASW, 24
hours), Aniline Blue (Wako Pure Chemical; 0.14% in ASW, 10 minutes), and
Coomassie Brilliant Blue R250 (CBB; Wako chemical, 0.25% with 2.5%
DMSO [Wako Pure Chemicals, Co. Ltd] in ASW, 15 minutes). This individual of
S. crosnieri was stained in 30–60 mL of ASW containing various
dyes at 4–5°C for a few minutes to one day. The period of
staining was desirable to be a few minutes for the in situ
staining, but it was prolonged when the staining was weak (e.g., Alizarin
Red-S). The stained animal was then washed in a 1-L beaker by changing
approximately 1-L of ASW 5–8 times, and incubated at 4–5°C in
approximately 800 mL ASW in the 1-L beaker. After the CBB-staining, it was
kept under this condition for 5 months; the ASW was changed 2–3 times a
week. After 5 months of culturing in the beaker, the galatheid crab was
transferred to a tank (l × w × h = 50 × 50 × 50 cm) for culturing S. crosnieri in the Enoshima
Aquarium. Sand-filtered natural sea water was replenished daily (200 L/day;
4°C; sulfide concentration was 400–1200 μM; pH was
6.8–8.0), and the galatheid crab was kept in this tank for an additional 3
months.
In situ staining of S. crosnieri was performed at the
Iheya North field (position of 27-47.484N; 126-53.804E, Jan. 03, 2016.
09:33-09:38) at a depth of 1,001 m using the ROV Hyper-Dolphin
during the R/V Natsushima NT16-01 cruise. The dye solution
(CBB-AB161; 100 g of Acid Blue 161 [Sigma Aldrich, final concentration of
0.5% w/v] and 25 g of Coomassie Brilliant Blue R250 [Wako Pure Chemicals.
0.125% w/v] and 250 mL DMSO [Wako Pure Chemicals. 1.25% v/v] was
dissolved in 20 L of natural sea water) was stored in a 20-L plastic
(Polyethylene) tank, which was connected to a specially designed staining
funnel with a square opening of 30 cm × 40 cm through a tubing
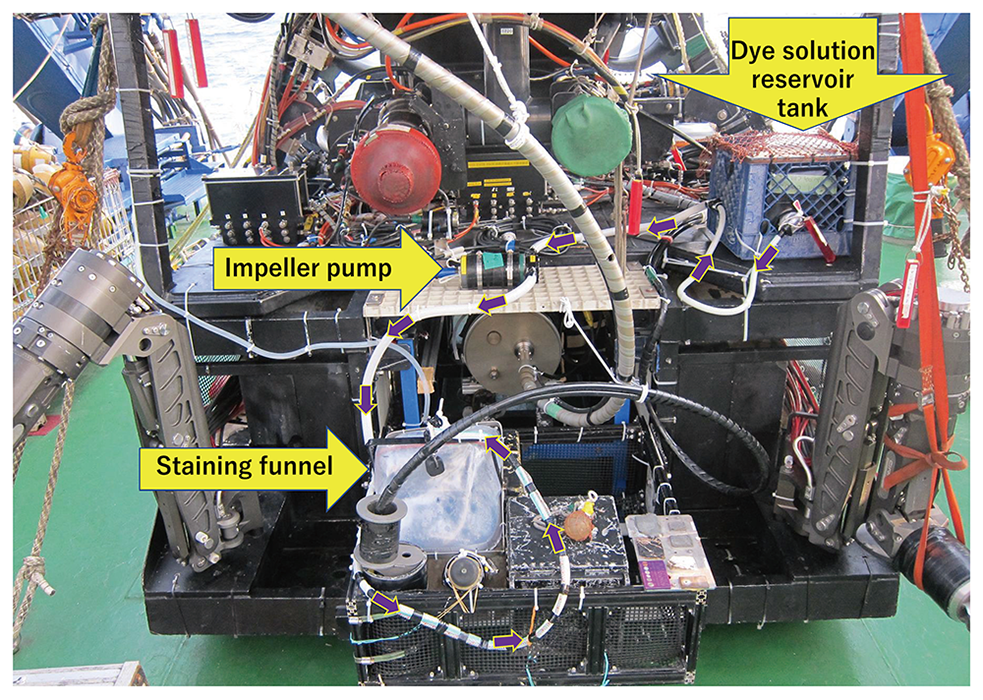
equipped with an impeller water pump (Fig.1). This staining facility had
been made by the operation team of the ROV. Using a manipulator of the ROV
Hyper-Dolphin, the funnel covered a small population of S. crosnieri in the hydrothermal vent field. The dye solution was then poured
on this population, and staining was performed for a few minutes. The funnel
was removed after the in situ staining, and the stained individuals
were left in their habitat.
