2021 Volume 28 Issue 4 Pages 349-355
2021 Volume 28 Issue 4 Pages 349-355
Aim: This study aims to describe the feasibility and safety of direct occluded vessel puncture as a new access site for complex peripheral artery occlusive disease.
Methods: Eleven consecutive patients with symptomatic peripheral artery disease underwent endovascular therapy using the direct occluded vessel puncture technique. The occluded vessel was punctured using a dedicated 20 G needle and the Hi-Torque Command 18 ST guidewire under duplex echo or fluoroscopic guidance, and a 6 Fr sheath was then inserted. Hemostasis was achieved with the Exoseal® Vascular Closure Device.
Results: Direct occluded vessel puncture was achieved in 10 of 11 cases (90.9%), and procedural success was achieved in all cases. There were no in-hospital deaths or any complications, including bleeding, pseudoaneurysms, thrombosis, or surgical conversion.
Conclusion:The direct occluded vessel puncture technique using a 20 G needle and the Hi-Torque Command 18 ST is feasible and safe. This technique may also be used as an alternative option when there are no appropriate approach sites.
In recent years, guidewire crossing techniques have been developed for peripheral artery occlusive disease (PAOD), and one of these techniques is the bi-directional approach for aortoiliac and femoropopliteal occlusive lesions 1- 3) . In general, puncturing a vessel to access the lumen is the standard choice in the antegrade and/or retrograde approach for PAOD 4) . However, some complex cases do not have appropriate approach sites because of multilevel lesions or anatomical issues. Although there are some technical difficulties, the direct occluded vessel puncture technique may be used to create an access site for recanalization in patients without an appropriate approach site. We will describe the procedures required to achieve this direct occluded vessel puncture technique on the basis of the cases we experienced.
This study was conducted to evaluate the feasibility and safety of the direct occluded vessel puncture technique in the creation of a new access site for complex PAOD.
A total of 82 symptomatic patients with aortoiliac and/or femoropopliteal chronic total occlusion (CTO) underwent endovascular therapy (EVT) in our hospital between August 2019 and February 2020. Direct occluded vessel puncture was attempted in 11 patients (5 men; mean age: 74.8±8.4 years) to recanalize the complex CTO lesion after unsuccessful standard antegrade recanalization because there was no appropriate approach site. These patients constitute the cohort for the analysis. Informed consent was obtained from each patient, and the institutional ethics committee reviewed the protocol and approved this study.
Direct Occluded Vessel PunctureAfter the administration of local anesthesia, the occluded superficial femoral artery (SFA) was punctured at the middle part with a dedicated 20 G needle (Medikit Co., Ltd., Tokyo, Japan) under fluoroscopic or duplex echo guidance depending on the presence or absence of calcification, respectively ( Figs.1A, 1B, and 1C) . The Hi-Torque Command 18 ST (Century Medical, Inc., Tokyo, Japan) was advanced directly into the occluded SFA ( Fig.2A) . The Hi-Torque Command 18 ST is a crossing wire with 10 cm nitinol at its distal end and provides high support and a flexible tip (4 g). It is designed for various lesions or for prolapse prevention in the occlusive plane. Its shapeable nitinol tip provides superb durability, and the stainless-steel body provides good torque, support, and pushability. Thereafter, the microcatheter Prominent® Raptor (Tokai Medical Products, Inc., Aichi, Japan) was set ( Fig.2B) inside the 10 cm 6 Fr sheath (Nipro Corporation, Osaka, Japan) to reduce the gap between the guidewire and the sheath ( Fig.2C) . After the 6 Fr sheath insertion, the 6 Fr Bright Tip® STR (Cordis, Dublin, USA) was used as a support catheter, and this approach enabled us to perform the intravascular ultrasound (IVUS)–guided wiring technique as previously reported 5) .

A: For fluoroscopic guidance, when the occluded vessel has calcification, advance the 20 G needle toward the center of both calcification lines by using the Seldinger technique under frontal view. B: Retract the 20 G needle until the tip is centered of both calcification lines under lateral view. C: For duplex echo guidance, when the occluded vessel does not have any calcification, advance the 20 G needle toward the center of the occluded vessel while watching the duplex image. The red arrow indicates the tip of the 20 G needle.

A: The Hi-Torque Command 18 ST is advanced directory into the occluded SFA with a J shape. B: The microcatheter Prominent® Raptor is inserted. C: A 6 Fr sheath is inserted into the occluded SFA.
The Exoseal® Vascular Closure Device (Exoseal; Cordis, Dublin, USA) was used under fluoroscopic guidance to achieve hemostasis 6, 7) after performing the direct occluded vessel puncture technique. The deployment button was depressed when the indicator wire exited the vessel ( Fig.3) because the reverse flow and the visual indicator are not useful in this situation.
