2021 Volume 28 Issue 6 Pages 611-621
2021 Volume 28 Issue 6 Pages 611-621
Aim: The mechanism underlying the stiffness of the aorta and iliofemoral artery that is required to maintain blood pressure (BP) is unclear. A new stiffness index of the aorta (aBeta) and iliac-femoral arteries (ifBeta) was defined by applying the cardio-ankle vascular index (CAVI). We compared changes in stiffness of the two arteries in response to reduced BP, due to the non-selective α adrenergic blocker phentolamine and the β1 adrenergic blocker atenolol, in rabbits.
Methods: Pressure waves at the origin (oA) and distal ends of the aorta (dA) and the distal end of the left femoral artery (fA) were recorded simultaneously using three pressure sensors in 25 anesthetized rabbits. Phentolamine (50 µg/kg/min) and atenolol (10 mg/kg/min) were infused for 2 min. The pulse wave velocity (PWV) in each artery was determined; aBeta, ifBeta, and whole Beta (aifBeta) were calculated by the following formula; Beta=2ρ/PP×ln(SBP/DBP)×PWV2 (ρ: blood density; SBP, SBP, and PP: systolic, diastolic, and pulse pressures, respectively).
Results: SBP and DBP at oA, dA, and fA decreased by the administration of phentolamine and atenolol, with and without decreased total peripheral vascular resistance. After phentramine infusion, cardiac output (CO), aBeta, and aifBeta increased, while ifBeta decreased. After infusion of atenolol, CO decreased, while aBeta, ifBeta, and aifBeta remained unchanged.
Conclusion: The contradictory reactions of aBeta and ifBeta to phentolamine suggest that the stiffness of the aorta and ilio-femoral artery is regulated separately during decreased BP induced by phentolamine, but not by atenolol.
See editorial vol. 28: 588-589
Arterial stiffness is not only a marker of vascular aging and arteriosclerosis progression 1) , but is also an index of the vascular function required to maintain efficient systemic circulation 2) . The latter function is called the Windkessel function, which reserves the blood ejected from the left ventricle at the systolic phase and sends it to peripheral tissue during the diastolic phase 3) , which plays a role as the afterload of the left ventricle. These functions are fulfilled by arterial stiffness, but there was no suitable arterial stiffness index to evaluate such vascular function, especially for clinical use. Pulse wave velocity (PWV) has been a widely-used index for reflecting arterial stiffness in clinical studies 1, 4- 7) . However, PWV does not properly reflect arterial stiffness because it is merely a velocity that depends on blood pressure (BP) at the time of measurement 5- 7) .
There have been efforts to eliminate the dependency of BP from the arterial stiffness index. Hayashi et al. proposed the stiffness parameter β 8) , which is not affected by BP during measurement. Subsequently, Kawasaki et al. 9) reported a method for calculating β using an ultrasonic diagnostic apparatus in humans by measuring caliber change brought by the difference between systolic and diastolic blood pressure at one part of the artery; this method is accepted widely. In 2004, the cardio-ankle vascular index (CAVI) was developed by applying the Bramwell–Hill equation 10) to Kawasaki’s β formula, using PWV as an index reflecting arterial stiffness of the arterial tree from the origin of the aorta to the ankle 11) . The index is defined in eq.1.
PWV: pulse wave velocity of the arterial tree from the origin of the aorta to the ankle, SBP: systolic blood pressure, DBP: diastolic blood pressure, ρ: blood density, PP: pulse pressure, a, b; coefficients.
The CAVI is widely accepted and is not only an index reflecting the degree of arteriosclerosis
11)
, but also reflecting functional arterial stiffness, which might be involved in regulating systemic circulation
2)
. Although the CAVI might represent a vascular function, the precise role of the vascular function itself is still unclear in response to some vasodilating drugs.
In this study, the arterial stiffness of the aorta (elastic artery) and the iliofemoral artery (muscular artery) were defined according to the theory of CAVI. Arterial stiffness in the aorta was called aortic Beta (aBeta), and that from the origin of the iliac artery to knee was called iliac to femoral Beta (ifBeta).
Stiffness of the whole artery from the origin of the aorta to the distal end of the iliac artery is called aortic, iliac, and femoral Beta (aifBeta).
The non-selective α adrenergic blocker phentolamine 12, 13) , and the β 1 adrenergic blocker atenolol 14) lower BP. The former agent lowers BP by decreasing vascular resistance through arteriole dilation 12, 13) , and the latter agent lowers BP by decreasing cardiac contraction 14) . Thus, the two drugs have different hypotensive mechanisms.
The changes of aBeta, ifBeta, and aifBeta, in response to lowered BP during the administration of non-selective α adrenergic blocker phentolamine and β 1 adrenergic blocker atenolol to rabbits, were studied to understand the regulatory system of the arterial stiffness of the aorta and iliofemoral artery to maintain general circulation.
This study aimed to understand the regulatory system of arterial stiffness of the aorta and the iliofemoral artery to maintain systemic circulation. Those arterial stiffnesses were defined according to the cardio-ankle vascular index theory. Arterial stiffness from the aorta was called aBeta, and that from the origin of the iliac artery to knee was called ifBeta. The changes of aBeta and ifBeta in response to lower BP during administration of non-selective α adrenergic blocker phentolamine and β 1 adrenergic blocker atenolol into rabbits were compared.
The experiment involved 25 male Japanese white rabbits (Japan Laboratory Animals, Inc. Tokyo, Japan) aged 10–12 months and weighing 3.2–3.9 kg. The animals were raised in individual stainless steel cages at a room temperature of 22℃ –25℃, relative humidity of 50%–60%, and light/dark cycle of 12 L and 12 D. The rabbits were fed 100 g of commercial rabbit chow (Labo R Grower, Nosan Corporation, Yokohama, Japan) daily with free access to tap water. The Experimental Animal Committee of Fukushima Medical University approved the study, which was performed according to the Guide for the Care and Use of Laboratory Animals, 8th Edition, supported by the National Institutes of Health.