This location of the staining was revisited by the ROV
Hyper-Dolphin the next day (24h post staining; day 2) and 2 days
after the staining (48h post staining; day 3). Images of the S. crosnieri population were captured with a high-definition video camera
installed on the ROV Hyper-Dolphin, and analyzed in the laboratory
after recovery by using softwares Adobe Photoshop CS4 version 11.0 and
ImageJ version 1.51j8 (http://imagej.nih.gov/ij/).
3. Results
3.1 Screening of dyes for in situ vital staining
In the screening of dyes for the in situ vital staining, we tested
Acid Blue 161 (AB161), Aniline Blue, Rose Bengal, Alizarin-Red S and
Coomassie Brilliant Blue R250 (CBB). AB161 was expected to stain the
calcified chitin of crustaceans. The galatheid crab was stained with this
dye at a concentraion of 0.5% w/v at 5°C for 4 minutes.
However, the staining was weak and the color almost completely faded away on
the next day. The galatheid crab was restained with the same dye at the
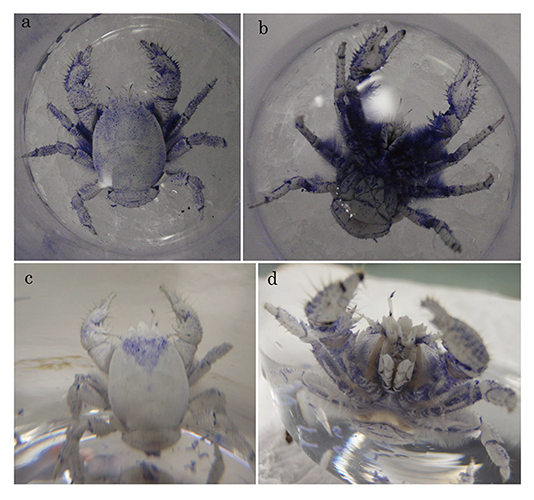
identical concentration and temperature for 1 hour. The ventral setae were
densely stained, while the carapace was only faintly stained (Fig.2a, b).
After 2 days, the color of the carapace faded but fainter blue color still
remained on the setae (Fig.2c, d). The carapaces and setae were also
stained with another chitin-staining dye, Aniline Blue (0.14%), for 10
minutes at 5°C. The staining profile was similar to that of
AB161, and the color faded within a few days and almost disappeared 7 days
post staining. Rose Bengal has been used for staining the members of the
phylum, Foraminifera (Bernhard et al., 2006). Although the ventral setae were
stained bright red with 0.5% Rose Bengal in ASW for 13 minutes at
5°C, the carapace was only faintly stained at its edge. The color
was not stable; even the color of the setae faded the next day and
disappeared after 3 days. After incubation in Alizarin-Red S in ASW (1.66
mg/L) for a day, the setae of the galatheid crab were only slightly stained,
while the carapace was not stained.

Coomassie Brilliant Blue R250 (CBB), which is usually used for staining
proteins in electrophoreses, stained S. crosnieri in blue
more densely than the other dyes examined; not only the ventral setae, but
also the carapaces (Fig.3a and b) were stained. The color faded very
slowly, and remained even after 5 months (the color after 55 days is shown
in Fig.3c and d). It is also noteworthy that the galatheid crab voided blue
feces even on 39 days post staining (Appendix1). After the staining
processes of any dyes, the animal did not show any wriggling or squirming
behavior; however, the behavior could not be observed during the staining
process. This suggested that it was not very perturbed by any of the dye
solutions.
After the staining, the stained S. crosnieri was kept in
approximately 800 mL of ASW in a 1-L beaker for 5 months at 4–5°
C, without a supply of sulfide or methane; the blue color of CBB was
recognizable even after 5 months. After the transfer to a tank in the
Enoshima Aquarium, the color gradually faded, and the ectosymbiotic
bacterial community on the setae seemed to grow. The galatheid crab
exuviated its shell after 8 months of cultivation post staining; however, it
died a month after the molting. The molt was white and looked just like an
intact living galatheid crab having ventral setae with ectosymbionts, but no
blue color was recognizable on the molt (data not shown), indicating that
the color was lost before molting.