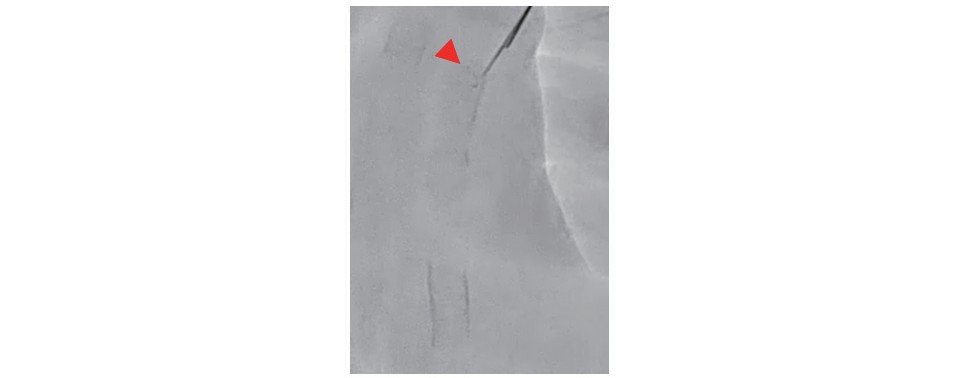
When the indicator wire exits the vessel wall, the plug deployment button is depressed. The red arrow indicates the indicator wire of Exoseal.
Exoseal; Exoseal® Vascular Closure Device
Technical puncture success was defined as the successful insertion of the 6 Fr sheath into the occluded vessel, and technical procedural success was defined as the revascularization of the target lesion. Clinical outcomes, including in-hospital death and procedural complications, were evaluated.
Statistical AnalysisContinuous data are given as means±SD as appropriate. Categorical data are presented as total amount (proportion), and calculations were performed using EZR 1.41.1 8) .
Table 1 presents the baseline of the patients and lesions characteristics. Four patients had intermittent claudication, and the remaining seven patients had critical limb ischemia. All lesions were classified into TransAtlantic Inter-Society Consensus II C or D. Table 2 shows the results of the procedure. In 8 of 11 cases (73%), the occluded SFA was retrogradely punctured to recanalize the complex CTO lesion, such as the aortoiliac and SFA CTO lesion with diseased common femoral artery ( Fig.4A) . On the other hand, in the other three cases (27%), the occluded SFA, which has complex infrainguinal lesion with some problems at the proximal, was antegradely punctured to recanalize the popliteal to the below the knee (BTK) artery lesion without a recanalization of the proximal SFA CTO lesion ( Fig.4B) . The punctures were achieved using fluoroscopy guidance and duplex echo guidance in seven cases (64%) and four cases (36%), respectively. The direct occluded vessel puncture technique was successful in 10 of 11 cases (90.9%).
| Patient Characteristics | |
|---|---|
| Age, y | 74.8±8.4 |
| Male gender | 5 (45) |
| Risk factors | |
| Hypertension | 8 (73) |
| Hyperlipidemia | 5 (45) |
| Diabetes mellitus | 5 (45) |
| Renal insufficiency | 5 (45) |
| (eGFR<30 mL/min/1.73 m2) | |
| Regular hemodialysis | 5 (45) |
| Current smoking | 2 (18) |
| Rutherford grade (3/4/5/6) | 4/4/2/1(36/36/19/9) |
| Lesion Characteristics | |
| TASC II classification (C/D) | 2/9 (18/82) |
| PACSS grade (0/1/2/3/4) | 3/1/2/2/3 |
| (27/10/18/18/27) | |
| Target lesion | |
| Aorto-iliac to SFA occlusion | 7 (64) |
| Popliteal-below the knee lesion | 4 (36) |
Data are presented as mean±SD or number (percentage).
Abbreviations:
eGFR, estimated glomerular filtration rate
TASC, TransAtlantic Inter-Society Consensus
PACSS, Proposed Peripheral Artery Calcium Scoring System
SFA, superficial femoral artery
| Procedural Characteristics | |
|---|---|
| Retrograde direct occlusive vessel puncture | 8 (73) |
| Antegrade direct occlusive vessel puncture | 3 (27) |
| Successful direct occlusive vessel puncture | 10 (90.9) |
| Puncture method | |
| Fluoroscopy guidance | 7 (64) |
| Duplex echo guidance | 4 (36) |
| Successful wire recanalization | 11 (100) |
| Successful hemostasis by Exoseal | 11 (100) |
| Hemostasis time, min | 4.8±3.8 |
| Contrast volume, ml | 107.3±74.2 |
| Ankle brachial index | |
| Before | 0.36±0.20 |
| After | 0.68±0.20 |
| Complication | |
| In-hospital death | 0 (0) |
| Bleeding | 0 (0) |
| Thrombosis | 0 (0) |
| Surgical conversion | 0 (0) |
Data are presented as mean±SD or number (percentage).
