2021 Volume 28 Issue 12 Pages 1340-1348
2021 Volume 28 Issue 12 Pages 1340-1348
Aim: The cardio-ankle vascular index (CAVI) consists of intrinsic and functional arterial stiffness mainly regulated by vasoactive compounds. A new stiffness index of the aorta (aBeta) and iliac-femoral arteries (ifBeta) was determined by applying the CAVI theory to the whole aorta and iliac-femoral arteries. We investigated the changes in aBeta and ifBeta in response to decreased blood pressure (BP) induced by the Ca2+ channel blocker nicardipine to elucidate the involvement of Ca2+ in aBeta and ifBeta.
Methods: Pressure waves at the origin of the aorta (oA), distal end of the abdominal aorta (dA), and left femoral artery (fA) as well as flow waves at the oA were simultaneously recorded before and after the infusion of nicardipine (50 µg/kg/min) for 2 min in 12 male rabbits under pentobarbital anesthesia. Beta was calculated using the following formula: Beta=2ρ / PP×ln (SBP / DBP)×PWV2, where ρ, SBP, DBP, and PP denote blood density and systolic, diastolic, and pulse pressures, respectively. aBeta, ifBeta, and aortic-iliac-femoral Beta (aifBeta) were calculated using aPWV, ifPWV, and aifPWV, respectively.
Results: SBP, mean arterial pressure (MAP), DBP, and total peripheral vascular resistance significantly decreased during the administration of nicardipine, whereas cardiac output significantly increased. aBeta and ifBeta significantly increased and decreased, respectively, whereas aifBeta did not change despite the decrease in BP. ifBeta and aBeta positively and negatively correlated with BP, respectively, whereas aifBeta did not correlate with SBP.
Conclusions: There were contradictory arterial responses to nicardipine between the elastic and muscular arteries. Unknown vasoconstriction mechanisms that are not involved in Ca2+ influx may function in the aorta in response to decreased BP.
Arterial stiffness is mainly determined by the intrinsic properties of the artery, peripheral vascular tone, and cardiac function 1, 2) . Peripheral vascular tone mainly affects blood pressure (BP) and cardiac output (CO) 3) . It is thought that arterial stiffness is not only a marker of atherosclerosis but also plays a partial role in cardiovascular hemodynamics, such as vascular resistance 2) .
The role of arterial stiffness in cardiovascular hemodynamics has not been elucidated as a proper index of arterial stiffness has not been contrived. Pulse wave velocity (PWV), a direct measure of arterial stiffness 4, 5) , depends on the BP at the time of measurement 6, 7) ; the cardio-ankle vascular index (CAVI) has been developed based on the theory of the stiffness parameter β 8, 9) and Bramwell–Hill’s equation 10) . CAVI reflects the overall stiffness from the origin of the aorta to the anterior and posterior tibial arteries, which contain the elastic and muscular arteries. The fine structure and function of the two types of artery are different 11, 12) .
It is well demonstrated that the aorta mainly functions as an auxiliary pump, whereas the muscular arteries have a contraction–dilation function for transporting and distributing blood to the peripheral tissues. It is supposed that there is a cross-talk function between the aorta and muscular arteries and that the arterial stiffness of the two arteries is involved in this function. However, such a function had not been precisely evaluated, because an adequate index for the estimation of arterial stiffness had not been established.
To investigate the stiffness of the elastic (aorta) and muscular (iliac and femoral) arteries, we determined a stiffness index Beta by applying the CAVI theory to the whole aorta (aBeta) and iliac-femoral arteries (ifBeta). Katsuda et al. 13) reported that BP decreased with the decrease in ifBeta and increase in aBeta when a nonselective α-adrenergic blocker, namely, phentolamine, was infused into rabbits. The mechanism and significance of the contradictory response remain unclear. One interpretation is that the increased aBeta might compensate for the decreased BP due to the dilation of the muscular arteries induced by the infusion of phentolamine. The question of whether this reciprocal response of arterial stiffness in the elastic and muscular arteries is induced by the infusion of α-adrenergic blocker or other vasodilator drugs, such as a calcium channel blocker, arises.