3.2 In situ staining of S. crosnieri at the Iheya North hydrothermal field.
A small group of S. crosnieri individuals in a wild population were
stained with the CBB-AB161 solution at the Iheya North hydrothermal field
(Fig.4). After staining with 20 L of the dye solution, at least 18
individuals were recognizably well-stained (Fig.5a). The appearance of the
stained galatheid crab in situ through a video camera was slightly
different from that of the laboratory-stained galatheid crab observed
directly. The whole body of the in situ-stained galatheid crabs
seemed to be dyed blue (Yellow arrowheads in Fig.5b). While the ventral
setae were stained much darker than the carapace in the laboratory
experiment (Fig.3a and b), the carapaces and the setae were similarly
stained after the in situ staining procedure (Yellow arrowheads in
Fig.5b). The galatheid crabs seemed to be a little disturbed by the moving
of the staining funnel, but most individuals became calm soon after. No
avoiding response was observed in the stained galatheid crabs and in those
in the surrounding area. This suggested that the staining process did not
greatly disturb their distribution.


This place was revisited 24 and 48 hours after the staining (Fig.5c and
d). The dyed galatheid crabs were recognizable in the in situ video
images taken by the ROV Hyper-Dolphin, although the blue color was
not very dark. Compared to the color observed immediately after the
staining, there appeared to be no change in color even a period of 48h (Fig.5d). However, the stained individuals were more easily recognizable
after changing the color and contrast parameters of the captured video
images (Appendix2). Twenty-four and 48h after the staining, most of the
stained galatheid crabs were found in approximately the same location that
they were originally stained at (Fig.5c and d).
4.
Discussion
In the laboratory staining, color of S. crosnieri stained by AB161
did not last long, but faded away within a few days (Fig.2). Color of the
acid blue (including AB161) that stains the chitin in the sheath of
vestimentiferan tubeworms has been reported to last stably for more than a
year (Bergquist et al., 2000, 2002; Fujiwara et al., unpublished data). Acid
blue stainability of calcified chitin may be much weaker than that of
uncalcified chitin.
The blue color of the CBB-stained S. crosnieri was the darkest
among the examined dyes and lasted for the longest period, which was more
than 5 months in the laboratory (Fig.3). Galatheid crab often wipe the back
portion of the carapace by using its pereiopods, and may feed on the
bacteria growing there (Miyake, personal communication). On the back portion
of carapace, blue color of CBB staining remained in the inverse triangle
region, where might be difficult to be scraped with the pereiopods (Fig.3c). CBB was probably the most effective dye in the dye mixture (CBB-AB161)
for the in situ staining of S. crosnieri (Fig.5).
However, the appearances of the in situ-stained galatheid crabs
were different from that of the laboratory-stained galatheid crab. There are
two possibilities for this difference: 1) differences between the
illuminations (including light path length) in the laboratory and deep-sea,
and 2) differences between the culture conditions of S. crosnieri
in the laboratory and natural habitat. In the natural habitat, there must be
some flows of sea water including woozing water movement from the bottom
sediments. In addition, S. crosnieri has been shown to produce
water flow, which plays an important role for the symbiotic bacterial growth
(Watsuji et al., 2017). These water movements in addition to the water
disturbance by the funnel movement, probably diluted the dye concentration
during the staining. Wild S. crosnieri seemed to have a denser
ectosymbiontic bacterial community on the setae than its laboratory-grown
counterpart (Figs.2d and 5b). The setae covered with a thicker bacterial
layer might be less stainable by the CBB-AB161 than one covered with a
thinner bacterial layer. It has been reported that crystal violet-stained
ectosymbionts on the setae were traced and detected in the intestines of
S. crosnieri (Watsuji et al., 2015). The feces of the
laboratory-stained galatheid crab were blue, (Appendix1), suggesting that
the blue color of the feces was derived from the stained bacteria, and/or
that inside of the digestive tract was stained and discharged after.
Therefore, the CBB-AB161mixture may also be used for detecting how
ectosymbionts are digested by the host animal, S. crosnieri.
In the field, the stained galatheid crabs were recognizable through the
video observations recorded on the ROV. Apparently, the darkness of the
color did not change for 2 days. We found the stained galatheid crabs in the
same place on day 3 post staining. This indicates that this method is useful
to observe the behavior and movements of the galatheid crabs for at least a
few days.
In the deep-sea environment outside of the Iheya North hydrothermal vent
area, very few or no S. crosnieri are usually observed. (Nakajima
et al., 2015). When a new artificial hydrothermal vent, which was 264 m away
from the nearest natural vent site, was established, S. crosnieri appeared 11 months after the drilling (Nakajima et al., 2015). It is deduced
that they migrated from their comfortable original habitat to the newly made
vent area (Nakajima et al., 2015). It is speculated that they have a
chemotactic behavior towards the new vent site (Nakajima, personal communication). From this report, their speed was estimated from the nearest
original habitat to the new one (distance = 264 m) as 264 m/11 months = 24 m/month $\approx $ 0.8 m/day. In the present study, the stained galatheid
crabs mostly stayed at the place of staining for 2 days, but their
distribution was slightly widened (Fig.5c and d). It is still not clear
whether or not they have a loose territory in the habitat and if they
compete for the methane and sulfide.
