2021 Volume 28 Issue 8 Pages 844-851
2021 Volume 28 Issue 8 Pages 844-851
Aim: Non-alcoholic fatty liver disease (NAFLD) is one of the most common chronic liver disorders associated with metabolic syndrome, and its prevalence has been on the rise. The pathogenesis of NAFLD has not yet been sufficiently elucidated due to the multifactorial nature of the disease, although the activation of macrophages/Kupffer cells is considered to be involved. We previously reported an animal model of NAFLD using MicrominipigsTM (µMPs) fed high-fat diets containing cholesterol with or without cholic acid. The aim of this study was to investigate the phenotypic changes of macrophages that occur during the development of NAFLD.
Methods: Immunohistochemistry of macrophages, lymphocytes, and stellate cells was performed using liver samples, and the density of positive cells was analyzed.
Results: The number of Iba-1-positive macrophages increased with increasing cholesterol content in the diet. The numbers of CD163-positive macrophages and CD204-positive macrophages also increased with increasing cholesterol content in the diet; however, the proportion of CD204-positive macrophages among Iba-1-positive macrophages was significantly reduced by cholic acid supplementation.
Conclusion: The results suggest that lipid accumulation induced macrophage recruitment in swine livers, and that the number of M2-like macrophages increased at the early stage of NAFLD, while the number of M1-like macrophages increased at the late stage of NAFLD, resulting in a liver condition like non-alcoholic steatohepatitis. We provide evidence of the phenotypic changes that occur in macrophages during the development of NAFLD that has never been reported before using µMPs.
Non-alcoholic fatty liver disease (NAFLD) is one of the most common chronic liver disorders worldwide, particularly in the Middle East and South America 1) . The prevalence of NAFLD has increased in the general population, and in Japan, the overall prevalence of NAFLD was around 30% from 2009 to 2010 2, 3) . Current epidemiological estimates indicate that NAFLD will soon be the leading causative liver disease of liver transplantation 4) . The spectrum of NAFLD ranges from non-alcoholic fatty liver (NAFL), hepatic steatosis without the presence of significant inflammation, to non-alcoholic steatohepatitis (NASH), a complex pattern with active lesions of hepatocyte injury, apoptosis, cell death, and inflammation in the absence of alcohol intake 5) . NASH potentially progresses to liver cirrhosis and hepatocellular carcinoma. The major risk factors for NAFLD are obesity, type 2 diabetes mellitus, hyperlipidemia, hypertension, and metabolic syndrome 1, 6) ; however, the pathogenesis of NAFLD is multifactorial and has not yet been sufficiently elucidated. Excess lipid accumulation in hepatocytes and subsequent hepatocellular injury, hepatic inflammation, and the activation of hepatic stellate cells (HSC) are induced by endoplasmic reticulum stress, oxidative stress, and inflammasome activation in hepatocytes 7) . This process is considered to be one of the causes of NAFLD/NASH pathogenesis. Macrophages (Kupffer cells) are the main population of inflammatory cells in the liver, and activated macrophages secrete several pro-inflammatory cytokines, including interleukin (IL)-1β, tumor necrosis factor-α, and IL-6 8) . In a previous report, macrophage depletion ameliorated liver damage in a murine NASH model 9) . This indicated that macrophage activation may be closely associated with the pathogenesis of NAFLD/NASH. Macrophage activation is broadly divided into two phenotypes 8) ; many researchers have reported that M1 and M2 macrophages have pro-inflammatory and anti-inflammatory functions, respectively, but the difference in functions between M1 and M2 macrophages remains controversial. Words such as “M1-like” or “M2-like” are used to explain the heterogeneity in macrophage phenotypes. The phenotypic change of macrophages into the M1-like phenotype has been reported in a mouse NAFLD/NASH model 10) , and an increased number of M1-like macrophages has also been reported in NASH liver samples in a human study 11) . However, the significance of M1-like macrophages in the pathogenesis of NAFLD/NASH remains unclear in humans.
We recently established an animal model using MicrominipigsTM (µMPs) for studying hyperlipidemia and NAFLD 12) . In this study, we tried to characterize the phenotype of macrophages/Kupffer cells according to the development of the pathological condition of NAFLD in the µMPs. We showed that lipid accumulated in the hepatocytes and macrophages, and that the number of macrophages attached to hepatic stellate cells (HSCs) increased with increasing severity of the liver condition. We also revealed changes in the numbers and phenotypes of macrophages that have never been reported before in swine.