Voltage-dependent L-type calcium channels play a major role in Ca2+ influx into vascular smooth muscle cells (VSMCs) 14, 15) . Nicardipine, a voltage-dependent L-type and T-type calcium channel blocker, lowers BP by dilating the peripheral muscular arteries and arterioles 16, 17) . We investigated the change in the stiffness of the aorta and iliac-femoral arteries using aBeta and ifBeta in response to decreased BP induced by the administration of nicardipine to rabbits to elucidate the involvement of Ca2+ in the change in aBeta and ifBeta.
A total of 12 male Japanese white rabbits weighing 3.39±0.19 kg and aged 10–12 months (Japan Laboratory Animals, Inc., Tokyo, Japan) were used in the present study. They were individually reared in stainless wire cage in an air-conditioned breeding room at a room temperature of 20℃–26℃, a humidity level of about 50%–70%, and a 12L/12D cycle. In addition, they were given a commercial rabbit food (Labo R Grower, Nosan Corporation, Yokohama, Japan) at about 100 g/animal/day and free access to water. The present study was approved by the Experimental Animal Committee of Fukushima Medical University and was conducted according to the Guidelines for Animal Care and Handling of the Japanese Association for Laboratory Animal Science.
2. Surgical ProcedureFig.1 shows the schematic arrangement of the experimental setup. The surgical procedure was similar to that previously reported 13) . The rabbits were anesthetized by intravenous administration of pentobarbital sodium (Somopentyl, Kyoritsu Seiyaku Corporation, Tokyo, Japan) at a dose of 30 mg/kg. Butorphanol tartrate (Vetorphal, Meiji Seika Pharma, Co., Ltd., Tokyo, Japan) was injected intramuscularly at a dose of 0.3 mg/kg for pain relief. The rabbit was fixed in supine and intubated trachea. Two catheter tip transducers (2.0 Fr, SPS-320, Millar Instruments, Inc., Huston, TX) were advanced to the origin of the aorta (oA) and distal end of the abdominal aorta (dA) through the left common carotid and right saphenous arteries, respectively. FISO catheter with a fiber optical pressure sensor at the tip (0.9 Fr, FPI-LS-10, FISO Technologies, Inc., Quebec, Canada) was introduced to the distal end of the left femoral artery (fA) through the left saphenous artery. An ultrasonic flow probe (6.0 mm, I.D.) was placed at the ascending aorta after carefully opening the chest under voluntary breezing to prevent injury to the pleura.

Schematic arrangement of the experimental setup
Nicardipine hydrochloride (Sawai Pharmaceutical Co. Ltd., Osaka, Japan) was infused into the ear vein for 2 min using a syringe pump at a dose of 50 µg/kg/min. Pulse waves at the oA, dA, and fA and blood flow at the oA were simultaneously measured using a polygraph (360 system, NEC Sanei Co., Ltd., Japan) and an ultrasonic blood flow meter (T206, Transonic Systems Inc., Ithaca, NY) and then recorded in a personal computer (PowerBook G4 M9691J/A, Apple Inc., Cupertino, CA, USA) using an analog-to-digital converter (PowerLab System 16/SP, AD Instruments, Inc., Sydney, Australia). After the measurement, the rabbits were euthanized with pentobarbital overdose. The distance between the two adjacent sensors at the catheter tip was accurately measured in situ with a string along the aorta and artery.
4. Statistical AnalysisPulse and blood flow waves were analyzed for 50 successive cardiac cycles. The foot of the pressure wave was defined as the peak of the second derivative of the original pressure waves, as previously reported 18) . PWV was calculated as the difference in the rising time of two pressure waves and the distance between two pressure sensors between the oA and dA (aortic PWV; aPWV), between the dA and fA (iliac-femoral PWV; ifPWV), and between the oA and fA (aortic-femoral PWV; aifPWV).