Abbreviation: Exoseal, The Exoseal® Vascular Closure Devise

A: Representative situation of the retrograde direct occluded vessel puncture. The schema shows an aortoiliac and SFA occlusion with a diseased common femoral artery. After the failed antegrade approach, the occluded SFA is punctured retrogradely. B: Representative situation of the antegrade direct occluded vessel puncture. The schema shows a long SFA occlusion with severe calcification at the ostium and a diseased popliteal BTK artery. After the failed antegrade approach, the occluded SFA is punctured antegradely in the above manner without the recanalization of the SFA ostium.
In the only unsuccessful retrograde case, the 6 Fr sheath could not penetrate the severely calcified wall along the microcatheter. After changing the approach site to a site that is more distal from the SFA with blood flow, this case succeeded in the recanalization of the target lesion. Therefore, target lesion recanalization was achieved in all cases. The hemostasis of the direct occluded vessel punctures was achieved by Exoseal in all cases. The mean hemostasis time of Exoseal was 4.8±3.8 minutes. There were no in-hospital deaths or complications, including bleeding, pseudoaneurysm, thrombosis, or surgical conversion.
Representative CaseAn 80-year-old female with a nonhealing ulcer of the first toe and severe rest pain of the right foot was admitted to our hospital ( Fig.5A) . Her skin perfusion pressure had decreased to 15 and 12 mmHg in the dorsal side and planter side, respectively. She had undergone two right femoropopliteal bypass surgeries in the past. Angiogram showed that her right SFA was occluded from the ostium to the P1 segment. Furthermore, the P3 segment was occluded, and the BTK segment was diseased. The collateral vessels from the deep femoral artery supplied the P2 and the BTK segment ( Fig.5B) . After the antegrade approach failed because of anatomical issues in the common femoral artery, we planned to perform an EVT for the outflow disease with the good collateral circulation from the deep femoral artery. The occluded right SFA was antegradely punctured at the middle part with a 20 G needle under fluoroscopic guidance, and a Hi-Torque Command 18 ST was advanced directly into the occluded SFA ( Fig.5C) . Thereafter, a Prominent® Raptor was set inside a 10 cm 6 Fr sheath to reduce the gap between the guidewire and the sheath. Finally, the 6 Fr sheath was placed in the occluded SFA ( Fig.5D) . The diseased lesions were dilated with a conventional balloon after guidewire crossing by using the IVUS guide and the rendezvous technique ( Figs.5E and 5F) . The hemostasis of the puncture site was achieved using Exoseal. Her skin perfusion pressure increased to 56 mmHg in the dorsal side and to 43 mmHg in the planter side one week after EVT. Debridement of the first toe was performed by a plastic surgeon. Finally, her wound completely healed, and she was discharged on foot ( Fig.5G) .

A: Nonhealing ulcer of the first toe at admission. B: Initial angiogram. C: The Hi-Torque Command 18 ST was advanced directly into the occluded SFA with a J shape. D: A 6 Fr sheath was inserted into the occluded SFA. E: Balloon angioplasty for the planter arch. F: Final angiogram. G: Healed ulcer after debridement at discharge.
The appropriate selection of the access site for PAOD is one of the most important factors for successful guidewire recanalization 4) . We first report the feasibility and safety of the direct occluded vessel puncture technique in combination with a dedicated 20 G needle and the Hi-Torque Command 18 ST for patients without appropriate access sites. The direct stent puncture technique was previously reported as an alternative option to the distal puncture technique for patients with SFA in-stent occlusion when an antegrade approach fails and when the distal flow for the retrograde transpopliteal approach is insufficient 9, 10) . This puncture technique ordinarily requires a micropuncture needle and a regular guidewire. The advantage of the direct stent puncture is that the needle easily stays within the stent owing to the visibility of the stent struts, and the guidewire easily advances inside the stent because of the stabilized stent wall. However, this technique is useful only for cases with SFA in-stent occlusion. On the other hand, the direct occluded vessel puncture can be applied in various situations (i.e., not only for femoropopliteal occlusive disease but also for aortoiliac occlusive disease). Furthermore, to avoid amputation and worsening claudication, it is important to note that the population in the current study had already experienced a failed antegrade crossing attempt and had no further endovascular option.
Puncture MethodsThere are two types of puncture methods.