Surgical ProceduresFig.1 shows the schematic arrangement of the experimental animals and apparatus. The rabbits were anesthetized by intravenous administration of pentobarbital sodium at a dose of 30 mg/kg. Butorphanol tartrate (Vetorphal, Meiji Seika Phrma Co., Ltd., Tokyo, Japan) was injected intramuscularly at a dose of 0.3 mg/kg for pain relief. Procaine chloride was applied to the incised areas to reduce pain. The animals were fixed at supine and intubated via the trachea by tracheotomy. Two catheter-tip transducers (2 Fr, SPS-320, Millar Instruments, Inc., Huston, TX) were advanced to the origin of the aorta (oA) and the distal end of the abdominal aorta (dA) through the left common carotid and the right saphenous arteries, respectively. A FISO catheter with a fiber optic pressure sensor at the tip (0.9 Fr, FPI-LS-10, FISO Technologies, Inc., Quebec, Canada) was introduced to the distal end of the left femoral artery (fA) through the left saphenous artery. An ultrasonic flow probe (6.0 mm, I.D.) was placed at the ascending aorta after the chest had been attentively opened under voluntary breezing to prevent from pleura injuring.
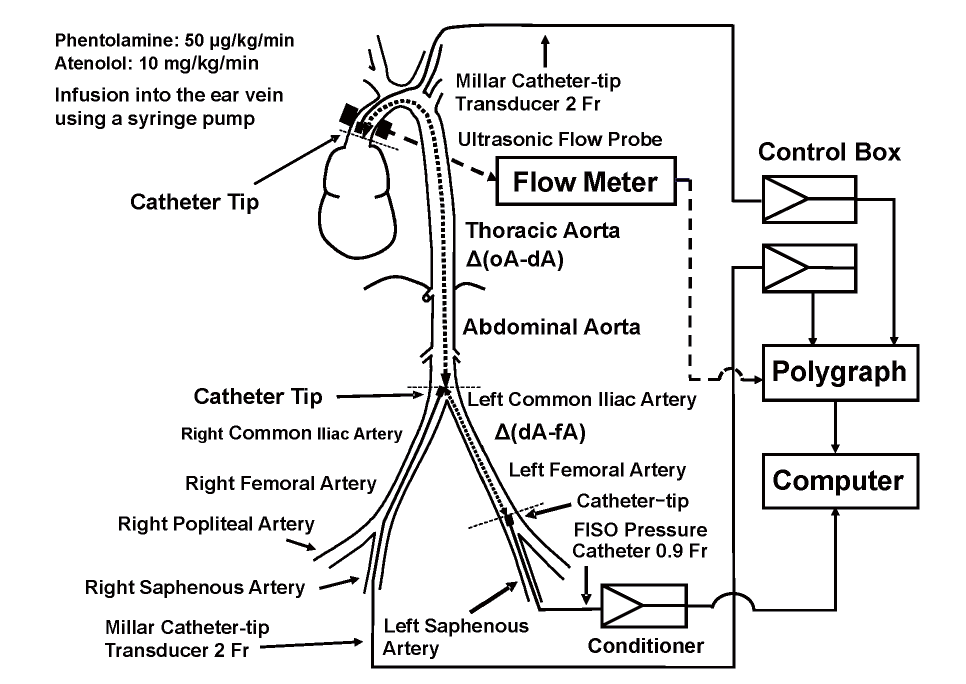
Phentolamine (Regitin, Novartis Pharma K.K., Tokyo, Japan) was infused through the ear vein at a dose of 50 µg/kg/min for 2 min using a syringe pump. Atenolol (Sigma-Aldrich Co., St. Louis, MO) was dissolved into physiological saline and infused into the ear vein at a dose of 10 mg/kg/min for 2 min using a syringe pump. We determined the phentolamine and atenolol doses in the preliminary experiment to investigate relation of change in BP with arterial stiffness. The present experiment’s purpose is not to investigate the dose-dependent effects of these drugs on BP and arterial stiffness, but to examine the effects of the difference in BP reduction mechanism due to the two drugs on arterial stiffness. Pressure waves at oA, dA, and fA, and flow waves at oA were measured simultaneously using a polygraph system (System 360, NEC-Sanei, Corporation, Tokyo, Japan) and an ultrasonic flow meter (T206, Transonic Inc., Ithaca, NY) through an analog-to-digital converter (PowerLab 16s/, AD Instruments Inc., Sydney, Australia) at intervals of 0.1 ms, and stored into a computer (PowerBook G4 M9691J/A, Apple, Cupertino, CA) before and after the infusion of phentolamine or atenolol. After the pressure and flow wave were recorded, the rabbits were sacrificed by overdose administration of pentobarbital sodium (100 mg/kg, i.v.).
Calculation of PWVThe length of the artery between the two pressure sensors, between oA and dA and between dA and fA, was measured carefully by fixing a thread to the artery. The peak of the second derivative of the pressure waves within a cardiac cycle was assigned to the increasing time of the original waves, as reported previously 15) . The PWV between oA and dA (aortic PWV; aPWV), between dA and fA (iliac-femoral PWV; ifPWV), and between oA and fA (aortic-femoral PWV; aifPWV) were determined by measuring the distance of the two pressure sensors between oA and dA, dA and fA, and oA and fA, and the differences in peak times of the two pressure waves between oA and dA, dA and fA, and Ao and fA, respectively.
Calculation of BetaaBeta, ifBeta, and aifBeta corresponding to aPWV, ifPWV, and aifPWV were calculated using the following equation:
Beta = 2ρ × ln (SBP/DBP)/PP × PWV 2
The blood density (ρ) was assumed to be 1.06. The mean values of SBP, DBP, and PP were used at oA and dA, dA and fA, and at oA and fA for calculation of aBeta, ifBeta, and aifBeta, respectively.