Arterial stiffness of the whole aorta (aBeta), iliac-femoral arteries (ifBeta), and whole aorta and iliac-femoral arteries (aifBeta) was measured by applying the CAVI theory. The CAVI is determined using the following formula 19, 20) :
CAVI=a [2ρ×ln (SBP / DBP) / PP×PWV2]+b,
where ρ, SBP, DBP, and PP denote the blood density and systolic, diastolic, and pulse pressures, respectively, and “a” and “b” denote the undisclosed coefficients. Takahashi et al. 21) defined heart-ankle Beta (haβ) as an index of the elasticity of the arteries without the coefficients “a” and “b” and demonstrated that there was no difference in the significance or interpretation between the CAVI and haβ in epidemiological and clinical studies. Therefore, we took the coefficients “a” and “b” for “1” and “0,” respectively, in calculating Beta.
aBeta, ifBeta, and aifBeta were determined using aPWV, ifPWV, and aifPWV, respectively. SBP, DBP, and PP were the average values of oA and dA for aBeta, dA, and fA for ifBeta, and oA and fA for aifBeta.
Heart rate (HR) was measured from the pulse waves at the oA using a cardiotachometer. The mean arterial pressure (MAP) was calculated using a pulse wave passed through a low-pass filter at 2.5 s. Total peripheral vascular resistance (TPR) was measured by dividing the cardiac output (CO) by the MAP.
These variables were determined before and after nicardipine infusion every 1 min for 5 min. The data were tested via one-way analysis of variance (ANOVA). When a significant difference was observed in the ANOVA, a post hoc test was conducted using Scheffe’s multiple comparisons test. We calculated Pearson’s correlation coefficient for each Beta and PWV against SBP, DBP, MAP, CO, and TPR, and the correlation coefficient was tested using the F-test. The significance level was set to p=0.05.
Fig.2 presents the time-dependent changes in SBP and DBP at the oA, dA, and fA (A); HR and CO (B); MAP and TPR (C); aBeta, ifBeta, and aifBeta (D); and aPWV, ifPWV, and aifPWV (E) during nicardipine infusion. SBP and DBP significantly decreased at the oA, dA, and fA due to the infusion of nicardipine. Moreover, CO increased significantly, whereas the MAP and TPR decreased significantly during the infusion of nicardipine. HR demonstrated a slight but significant decrease 3–5 min after the administration. We investigated the changes in aBeta, ifBeta, and aifBeta concomitant with the decrease in BP during nicardipine infusion. ifBeta decreased significantly, whereas aBeta increased. aifBeta, which corresponds approximately to the CAVI, did not exhibit a significant change. aPWV, ifPWV, and aifPWV decreased significantly in a pressure-dependent manner during the infusion of nicardipine.

SBP, systolic blood pressure; DBP, diastolic blood pressure; CO, cardiac output; HR, heart rate; TPR, total peripheral vascular resistance; MAP, mean arterial pressure; aBeta, aortic Beta; ifBeta, iliac-femoral Beta; aifBeta, overall aorta and iliac-femoral Beta; PWV, pulse wave velocity; aPWV, aortic PWV; ifPWV, iliac-femoral PWV; aifPWV, overall aorta and iliac-femoral PWV.
Fig.3 presents the correlation between Beta and SBP (A), Beta and MAP (C), Beta and DBP (E), PWV and SBP (B), PWV and MAP (D), and PWV and DBP (F) during the infusion of nicardipine. aBeta was significantly negatively correlated with SBP, MAP, and DBP, whereas ifBeta significantly positively correlated with SBP, MAP, and DBP. aifBeta did not demonstrate a significant correlation with SBP; however, it exhibited a significant negative correlation with MAP and DBP. aPWV, ifPWV, and aifPWV showed a significant positive correlation with SBP, MAP, and DBP during the infusion of nicardipine.