The present vital staining method provides a unique and practical technique
to understand how the deep-sea galatheid crabs move within jostling crowds,
and how they interact each other, without any lethal effects to the animal.
However, the stained color was not contrasting enough with the non-stained
ones for easier recognition through video observations in seawater. It is
better to find a better dye or method to improve the intensity and longevity
of the staining. In the present study, the setae were stained darker in the
laboratory than in case of the in situ-stained wild galatheid
crabs. If the ectosymbionts could be stained without damaging them,
it would be possible to monitor the growth of these bacteria in this
galatheid crab in situ, in which the newly grown parts of the
fibrous bacterial cells would be unstained, but the older stained parts
should remain colored.
5.
Conclusion and perspectives
The present study showed that: 1) the deep-sea crustacean Shinkaia crosnieri could be marked by a vital staining without apparent damage to
its survival or activity. Neither an avoiding nor an escaping reaction was
observed. They were recognizable at least for a few days in the deep-sea
habitat. 2) the marked individuals seemed to stay at the place where they
were stained, for two days. This may indicate that S. crosnieri does not move around much and remains localized.
Vital staining is a mild marking method for invertebrates in various
habitats. However, the blue color of the stain did not give a very high
contrast for recognizing the stained galatheid crab in the deep-sea habitat.
It is desirable to search for more suitable dyes, including fluorescent
dyes, which may have high stainability for various organisms, a more easily
recognizable color, and more stable and long-lasting color in the dark
deep-sea environments, than the dyes being used currently, though we may need a
strong excitation light source on the ROV. The present vital staining
approach may be applicable to not only crustaceans with chitinous
exoskeletons, but also to other various invertebrates that have
proteinaceous outer coats.
In the present study, staining and tracing S. crosnieri were
performed by using an ROV. Since the monitoring of the dyed galatheid crabs
was performed visually, unmanned platforms such as autonomous underwater
vehicles (AUV) equipped with visual mapping instruments to efficiently
analyze the distribution of dyed individuals would be used. Such new
technologies using unmanned robotics are becoming more and more important
for future studies about marine ecology.
Acknowledgments
We are grateful to the captain and cruise members of NT06-01 of R/V
Natsushima, and to the operation team of the ROV
Hyper-Dolphin. The R/V Natsushima retired in February
2016. The present study was supported by the Japan Science and Technology
Agency for the CREST project, “Synthesis of an autonomous underwater
vehicle (AUV) fleet for bio-sampling using 3D reconstructions of the
seafloor,” lead by Prof. T. Ura. Dr. R. Nakajima is acknowledged for his
critical comments on the manuscript and the information about the migration
in of S. crosnieri around the newly and artificially made
hydrothermal vent area. We would like to thank Dr. H. Miyake for his
critical comments of the manuscript and useful discussion about the behavior
of S. crosnieri. Ms. M. Kitajima of Enoshima Aquarium is
acknowledged for the information about the feeding behavior of S. crosnieri. Dr. S. Sakai is acknowledged for his advice in writing the
manuscript.
References
-
Baba, K.
and
A.B. Williams
(1998), New Galatheoidea (Crustacea, Decapoda,
Anomura) from hydrothermal systems in the West Pacific Ocean: Bismarck
Archipelago and Okinawa Trough, Zoosystema, 20, 143-156.
-
Bergquist, D.C.
,
F.M. Williams
, and
C.R. Fisher
(2000), Longevity record for
deep-sea invertebrate, Nature, 403, 499-500.
-
Bergquist, D.C.
,
I.A. Urcuyo
, and
C.R. Fisher
(2002), Establishment and
persistence of seep vestimentiferan aggregations on the upper Louisiana
slope of the Gult of Mexico, Mar. Ecol. Prog. Ser., 241,
89-98.
-
Bernhard, J.M.
,
D.R. Ostermann
,
D.S. Williams
, and
J.K. Blanks
(2006),
Comparison of two methods to identify live benthic foraminifera: A test
between Rose Bengal and CellTracker Green with implications for stable
isotope paleoreconstructions, Pleoceanography, 21, PA4210,
doi:10.1029/2006PA001290.