The swine and diets used were as described previously 12) . The swine were fed a normal chow diet (NcD; Kodakara 73, Marubeni Nisshin Feed, Tokyo, Japan) or one of the special diets on a daily basis (3% of body weight/day) for 8 weeks. The special diets were high-fat diets with cholesterol (HcDs); they consisted of NcD supplemented with 12% lard (Miyoshi Oil & Fat, Tokyo, Japan) and 0.2%, 0.5%, or 1.5% cholesterol (Wako Pure Chemical, Osaka, Japan). Twenty-five µMPs were divided into five groups ( n= 5 each) as follows: the 1) NcD group; 2) HcD (0.2% cholesterol) group; 3) HcD (0.5% cholesterol) group; 4) HcD (1.5% cholesterol) group; and 5) HcD (1.5% cholesterol) with 0.7% cholic acid (CA; Wako Pure Chemical) group.
SamplesFormalin-fixed paraffin-embedded µMP liver samples, which were prepared in a previous study 12) , were used for hematoxylin and eosin staining, Gömöri’s silver impregnation, and immunohistochemistry (IHC). All protocols were approved by the Ethics Committee of Animal Care and Experimentation, Kagoshima University, and were performed according to the Institutional Guidelines for Animal Experiments and the Laws (no. 105) and Notification (no. 6) 12) .
IHCSpecimens were cut into 3-µm sections. For immunostaining, anti-Iba-1 (#019-19741, rabbit polyclonal, FUJIFILM Wako Pure Chemical Corp., Tokyo, Japan), anti-CD163 (#ab182422, rabbit monoclonal, clone EPR14336, Abcam, Cambridge, UK), and anti-CD204 (#KMU-MA01, mouse monoclonal, clone SRA-E5, Cosmo Bio, Tokyo, Japan) were used as the primary antibodies to detect macrophages. Anti-CD3 antibody (#413591, rabbit monoclonal, clone SP7, Nichirei Biosciences, Tokyo, Japan) was used as the primary antibody to detect lymphocytes. Anti-alpha smooth muscle actin (αSMA; #M0851, mouse monoclonal, clone 1A4, Agilent Technologies, Inc., Santa Clara, CA, USA) was used as the primary antibody to detect activated HSCs (myofibroblasts). The samples were incubated with horseradish peroxidase-labeled goat anti-mouse or rabbit secondary antibodies (#424131 for anti-mouse and #424141 for anti-rabbit, Histofine, Nichirei Biosciences). We visualized immunoreactions using a diaminobenzidine substrate kit (#425011, Nichirei Biosciences). For double-IHC, HistoGreen (green color) substrate (#AYS-E109, Eurobio Scientific, Les Ulis, France) was used for peroxidase-based immunostaining. For triple-IHC, HIGHDEF blue IHC chromogen (#ADI-950-150, Enzo Life Sciences, Inc., Farmingdale, NY, USA) was used for alkaline phosphatase-based immunostaining. The detailed methods, such as antigen retrieval and the dilution of antibodies, are described in our previous report 13) .
Image Processing and Cell CountingTwo investigators (Y.K. and D.Y.), who were blinded to any sample information, counted the cell numbers using microscopy in four non-overlapping randomly selected high-power fields (400× magnification; eight selected fields in total), and the results were averaged. For cell size measurements, five cells were randomly selected per µMP, and a total of 25 cells per group were measured and then compared. The proportion of immunostained area per field and the cell size were quantified by ImageJ 1.46 (National Institutes of Health, Bethesda, MD, USA).
StatisticsWe used the Mann-Whitney U test and Kruskal-Wallis test to perform comparisons between groups. All tests were two-sided, and we considered P values <0.050 to be statistically significant. Statistical analyses were performed with GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA).