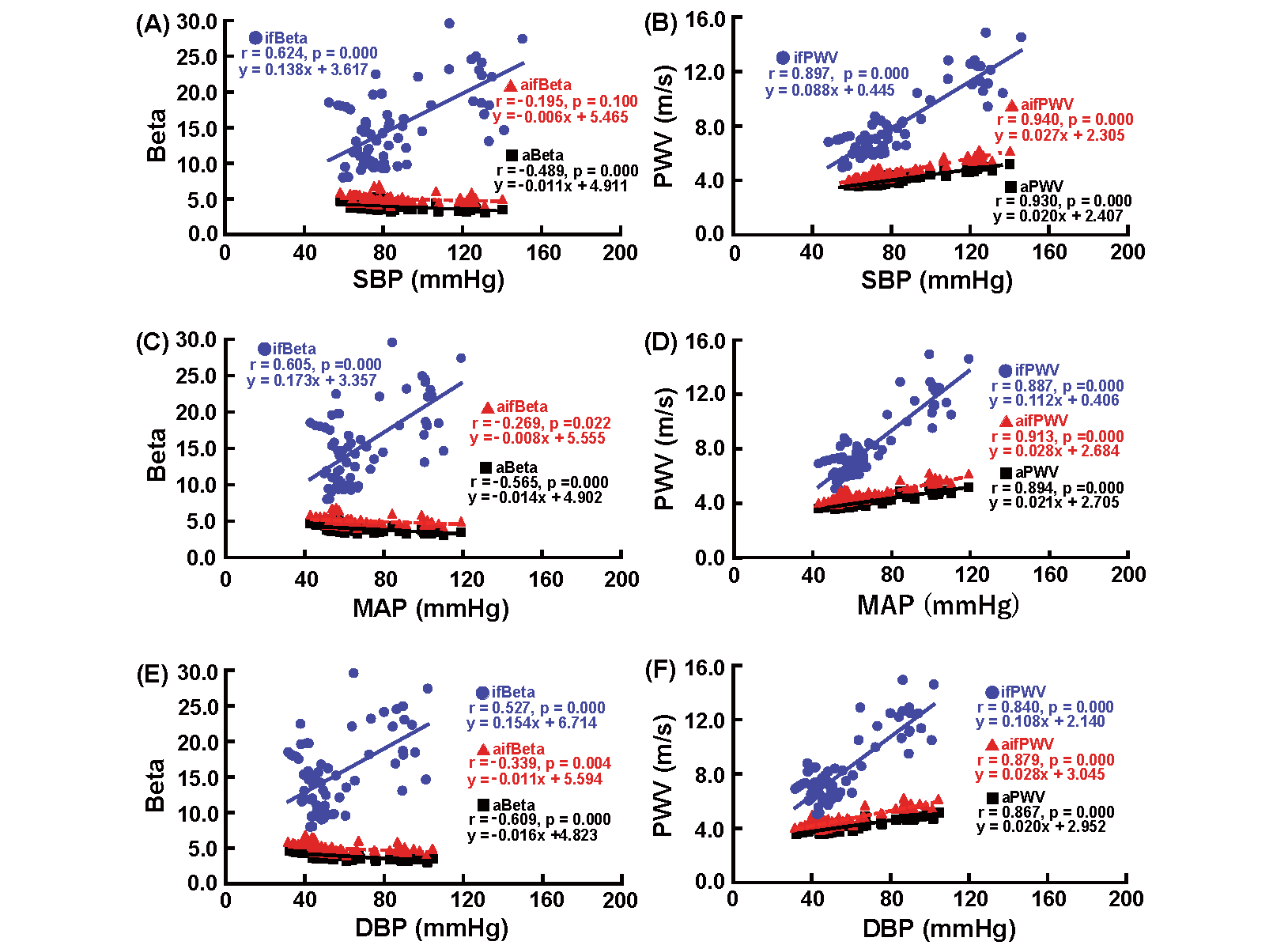
See the legend of Fig. 2.
aBeta significantly negatively correlated with SBP, MAP, and DBP, whereas ifBeta significantly positively correlated with SBP, MAP, and DBP. aifBeta did not correlate SBP. aPWV, ifPWV, and aifPWV demonstrated a significant positive correlation with SBP, MAP, and DBP.