Just for reference, CAVI was determined by the following formula 2, 11, 16) :
CAVI = a [2ρ × ln (SBP/DBP)/PP × PWV 2] + b,
where “a” and “b” are undisclosed coefficients.
We determined Beta by excluding the coefficients “a” and “b” from the CAVI equation because they were undisclosed. Takahashi et al. 17) demonstrated that there was no significance or interpretation between CAVI and CAVI without the coefficients in the epidemiological and clinical studies. Both indices are considered equivalent for evaluating arterial stiffness.
Calculation of Other Cardiovascular ParametersThe HR was determined from the period of pressure waves. The TPR was calculated as MAP/CO, where MAP and CO are the mean arterial pressure and cardiac output, respectively.
Data ProcessingThe cardiovascular parameters were determined before and after the infusion of phentolamine or atenolol every 1 min for 5 min. The data were analyzed by one-way analysis of variance (ANOVA) for each drug since the present study was not performed to compare the hypotensive effect between phentolamine and atenolol. If there was a significant difference in the one-way ANOVA analysis, a post-hoc test was performed using Scheffe’s multi-comparison test with commercial software (StatFlex Ver. 6, Artech Inc., Osaka, Japan). The significance level was set at p= 0.05.
We did not test the statistical significance of each cardiovascular parameter between phentolamine and atenolol because we were not comparing the pharmacological effects of the two drugs.
Fig.2 depicts the changes in SBP and DBP at oA, dA, and fA (A and B); CO and HR (C and D); MAP and TPR (E and F); aBeta, ifBeta, and aifBeta (G and H); and aPWV, ifPWV, and aifPWV (I and J) during the infusion of phentolamine (left figures) and atenolol (right figures).
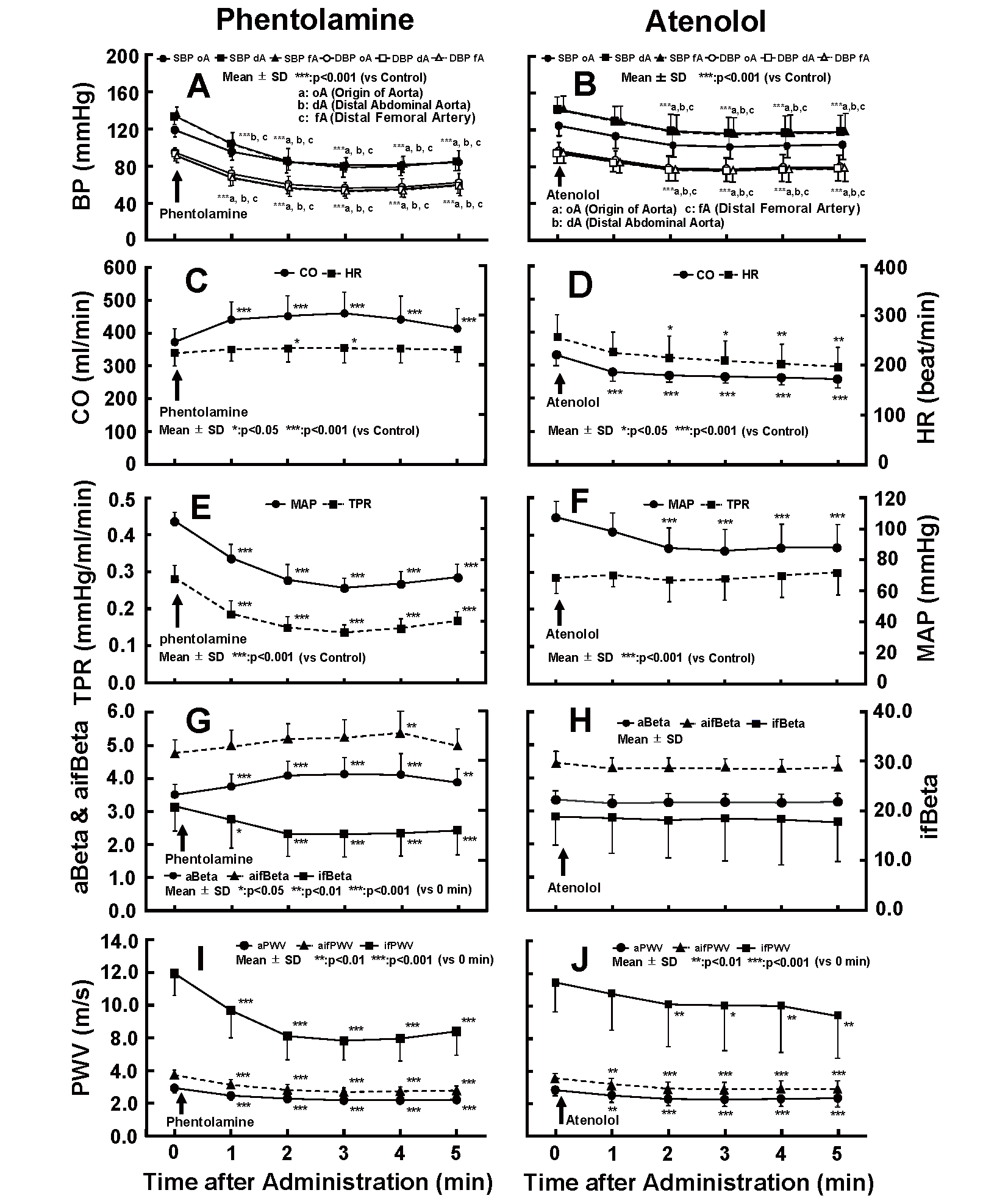
“0” means before the infusion. SBP: Systolic blood pressure, DBP: Diastolic blood pressure, CO: Cardiac output, HR: Heart rate, MAP: Mean arterial pressure, TPR: Total peripheral vascular resistance, PP: Pulse pressure, oA: Origin of the aorta, dA: Distal end of the abdominal aorta, fA: Distal end of the femoral artery, aBeta: Aortic Beta, ifBeta: Iliac to femoral Beta, aifBeta: Aortic, iliac and femoral Beta, aPWV: Aor- tic pulse wave velocity, ifPWV: Iliac to femoral PWV, aifPWV: Aortic, iliac and femoral PWV.