-
Chan, T.
,
D. Lee
, and
C. Lee
(2000), The first deep-sea hydrothermal animal
reported from Taiwan: Shinkaia crosnieri Baba and Williams, 1998
(Crustacea: Decapoda: Galatheidae), Bull. Mar. Sci., 67,
799-804.
-
Haddaway, N.R.
,
R.J.G. Mortimer
,
M. Christmas
, and
A.M. Dunn
(2011), A
review of marking techniques for Crustacea and experimental appraisal of
electric cauterisation and visible implant elastomer tagging for
Austropotamobius pallipes and Pacifastacus leniusculus,
Freshwater Crayfish, 18, 55-67.
-
Kitajima, M.
,
M. Sugimura
,
S. Nemoto
, and
S. Nakagawa
(2012), Feeding behavior of the captured deep-sea galatheid crab, Shinkaia crosnieri, observed during cultivation, Proceedings of Blue Earth 2012, (in Japanese), <https://www.jamstec.go.jp/maritec/j/blueearth/2012/pdf/poster/BE12-P20.pdf>.
-
Nagasawa, H.
(2012), The crustacean cuticle: structure, composition and
mineralization, Frontiers in Bioscience, E4, 711-720.
-
Nakajima, R.
,
H. Yamamoto
,
S. Kawagucci
,
Y. Takaya
,
T. Nozaki
,
C. Chen
,
K. Fujikura
,
T. Miwa
, and
K. Takai
(2015), Post-drilling changes in seabed landscape and
megabenthos in a deep-sea hydrothermal system, the Iheya North Field,
Okinawa Trough, PLoS ONE, 10, 0123095.
-
Tokeshi, M.
(2011), Spatial structures of hydrothermal vents and
vent-associated megafauna in the back-arc basin system of the Okinawa
Trough, western Pacific, J. Oceanogr., 67, 651-665.
-
Tsuchida, S.
,
Y. Fujiwara
, and
K. Fujikura
(2003), Distribution and population
structure of the Galatheid crab Shinkaia crosnieri (Decapoda: Anomura: Galatheidae) in the southern Okinawa Trough, Jpn. J. Benthol., 58, 84-88.
-
Tsuchida, S.
,
Y. Suzuki
,
Y. Fujiwara
,
M. Kawato
,
K. Uematsu
,
T. Yamanaka
,
C. Mizota
, and
H. Yamamoto
(2011), Epibiotic association between filamentous
bacteria and the vent-associated galatheid crab, Shinkaia crosnieri
(Decapoda: Anomura), J. Mar. Bio. Ass. UK, 91, 23-32.
-
Van Dover, C.L.
(2000), The Ecology of Deep-Sea Hydrothermal Vents,
Princeton University Press., pp. 424.
-
Watsuji, T.
,
S. Nakagawa
,
S. Tsuchida
,
T. Toki
,
A. Hirota
,
U. Tsunogai
, and
K. Takai
(2010), Diversity and function of epibiotic microbial communities
on the galatheid crab, Shinkaia crosnieri, Microb. Environ., 25, 288-294.
-
Watsuji, T.
,
M. Nishizawa
,
Y. Morono
,
H. Hirayama
,
S. Kawagucci
,
N. Takahata
,
Y. Sano
, and
K. Takai
(2012), Cell-specific thioautotrophic
productivity of epsilon-proteobacterial epibionts associated with
Shinkaia crosnieri, Plos One, e46282.
-
Watsuji, T.
,
A. Yamamoto
,
K. Motoki
,
K. Ueda
,
E. Hada
,
Y. Takaki
,
S. Kawagucci
, and
K. Takai
(2015), Molecular evidence of digestion and
absorption of epibiotic bacterial community by deep-sea crab
Shinkaia crosnieri, ISME J., 9, 821-831.
-
Watsuji, T.
,
R. Tsubaki
,
C. Chen
,
Y. Nagai
,
S. Nakagawa
,
M. Yamamoto
,
D. Nishiura
,
T. Toyofuku
, and
K. Takai
(2017), Cultivation mutualism between a
deep-sea vent galatheid crab and its chemosynthetic epibionts,
Deep-sea Res. Part I, 127, 13-20.
-
Yang, C-H.
,
S. Tsuchida
,
K. Fujikura
,
Y. Fujiwara
,
M. Kawato
, and
T-Y. Chan
(2016), Connectivity of the squat lobsters Shinkaia crosnieri (Crustacea: Decapoda: Galatheidae) between cold seep and hydrothermal vent
habitats, Bul. Mar. Sci., 92, 17-31.