When the occluded vessel is calcified, the fluoroscopic guided puncture is effective. First, the 20 G needle is advanced toward the center of both calcification lines with the Seldinger technique under frontal view ( Fig.1A) , and secondly the 20 G needle is retracted under lateral view until the tip is centered on both calcification lines ( Fig.1B) . When the occluded vessel does not have any calcification, the duplex echo guided puncture is effective. To maintain the advancement of the tip of the 20G needle at the center of the occluded vessel, it is important to obtain a successful direct occluded vessel puncture ( Fig.1C) .
Puncture SystemsThis direct occluded vessel puncture technique requires a dedicated puncture system.
When a combination of an intravenous catheter and a 0.035-inch guidewire is used, the outside plastic sheath tends to withdraw from the vessel against the resistance when a 0.035-inch guidewire is advanced to the occluded vessel. The wire prolapses because the outside plastic sheath is invisible and has weak supportability ( Fig.6A) . When a combination of a dedicated 20 G needle and a 0.014-inch soft-type guidewire is used, the guidewire does not easily advance forward against the resistance and finally breaks ( Fig.6B) . On the other hand, the combination of a 20 G needle and the Hi-Torque Command 18 ST offers the best balance of adequate visibility, durability, and pushability. Furthermore, it allows the advancement of the guidewire into the occluded vessel ( Fig.6C) .

A: Combination of an intravenous catheter and 0.035-inch guidewire. B: Combination of a dedicated 20 G needle and 0.014-inch soft-type guidewire. C: Combination of the 20 G needle and Hi-Torque Command 18 ST.
To facilitate the smooth insertion of the sheath, we need to prepare in advance the microcatheter Prominent® Raptor inside the 10 cm 6 Fr sheath. This approach enables the easy insertion of the sheath because of the reduction in the gap between the guidewire and the sheath. In one case in this study, the 6 Fr sheath could not penetrate the calcification wall along the Hi-Torque Command 18 ST and the microcatheter after successful occluded vessel puncture. We defined technical puncture success as the successful insertion of a 6 Fr sheath into the occluded vessel and not the successful insertion of a microcatheter into the occluded vessel. If the Hi-Torque Command 18 ST advances into the subintimal space of the occluded vessel, it will be very difficult to introduce the guidewire to the intraluminal space by using only a microcatheter. The failure of the 6 Fr sheath insertion can be attributed to our choice of an occluded vessel with severe calcification as a puncture site. Therefore, choosing a puncture site with less calcification is important to avoid this type of sheath insertion failure.
IVUS-Guided WiringAs we previously reported 5) , the IVUS-guided wiring technique enables us to treat the occluded lesions of the iliac and femoropopliteal arteries with a high initial technical success rate. It is feasible, safe, and useful to reduce the contrast medium. Therefore, it is necessary to use 6 Fr sheath insertion rather than microcatheter insertion for IVUS-guided technique after direct occluded vessel puncture.
Hemostasis after the Direct Occluded Vessel PunctureHemostasis is the most important issue in this procedure. We usually use Exoseal 6, 7) for hemostasis at the direct occluded vessel puncture site. In general, the deployment button is depressed according to the presence or absence of backflow and the change in the color of the indicator. However, the use of Exoseal after occluded vessel puncture prevents the application of these general steps because there is no blood flow at the puncture site. The deployment of a fluoroscopic guided extravascular plug is useful for this type of situation. After the adjustment of the C arm to a lateral position, we pull back the Exoseal carefully and slowly while watching the indicator wire. When the indicator wire exits the vessel wall ( Fig.3) , the plug deployment button is depressed. All 10 cases that used this hemostasis technique successfully achieved hemostasis by Exoseal without any complications such as intravascular plug ejection, bleeding, or pseudoaneurysm. The mean hemostasis time of Exoseal was 4.8±3.8 minutes. The use of Exoseal for an occluded vessel and not for a vessel with a lumen is reasonable for achieving easy and quick hemostasis. In this study, we did not treat the lesions of the puncture site at the same session. Therefore, it is important not to leave any material in the occluded vessel after hemostasis with Exoseal when we schedule a second-stage EVT.
This study has some limitations. First, this study is an analysis of a relatively small number of patients. Second, we did not evaluate the long-term outcome after the direct occluded vessel puncture in this study. Further studies are needed to confirm the effectiveness and safety of the direct occluded vessel puncture.
Direct occluded vessel puncture can be effectively achieved using a 20 G needle and the Hi-Torque Command 18 ST. The direct occluded vessel puncture may also be used as an alternative option when there are no appropriate approach sites.
Support from institutional sources only.
The authors declare that there are no conflicts of interest.