The SBPs and DBPs at oA, dA, and fA (A), and the MAP at oA (E) decreased significantly after the administration of phentolamine. CO and HR increased significantly at 2–5 min and 2–3 min after the administration (C). TPR decreased significantly in combination with the MAP (E).
These results are consistent with the hypothesis that the non-selective α adrenergic blocker phentolamine would stimulate arterial smooth muscle relaxation, decrease arterial resistance, and increase cardiac output. A decrease in ifBeta indicates the dilatation of arterial smooth muscle cells, while a reciprocal increase in aBeta might represent a compensation of decreased peripheral arterial resistance and blood pressure (BP).
After infusion of atenolol, the SBPs and DBPs at oA, dA, and fA (B), and the MAP at oA (F) showed a significant decrease within 2–5 min after administration. HR and CO declined significantly 2–5 min and 1–5 min after administration, respectively (D). There was no significant change in the TPR following the infusion (F).
With regards to arterial stiffness, when phentolamine was infused, ifBeta decreased significantly immediately after the infusion and maintained a lower value after 2 min of infusion (approximately 26% smaller than the control value), while aBeta inversely increased significantly after 2 min of the infusion (G). The maximum increase in aBeta was about 18% greater than the control value 3 min after the infusion (G). aifBeta also increased significantly 4 min after the infusion (approximately 13% greater than the control value) (G). The aPWV, ifPWV, and aifPWV decreased significantly 1 min after the infusion and remained at a lower value after 2 min of infusion (I).
Following administration of atenolol, aBeta, ifBeta, and aifBeta showed no significant change despite a significant decrease in BP (H). The aPWV, ifPWV, and aifPWV fell significantly concomitant with the decrease in BP after the administration (J). These results are consistent with the hypothesis that the β 1 adrenergic blocker atenolol would not affect the arterial smooth muscle but would decrease cardiac function and, subsequently, CO, without altering arterial resistance. The correlations between various circulation factors were analyzed as follows to confirm the results:
Relationship Between BP and BetaFig.3 illustrates the correlation of SBP (A and B), MAP (C and D) and DBP (E and F) with Beta during the infusion of phentolamine (left figures) and atenolol (right figures). ifBeta correlated positively with SBP (A), MAP (C) and DBP (E) during the infusion of phentolamine; however, a significant negative correlation was found between SBP and aBeta (A), between MAP and aBeta (C), between DBP and aBeta (E). There was a similar negative relationship between SBP and aifBeta (A), between MAP and aifBeta (B), between DBP and aifBeta (C). aBeta, ifBeta, and aifBeta had no significant correlation with SBP (B), MAP (D) and DBP (F), while ifBeta scattered across a wide range at different SBP, MAP and DBP during the administration of atenolol.
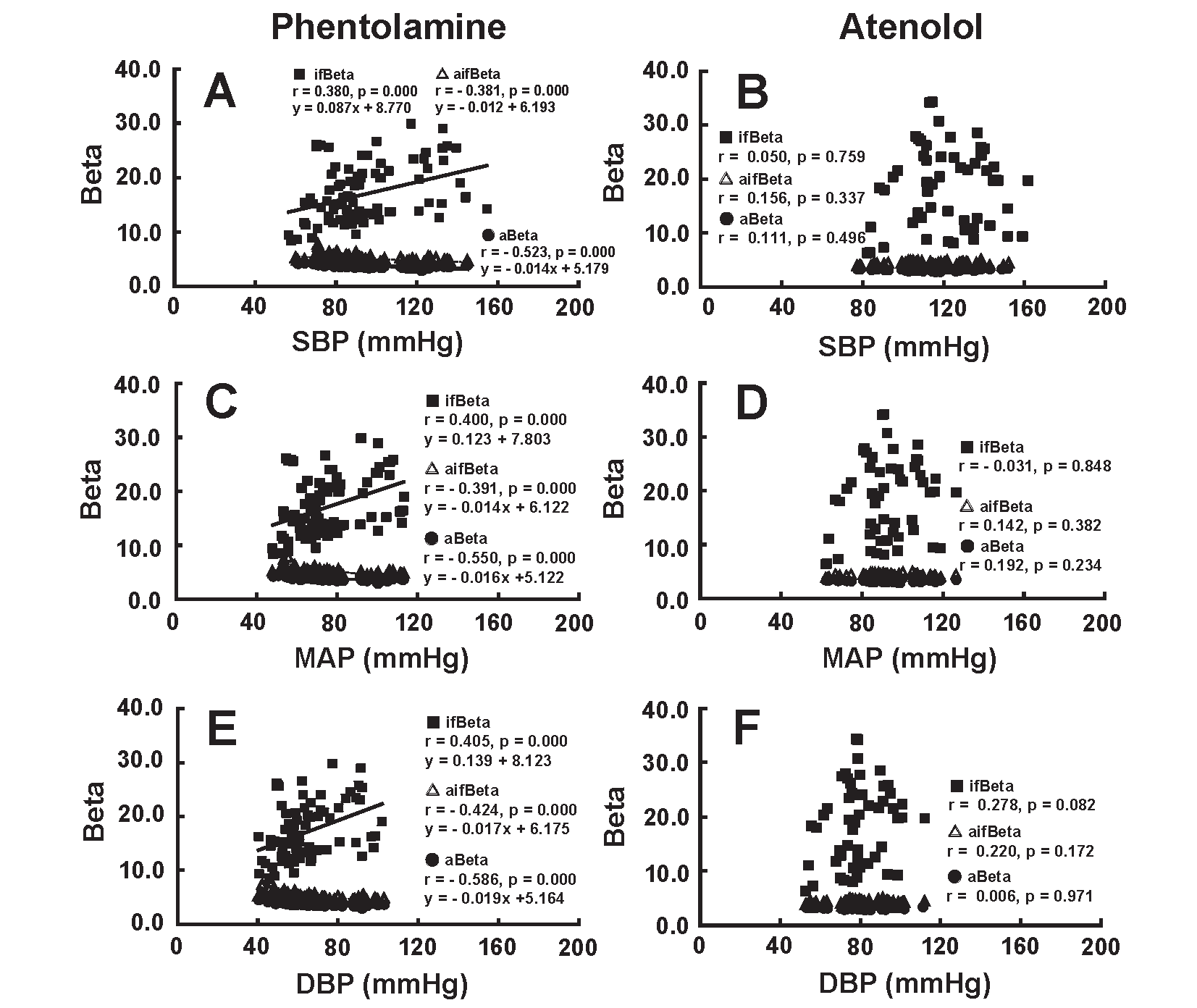
aBeta and aifBeta showed a significant negative correlation with SBP (A), MAP (C) and DBP (E), whereas ifBeta had a significant positive correlation with SBP (A), MAP (C) and DBP (E) during the administration of phentolamine. aBeta, ifBeta, and aifBeta had no significant correlation with SBP (B), MAP (D) and DBP (F) during the administration of atenolol.