Hepatic steatosis could be seen in the HcD (0.5% cholesterol) group to the HcD (1.5% cholesterol) group, and hepatic lobular inflammation and foamy macrophages were seen in the HcD (1.5% cholesterol) group. Hepatocyte ballooning, the characteristic finding of NASH, was seen only in the HcD (1.5% cholesterol) with CA group ( Fig.1A) . We then performed IHC for Iba-1, a pan-macrophage marker, to detect macrophages/Kupffer cells in the livers. The number and staining area of Iba-1-positive macrophages increased with increasing content of cholesterol in the diet, and they were significantly elevated by CA administration ( Fig.1A and 1B) . The size of macrophages also increased with increasing cholesterol content in the diet ( Fig.1B) . These results suggested that macrophage accumulation is stimulated by cholesterol intake, and that the level of accumulation is dependent on the concentration of cholesterol in the daily diet.
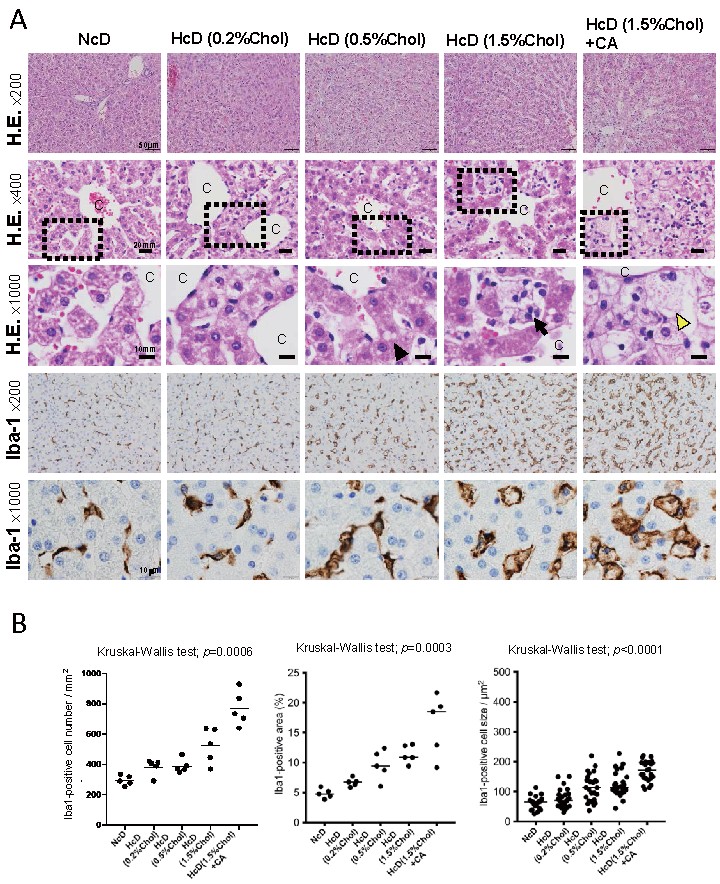
(A) Representative hematoxylin and eosin (H.E.) and immunohistochemistry (IHC) stainings of liver specimens in each group. The black arrowhead shows a lipid droplet, the black arrow shows a foamy macrophage, and the yellow arrowhead shows a ballooning hepatocyte. C, central vein. (B) Dot plots of the Iba-1-positive area, cell number, and cell size. All horizontal lines in the dot plots indicate median values. Chol, cholesterol; CA, cholic acid.
CD163 and CD204 are well-known M2-like markers that are specifically expressed on macrophages 8) . To investigate the M1/M2 balance in the livers of µMPs, we performed IHC of Iba-1, CD163, and CD204 using serial sections. Interestingly, CD163- and CD204 expression in Iba-1-positive macrophages were decreased by CA administration ( Fig.2A) . The densities and percentages of CD163-positive macrophages and CD204-positive macrophages increased with increasing cholesterol content in the groups without CA; however, CA administration reduced the densities and percentages of CD163-positive macrophages and CD204-positive macrophages ( Fig.2B and 2C) . In particular, a statistically significant reduction in the proportion of CD204-positive macrophages among Iba-1-positive macrophages was found (P=0.0367). However, similar to the Iba-1-positive macrophages, the sizes of CD163-positive macrophages and CD204-positive macrophages also increased with increasing cholesterol content in the diet ( Fig.2B and 2C) . The results suggested that macrophages changed to a non-M2-like (M1-like) phenotype with increasing cholesterol content and the addition of CA.

(A) Representative images of IHC staining of Iba-1-, CD163-, and CD204-positive macrophages in each group at the same site of serial sections. (B) Dot plots of the CD163-positive cell number, cell size, and the proportion of CD163/Iba-1 cell numbers. (C) Dot plots of the CD204-positive cell number, cell size, and the proportion of CD204/Iba-1 cell numbers. All horizontal lines in the dot plots indicate median values.