Fig.4 presents the correlation between Beta and CO (A), PWV and CO (B), Beta and TPR (C), and PWV and TPR (D) during the infusion of nicardipine. aBeta and aifBeta did not exhibit a significant correlation with CO, whereas ifBeta demonstrated a significant negative correlation with CO. aBeta had a significant negative correlation with TPR, whereas ifBeta positively correlated with TPR. No significant correlation was observed between aifBeta and TPR. aPWV, ifPWV, and aifPWV significantly negatively correlated with CO, whereas aPWV, ifPWV, and aifPWV showed a significant positive correlation with TPR.
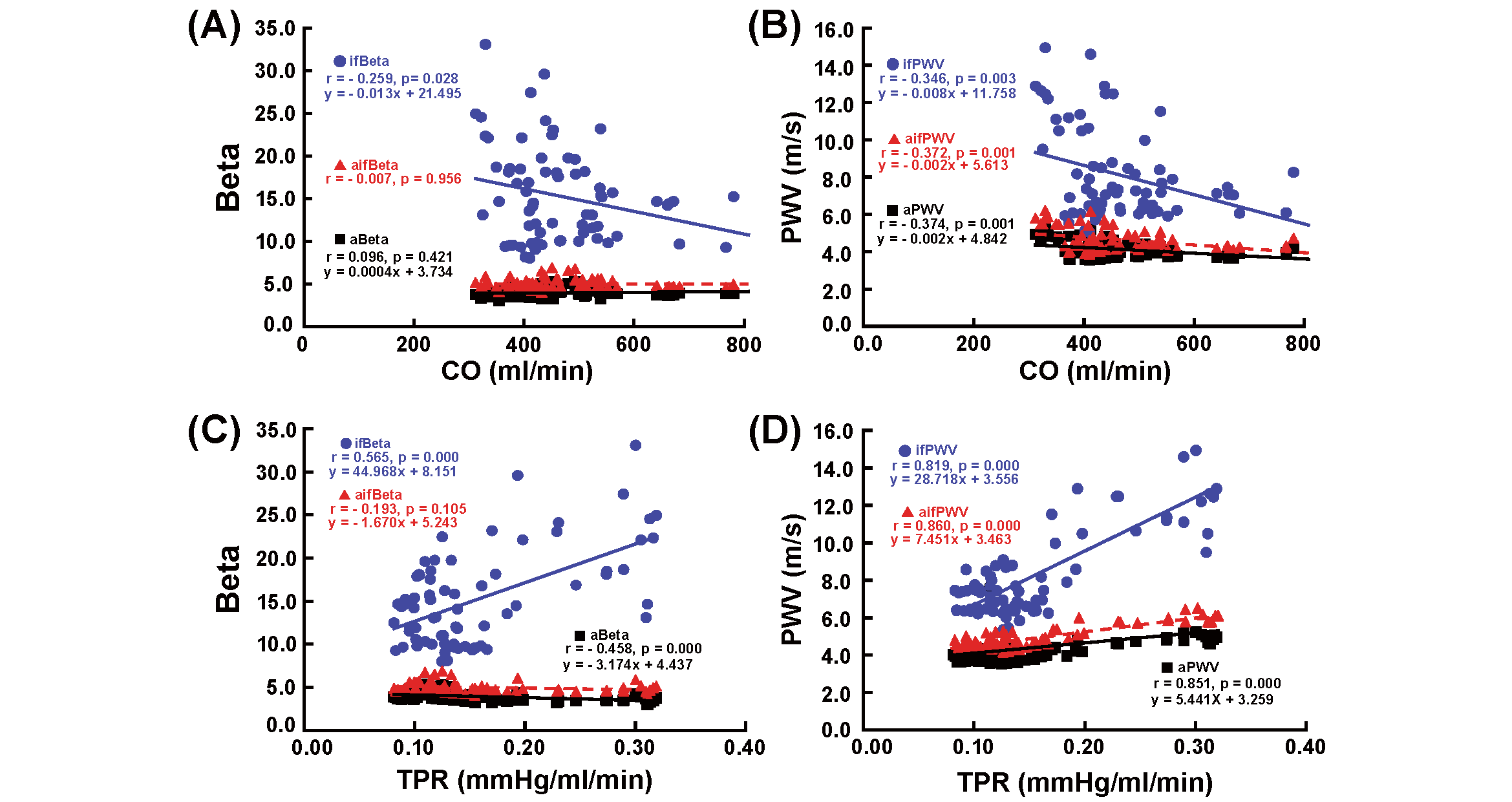
See the legend of Fig. 2.