These results are consistent with the hypothesis that the non-selective α adrenergic blocker phentolamine would decrease muscular arterial stiffness, accompanied by a decrease in BP, but would not affect the arterial stiffness of the elastic artery. The β 1 adrenergic blocker atenolol had no significant effect on the arterial stiffness of the elastic artery and muscular artery but did decrease BP as a result of a decrease in CO.
Relationship Between BP and PWVFig.4 shows the correlation of SBP (A and B), MAP (C and D) and DBP (E and F) with PWV due to the administration of phentolamine (left figures) and atenolol (right figures). aPWV, ifPWV, and aifPWV correlated positively with SBP, MAP and DBP due to the administration of phentolamine and atenolol.
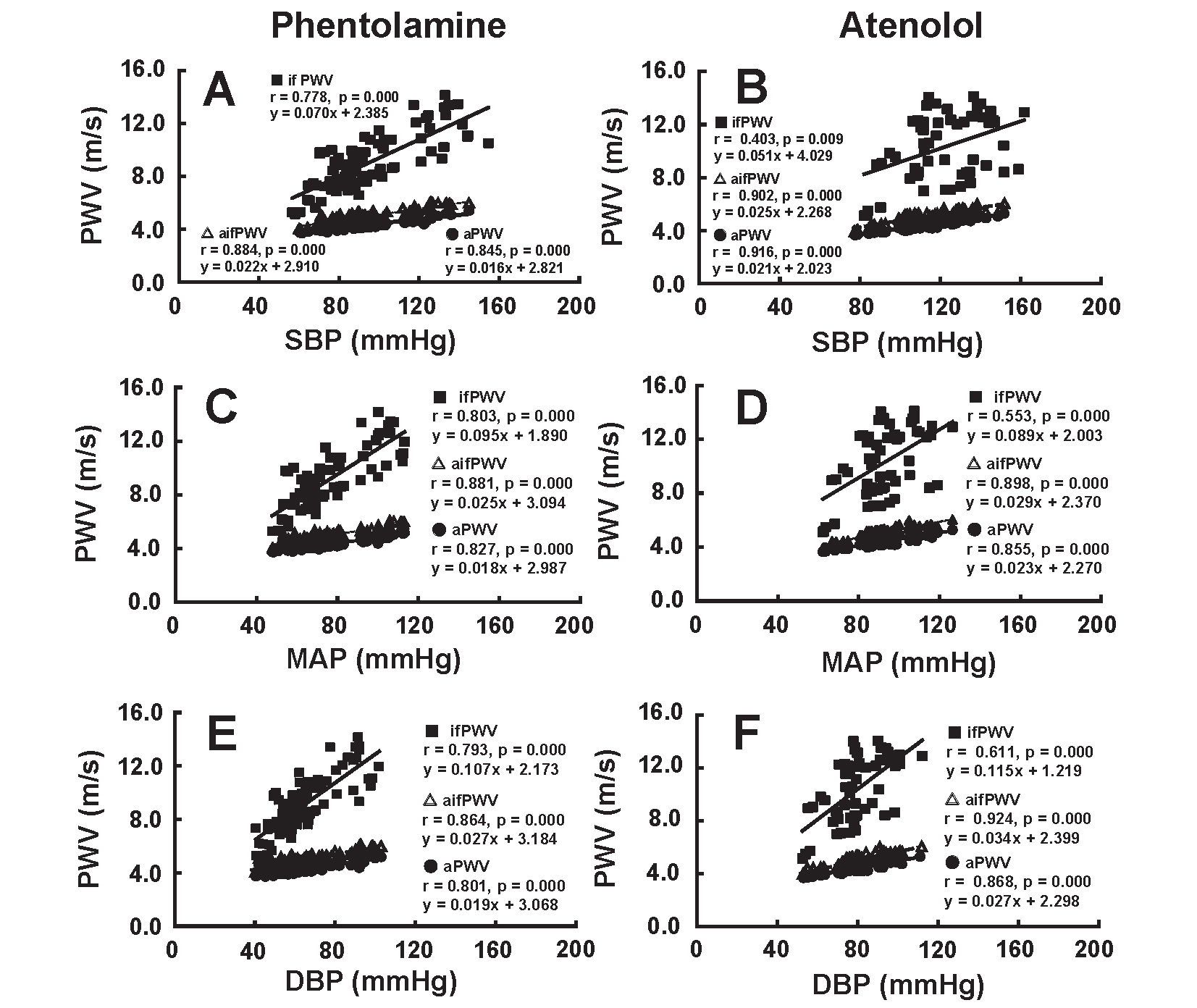
aPWV, afPWV, and if PWV showed a significant positive correlation with SBP, MAP and DBP during the administration of phentolamine and atenolol.