Progressive fibrosis is the hallmark of NASH, and HSCs are responsible for fibrogenesis in liver disease. To assess perisinusoidal fibrosis, we stained the sections using Gömöri’s silver impregnation staining technique. There was no significant difference in perisinusoidal fibrosis between the NcD group, HcD (0.2% cholesterol) group, and HcD (0.5% cholesterol) group; however, perisinusoidal fibrosis was more evident in the HcD (1.5% cholesterol) group and HcD (1.5% cholesterol) with CA group ( Fig.3) . Next, to explore the microenvironmental interactions between macrophages, HSCs, and immune cells, we performed double-IHC for macrophages and activated HSCs, and triple-IHC for macrophages, activated HSCs, and lymphocytes. The number of αSMA-positive activated HSCs increased with increasing cholesterol content and CA administration ( Fig.4A, 4B) . Double-IHC suggested that the number of Iba-1-positive macrophages attached to αSMA-positive activated HSCs increased with increasing cholesterol content and CA administration; however, the number of CD163-positive macrophages attached to αSMA-positive activated HSCs seemed not to be changed with increasing cholesterol content and CA administration ( Fig.4A) . Triple-IHC demonstrated the microenvironmental interactions between macrophages, activated HSCs, and CD3-positive lymphocytes ( Fig.4C) .

The red arrowheads show perisinusoidal fibrosis, and the yellow arrowheads show pericellular fibrosis. Scale bars = 10 µm.

(A) Representative double-IHC staining of Iba-1 and αSMA-positive cells and CD163 and αSMA-positive cells in each group. The black arrowheads show lipid droplets in activated HSCs. The yellow arrowheads show Iba-1-positive macrophages attached to αSMA-positive activated HSCs, and CD163-positive macrophages attached to αSMA-positive activated HSCs. (B) Dot plots of the activated HSC number. Horizontal lines in the dot plots indicate median values. (C) Representative triple-IHC staining of Iba-1-, αSMA-, and CD3-positive cells in the group fed a high-fat/high-cholesterol diet with cholic acid. The red arrowheads show CD3-positive cells.
In this study, we investigated the changes in macrophage characteristics using µMPs fed a high-fat/high-cholesterol diet with or without CA as an animal model of NAFLD. We found that the expression of CD163/CD204 and the proportions of CD163-positive macrophages and CD204-positive macrophages among Iba-1-positive macrophages were reduced in the HcD (1.5% cholesterol) with CA group. CD163 and CD204, known as M2 markers, are expressed in normal tissue-resident macrophages (liver Kupffer cells) 14, 15) . In particular, the scavenger receptor SR-A (CD204) is generally known to be an essential component for foam cell formation 16) . Anti-inflammatory M2 macrophages have been reported to be more sensitive to foam cell formation than inflammatory M1 macrophages 17) , indicating that foam cell formation occurs predominantly in anti-inflammatory M2 macrophages. Scavenger receptors bind and internalize modified plasma low-density lipoproteins (LDL) and high-density lipoproteins, causing cholesterol ester accumulation in the macrophages. M2 macrophages then become pro-inflammatory (M1-like phenotype) upon exposure to oxidized LDL 18, 19) . It has been reported that exposure to oxidized LDL induced M1-like polarization by suppressing Krüppel-like factor 2 17) . In addition, macrophages that newly invade the liver due to the release of chemotactic factors are monocyte-derived CD163-negative cells 8, 14) . Therefore, it may be considered that the ratio of M2-like macrophages decreased not only because M2-like macrophages (Kupffer cells) changed their polarity to be M1-like, but also because monocytes that expressed low levels of CD163 and CD204 invaded.