aBeta and aifBeta did not correlate with CO, whereas ifBeta correlated negatively with CO. aBeta and ifBeta demonstrated significant negative and positive correlations with TPR, respectively. aPWV, ifPWV, and aifPWV exhibited significant negative and positive correlations with CO and TPR, respectively.
Nicardipine, a calcium channel blocker, relaxes the VSMCs of the muscular arteries and arterioles by blocking L-type and T-type Ca 2+ channels 16, 17) , which reduces peripheral vascular resistance, leading to a decrease in BP 22) . Nicardipine also dilates the coronary artery 23) , which results in an improved left ventricular function without producing any negative ionotropic effects 24) . The α1 subunit of the vascular smooth muscle Ca2+ channel has a higher affinity for dihydropyridine drugs than the α1 subunit of the heart. The selectivity of the action of nicardipine on the vascular smooth muscle is more than 10,000 times that on the cardiac muscle 25) .
In this study, we observed a significant decrease in TPR, BP, and HR and a significant increase in CO during nicardipine infusion. These findings are almost consistent with those of other investigators, except that with regard to HR 22, 24) . Satoh 26) also reported that a high dose of nicardipine inhibited tachycardia induced by sympathetic nerve stimulation mainly by suppressing slow inward Ca2+ currents. The significant increase in CO is considered to be mainly caused by the decrease in peripheral vascular resistance.
aPWV, ifPWV, and aifPWV demonstrated a significant decrease concomitant with the decrease in BP during the infusion of nicardipine as well as a significant positive correlation with SBP, MAP, and DBP. PWV has been shown to change depending on BP at the time of measurement. Thus, it was difficult to determine whether decreased PWV was derived from a decrease in intrinsic stiffness or just by accompanying with the decreased BP during the infusion of nicardipine.
ifBeta significantly decreased during the administration of nicardipine, which was thought to result from the relaxation of smooth muscle cells in the iliac-femoral artery due to the infusion of nicardipine. ifBeta exhibited a significant positive correlation with SBP, DBP, and TPR and a significant negative correlation with CO. In other words, nicardipine-induced dilation of the muscular artery decreased TPR, which led to the decrease in BP and the increase in CO. These findings suggest that ifBeta reflects part of the resistance of the muscular artery in the systemic circulation.
However, aBeta increased during the decrease in BP induced by the administration of nicardipine. aBeta negatively correlated with SBP, MAP, DBP, and TPR but did not correlate with CO. The response of the elastic arteries to nicardipine is completely opposite to that of the muscular arteries. This indicates that elastic arteries stiffen, contrasting the dilation of the peripheral arteries and decrease in BP, which is a very interesting and unexpected phenomenon. Why don’t both aBeta and ifBeta change in the same direction among the arterial tree by the infusion of nicardipine which lowers BP by dilating the peripheral muscular arteries and arterioles? Similar contradictory responses of aBeta and ifBeta were also observed during the decrease in BP induced by the infusion of phentolamine 13) . There exists a certain mechanism that the decrease in BP induced by the dilation of the muscular arteries might enhance the contraction of the smooth muscle cells in the elastic artery; however, such a mechanism should be elucidated in the future. This phenomenon might be one of the cross talks between the aorta and muscular arteries to maintain systemic hemodynamics, for example, to keep the blood supply to the brain when BP decreases.
aifBeta did not change significantly in response to the decreased BP induced by the infusion of nicardipine and did not correlate with SBP, CO, or TPR. aifBeta almost corresponds to the CAVI as aifBeta reflects the stiffness from the origin of the aorta to the distal end of the femoral artery. aifBeta seems to function as a pressure-independent index of arterial stiffness at a glance.