Generally, the PWV at each artery decreased along with a decrease in BP during the administration of phentolamine and atenolol. These results indicate that PWV cannot be used as an index to evaluate the role of arterial stiffness in systemic circulation.
Relationship of Beta with CO and TPRFig.5 illustrates the relationship between Beta and CO (A and B) and between Beta and TPR (C and D) during the administration of phentolamine (left figures) and atenolol (right figures). aBeta and aifBeta had a significantly positive correlation with CO (A), while ifBeta did not correlate with CO (A) during the infusion of phentolamine. ifBeta and aifBeta did not show a significant correlation with CO (B), although aBeta correlated negatively with CO (B) during the infusion of atenolol. aBeta and aifBeta showed a significant negative and positive correlation with TPR during the administration of phentolamine (C) and atenolol (D), respectively. ifBeta had positive and no correlation with TPR due to the administration of phentolamine (C) and atenolol (D), respectively.
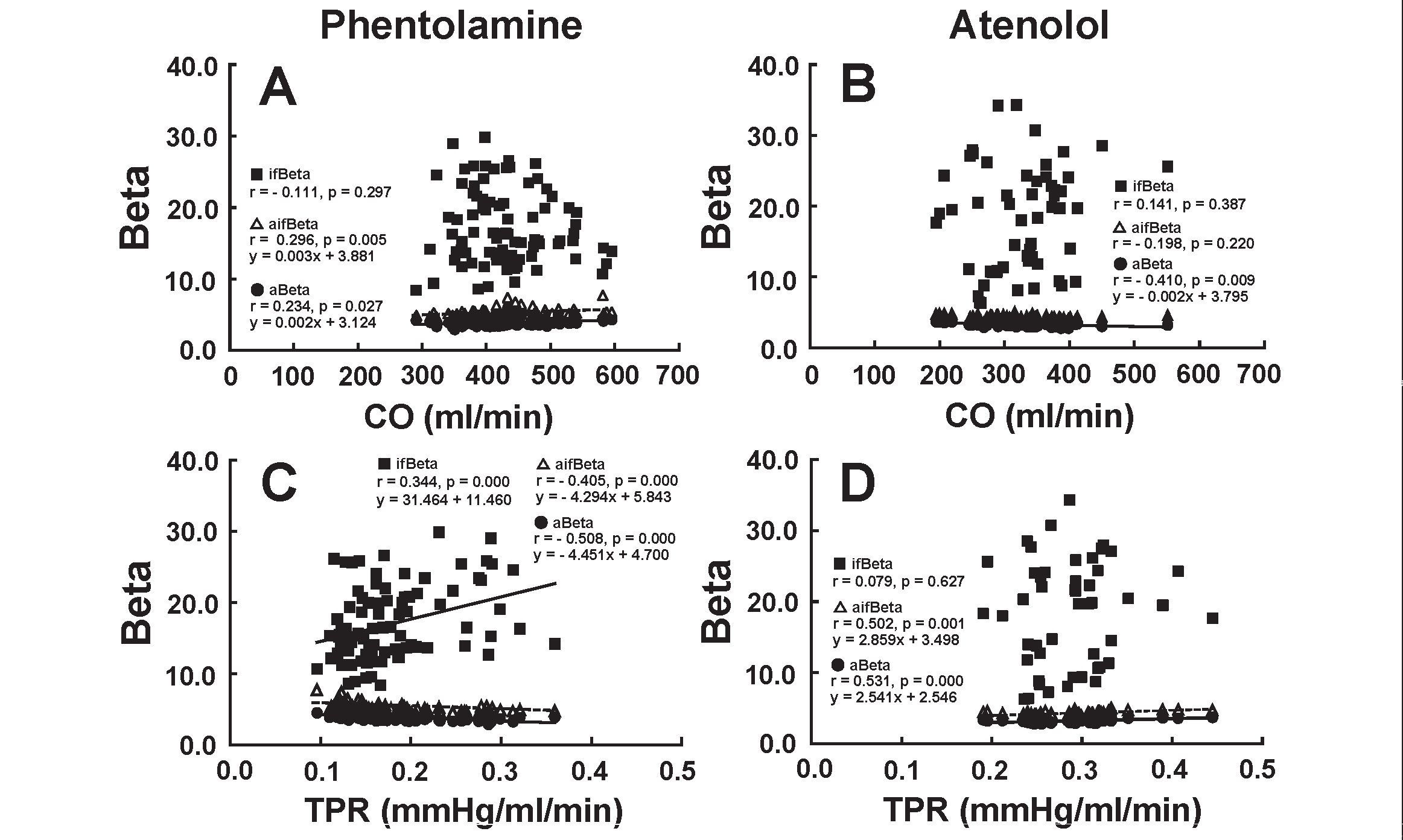
aBeta and aifBeta showed a significant positive and negative correlation with CO (A) and TPR (C), respectively, due to the infusion of phentolamine. ifBeta correlated significantly not with CO (A) but with TPR (C) during the infusion of phentolamine. When atenolol was administered, there was no significant correlation between ifBeta and CO (B), between aifBeta and CO (B), and between ifBeta and TPR (D). aBeta correlated negatively with CO (B), whereas aBeta and aifBeta correlated positively with TPR (D).
These results are consistent with the hypothesis that the non-selective α adrenergic blocker phentolamine would decrease arterial stiffness of the muscular artery, accompanied by a decrease in TPR, but that the β 1 adrenergic blocker atenolol would not affect the arterial stiffness of the elastic artery and muscular artery.
Relationship of PWV With CO and TPRFig.6 depicts the relationship between PWV and CO (A and B) and between PWV and TPR C and D) during the administration of phentolamine (left figures) and atenolol (right figures).