The contribution of macrophages to the development of NAFLD has been reported by many groups 4) . In this study, high-fat diets (12% lard) with cholesterol were fed, and lard consists of fatty acids. Excess lipids (fatty acids and cholesterol) are responsible for the pathogenesis of NAFLD 2, 7) . Excess lipids can contribute to the formation of lipotoxic lipids in hepatocytes, which leads to ER stress, oxidative stress, and inflammasome activation 2) . These processes cause hepatic injury, and the production of cytokines (e.g., IL-1β, IL-6, IL-18, tumor necrosis factor-α, and transforming growth factor-β) 2) and macrophage-recruiting chemokines (e.g., CCL2 and CXCL10) 20) . Activated macrophages and HSCs also produce various chemokines for increasing macrophage recruitment 21) . Macrophages are activated into M1-like macrophages by the uptake of lipids 17- 19, 22, 23) , and the activated M1-like macrophages produce pro-inflammatory cytokines that can cause hepatocyte steatosis and hepatocellular damage 2) . M2-like macrophages produce anti-inflammatory mediators (e.g., IL-10) 24, 25) , and are considered to play a protective role in the development of metabolic diseases 8) . In atherosclerosis, M2-like macrophages are dominant at the early stage, and the macrophages preferentially shift to the M1-like phenotype at the late stage 26, 27) . In other words, the decreasing proportion of M2-like macrophages due to CA administration may reflect the progression toward a NASH-like liver condition. The development of NAFLD may occur as a consequence of an imbalance between M1-like and M2-like activated macrophages in the liver.
We demonstrated that there was an increased number of αSMA-positive activated HSCs and an increased number of Iba-1-positive macrophages attached to HSCs ( Fig.4A and B) . HSCs are the cells most responsible for fibrogenesis. Perisinusoidal collagen deposition is a histological finding of NASH. Injured or stressed hepatocytes and activated macrophages induce the activation of HSCs into collagen-producing myofibroblasts 3) . By double-IHC, we demonstrated the enhanced crosstalk between Iba-1-positive macrophages and activated HSCs; however, the crosstalk between CD163-positive macrophages and activated HSCs was not enhanced ( Fig.4A and B) . CD163-negative cells consist of Kupffer cells with a pro-inflammatory phenotype, and monocytes recruited from the bone marrow may be responsible for the activation of HSCs and act as pro-fibrotic macrophages 28) . Macrophages and HSCs are closely related to inflammatory cells, such as lymphocytes and neutrophils, in the pathogenesis of NAFLD 29- 31) , and we could show the microenvironmental interactions by triple-IHC ( Fig.4C) .
We recently established a swine model for the investigation of atherosclerosis and NAFLD 12, 32) . Although foamy macrophages were found in groups fed high-fat/high-cholesterol diets, hepatocyte ballooning was found only in the group supplemented with CA in this study ( Fig.1A) . Hepatocyte ballooning is one of the most important pathological findings of NASH 33- 35) . Bile acids are essential for absorbing lipids, and CA is the best among the bile acids in promoting the absorption of cholesterol 33) ; therefore, CA supplementation is important for producing an animal model of NAFLD, and we can reproduce the pathological condition of NAFLD in CA-supplemented µMPs. Regarding the direct effect of CA administration, CA is a hydrophilic bile acid that is considered to have relatively low cytotoxicity when compared to hydrophobic bile acids 36, 37) . In in vitro experiments, it was reported that bile acid itself had no hepatotoxicity 36) . Taken together with these results, the administration of the hydrophilic bile acid CA may not directly contribute to the pathophysiology of NAFLD, although it may indirectly influence the pathophysiology. To clarify this point, the inclusion of an NcD with CA group or HcD (0.2%/0.5% cholesterol) with CA group is needed to rule out the direct effects of CA, and this issue is a limitation of the current study.
In conclusion, we demonstrated the characteristics of macrophages in the µMP model of NAFLD, and the observed phenotypic change of macrophages was consistent with that in human NAFLD. µMPs fed a high-fat/high-cholesterol diet with CA supplementation represent a useful animal model for the elucidation of the pathogenesis of NAFLD, and for the development of novel therapies.
This work was supported in part by a grant from the Kodama Memorial Fund for Medical Research, Kagoshima, Japan (to S.Y.), Health Labour Sciences research Grant (No. 33361105) from the Ministry of Health, Labour and Welfare of Japan (to A.T.), the Adaptable and Seamless Technology Transfer Program Grant (A-Step No. AS2316907E) from the Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan (to A.T. and H.K.), the SENSHIN Medical Research Foundation (to H.K.), and KAKENHI (16H05162, to Y.K.).
The authors are grateful to Ms Takana Motoyoshi (K.I. Stainer Inc.) for assistance with immunostaining analysis.
We have no conflicts of interest to declare.