Chiba et al. 27) reported that the CAVI determined by the heart-ankle PWV (haPWV) in rabbits significantly increased after the continuous administration of nicardipine (10 µg/kg) for 10 min, which was fundamentally consistent with our results. However, there are some differences in the anesthetic regimen, dose and administration time, and BP level before the administration. They suggested that the increase in the CAVI was associated with the baroreflex-mediated increase in sympathetic activity in response to the decrease in BP induced by the infusion of nicardipine. Katsuda et al. 13) previously demonstrated that aifBeta showed a slight but significant increase in response to the decrease in BP induced by the infusion of phentolamine, which is a nonselective α-adrenergic blocker. This indicates that sympathetic activation mainly through the baroreflex is not associated with the increased aifBeta in our study.
In humans, Sasaki et al. 28) demonstrated that efonidipine, an L-type and T-type calcium channel blocker, significantly decreased the CAVI concomitant with the decrease in BP in patients with type 2 diabetes mellitus accompanied by hypertension and nephropathy. This is incompatible with the change in aifBeta in rabbits. aifBeta corresponds to the stiffness of the overall aorta and iliac-femoral arteries, which almost corresponds to the CAVI. One reason of the inconsistency could be the difference in the proportion of the elastic and muscular arteries between the rabbits and humans as well as the rate of change in aBeta and ifBeta. aBeta increased by 17.5% from the control value, whereas ifBeta decreased by 41.0% from the control 2 min after the onset of administration. We estimated aifBeta by considering the percentage of the length of the two arteries and that of the increase in aBeta and decrease in ifBeta. The lengths of the aorta and iliac-femoral arteries were 261.4±9.8 mm and 80.5±8.3 mm (mean±SD), respectively. In the present study, the elastic and muscular arteries accounted for 76.5% and 23.5% of the studied length, respectively. The estimated aifBeta on a simple calculation ([0.175×0.765×100−0.410×0.235×100]+100) was 103.7% of the control value, although the estimated value was smaller than the actual aifBeta (106.7% of the control value) 2 min after administration.
The regulatory mechanisms of the vascular tone may be different between humans and rabbits; the former is two-legged, whereas the latter is four-legged. Pathological alterations in the arteries and heart could also affect the CAVI. Kirigaya et al. 29) demonstrated that CAVI was an independent long-term predictor of major adverse cardiovascular events in patients with acute coronary syndrome. Niwa et al. 30) showed the relationship of CAVI with microangiopathy in patients with type 2 diabetes mellitus. Sugiura et al. 31) also reported the association of obesity-related indices and metabolic syndrome with subclinical atherosclerosis in middle-aged Japanese workers. In clinical setting, the comprehensive evaluation of CAVI is required.
Yamamoto et al. 32) demonstrated that the sublingual administration of nitroglycerine to healthy subjects led to the significant decrease in arterial stiffness in the muscular arteries compared with the elastic arteries. This was more prominent in patients with atherosclerosis. Their results did not coincide with our findings. Such a discrepancy may be partly due to the difference in species and also the decrease in BP.
In the future, it might be meaningful to study separately the responses to vasoactive agents in the elastic and muscular arteries in humans.
The CAVI employed in the clinical examination includes the stiffness of the elastic and muscular arteries. The present data are not obtained from humans to whom nicardipine was administered orally but from rabbits to which nicardipine was administered intravenously in an acute experiment. It is important to elucidate the effect of the long-term administration of nicardipine to patients with cardiovascular disease on the change in stiffness in the elastic and muscular arteries in the future.
We observed a contradictory response between the elastic and muscular arteries to the decrease in BP induced by nicardipine infusion. The increased stiffness of the elastic artery in response to the decrease in BP is considered to be caused by the contraction of the aortic smooth muscles. Unknown contractile mechanisms may exist other than the sympathetic nervous system and the inhibition of Ca2+ influx. The mechanism should be elucidated in the future.
The authors express our gratitude to Mr. H. Wago for his kind support of the present study.
The authors have no conflict of interest concerning the present study.