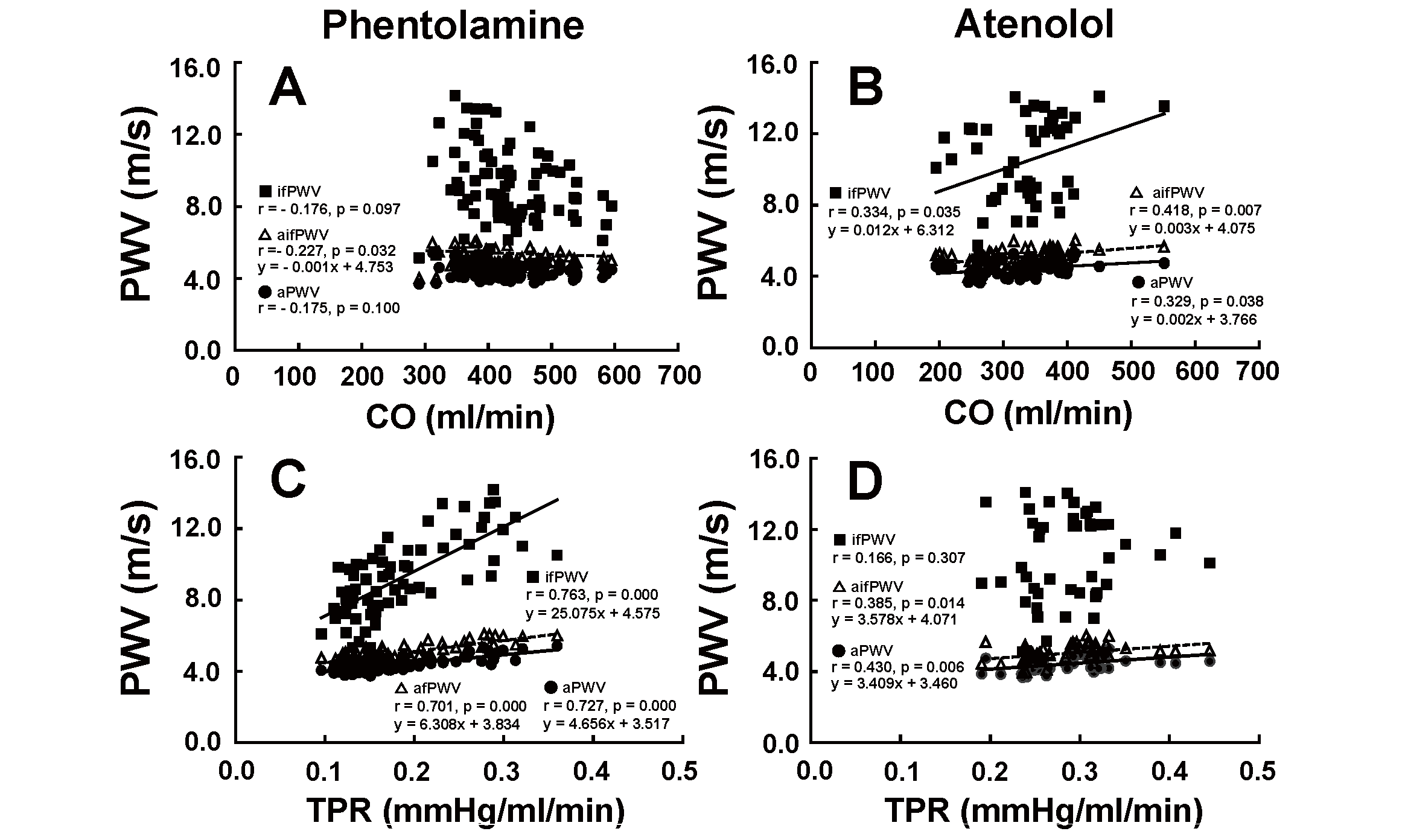
When phentolamine was infused, only aifPWV showed a significant negative correlation with CO (A). aPWV and ifPWV did not correlate with CO (A). aPWV, aifPWV, and ifPWV had a significant positive correlation with TPR (C). When atenolol was administered, aPWV, ifPWV and aifPWV correlated positively with CO (B). aPWV and aifPWV also correlated positively with TPR (D), while ifPWV had no significant correlation with TPR (D).
ifPWV and aPWV did not have a significant correlation with CO (A), while aifPWV showed a weak negative correlation with CO (A) during the infusion of phentolamine. ifPWV, aifPWV, and aPWV correlated positively with CO due to the administration of atenolol (B). ifPWV, aifPWV, and aPWV correlated positively with TPR (C) during the infusion of phentolamine. aPWV and aifPWV correlated positively with TPR (D), while ifPWV did not correlate with TPR (D) during the administration of atenolol.
The regulatory roles of stiffnesses of the aorta and iliofemoral artery in maintaining systemic circulation were studied. We defined new stiffness indices of the aorta (aBeta), iliac-femoral arteries (ifBeta), and aortic-iliac-femoral artery (aifBeta) by applying the CAVI theory and established the measuring system of them in the rabbit.
When the α-adrenergic blocker phentolamine was administered to rabbits, the BP decreased with increasing CO, decreasing TPR, and increasing HR. These changes were consistent with the pharmacological effects of the non-selective α-adrenergic blocker phentolamine 12, 13) . That is, phentolamine reduces TPR and increases CO due to relaxing arterial smooth muscles. HR is thought to increases due mainly to baroreflex in response to the fall in BP. In this condition, ifBeta was decreased significantly. The decrease in ifBeta might reflect the relaxation of the smooth muscle cells of the iliac-femoral artery due to phentolamine.
On the contrary, atenolol significantly reduced BP with decreasing CO and HR without affecting TPR. These changes were different from those induced by α-adrenergic blocker phentolamine 12, 13) . That is, β 1-adrenergic blocker atenolol prevents adrenergic stimulation by binding to β 1 receptors, diminishing HR and contractility of the heart muscles without affecting contraction of arterial smooth muscle cells 14) . In this condition, ifBeta, aBeta, and aifBeta did not change. These results are consistent with the action of atenolol in the systemic circulation.
As for PWV as an index of arterial stiffness, when either phentolamine or atenolol was administered, aPWV, aifPWV, and ifPWV declined with decreasing BP level, uniformly. Those data were the natural outcome because PWV depends on the BP at a time of measurement and is not indicative of proper stiffness of the arterial wall. Therefore, PWV is not an appropriate index of arterial stiffness for the studies on the role of arterial stiffness in the analysis of vascular function, as shown in the analysis in Fig.4 and Fig.6 .
By the way, when phentolamine was administered, the aortic stiffness (aBeta) increased according to a decrease in SBP, DBP, and MAP, whereas ifBeta decreased. Also, the relationship between aBeta and SBP, aBeta and DBP, aBeta and MAP, and aBeta and TPR were significantly negative during phentolamine administration, as shown in Fig.3A, C and E, and Fig.5C . In contrast, the relations between ifBeta and SBP, DBP and MAP were significantly positive. These results indicate enhanced aortic stiffness in parallel to the decreased stiffness of the iliac and femoral arteries. This reciprocal effect is unexpected.
Gnus et al. 18) demonstrated in an in vitro study that α 1A receptors played a major role in the contraction of the rabbit aortic wall. The non-selective α blocker phentolamine may dilate the arteries through the α 1-adrenergic receptor 12, 13) . However, the current results indicate that the stiffness of aorta and iliac-femoral arteries is not uniformly regulated, but regulated separately during the administration of phentolamine. The mechanism of this contradictory response between aBeta and ifBeta during the administration of phentolamine was unclear now, but several mechanisms are proposed. One possible mechanism is that the decrease in BP might stimulate the endogenous sympathetic nerve and contract the aortic medial smooth muscle cells, increasing aBeta. This hypothesis is based on the presence of different sensitivity of both smooth muscle cells for α adrenostimulant. The second possible mechanism is that a decrease in BP might intrinsically stimulate aortic medial smooth muscle cell contraction, thereby increasing aBeta. Anyway, the contractions of arterial smooth muscle cells in the elastic artery and aorta, and in the muscular artery and iliac-femoral artery, were regulated differently. Furthermore, there remains the question of whether this phenomenon occurs only in the rabbit. Further studies are required to elucidate those problems.
The more precise co-relationships among various Betas and vascular parameters such as CO and TPR were analyzed, as shown in Fig.5 . When phentolamine was administered, ifBeta was positively correlated with TPR, as shown in Fig.5C . This might indicate that phentolamine induced relaxation of the peripheral arterial smooth muscle cell. As a result, TRP and ifBeta decreased. During this process, aBeta was negatively related to TPR, as shown in Fig.5C , and positively related with CO in Fig.5A . There may be some contraction-inducing factor promoted by enhanced CO for aortic smooth muscle cells.
When atenolol was administered, aBeta and CO were negatively co-related latently even though aBeta decreased only slightly, as shown in Fig.5B , and aBeta and TPR were positively related latently, as shown in Fig.5D . The decreased CO and BP induced by atenolol would be recovered by contracting arterial smooth muscle cells. There are some mechanisms to raise BP and CO such as baroreflex control for vascular tone or unknown vascular intrinsic mechanism.
In this study, the changes in aifBeta are almost parallel to those of aBeta. Furthermore, aifBeta almost corresponds to the CAVI, which is used clinically in humans, and includes the aorta, iliac, femoral, and tibial artery. Shirai et al. 2) reported in healthy male volunteers that the administration of doxazosin, an α 1-adrenergic blocker, decreased BP and CAVI. The effect of the blockade of α 1-adrenergic receptor on CAVI and aifBeta is contradictory. This discrepancy might be the difference in the percentages of elastic and muscular arteries between humans and rabbits.
The percentage of muscular arteries from AA to fA is approximately 24% (dA to fA) in rabbits in the present study, and that from AA to the anterior and posterior tibial arteries is >50% in humans. The increase in aBeta and decrease in ifBeta are approximately 18% and 26% to the control value 3 min after phentoplamine administration. Considering the percentage of elastic (about 76%) and muscular arteries (about 24%) from AA to fA, the aifBeta increases about 7.4% compared to the control value (0.18×0.76×100 - 0.26×0.24×100=7.4), which is slightly smaller than the actual increase (9.6%). Therefore, the aifBeta mainly reflects aortic stiffness in rabbits, but this requires further studies in humans. It will be necessary to examine in future studies whether the contradictory effects of vasodilating drugs to aBeta and ifBeta appear in human. Our finding raises questions concerning the evaluation of the effects of antihypertensive drugs.
This study was performed on rabbits under anesthesia; thus, the observation time was relatively short. Furthermore, the results were not easily translated into humans after long term phentolamine and atenolol administration.
When phentolamine decreased BP, ifBeta decreased, but aBeta increased. However, when atenolol decreased BP, both ifBeta and aBeta remained unchanged. These results suggest that the elastic and muscular arteries are regulated differently in response to systemic circulatory conditions. Further studies are required to clarify the mechanism and meaning of this contradictory response.
The authors thank Mr. H. Wago for his kind support to the work of the present study.
The authors have no conflict of interest concerning the present study.
aBeta: aortic Beta, ifBeta: iliac to femoral Beta, aifBeta: aortic, iliac and femoral Beta, CAVI: cardio-ankle vascular index, aPWV: aortic PWV, ifPWV: iliac to femoral PWV, aifPWV: aortic, iliac and femoral PWV, BP: blood pressure, SBP: systolic blood pressure, DBP: diastolic blood pressure, PP: pulse pressure, MAP: mean arterial pressure, CO: cardiac output, TPR: total peripheral vascular resistance, HR: heart rate, oA: origin of the aorta, dA; distal end of the aorta, fA: distal end of the femoral artery