2022 Volume 29 Issue 1 Pages 82-98
2022 Volume 29 Issue 1 Pages 82-98
Aim: Feedback activation of factor XI (FXI) by thrombin is believed to play a critical role in the amplification phase of thrombin generation and to contribute to thrombosis development and hemostasis. However, the activation of FXI by thrombin has been shown in vitro to require a cofactor. In this study, the role of thrombin in activated FXI (FXIa) formation in vivo is investigated.
Methods: The study population comprised probands in whom coagulation activation was triggered by low-dose (15 µg/kg) recombinant activated factor VII (rFVIIa, n=89), of whom 34 with (VTE+) and 45 without a history of venous thromboembolism (VTE−), and patients undergoing major orthopedic surgeries (n=45). FXIa was quantified via an enzyme capture assay using a monoclonal FXI-specific antibody. Thrombin formation was monitored using an oligonucleotide-based enzyme capture assay and the thrombin activation markers prothrombin fragment 1+2 (F1+2) and thrombin–antithrombin complex (TAT).
Results: In the rFVIIa cohort, FXIa and thrombin remained below their lower limit of quantification of 3.48 and 1.06 pmol/L, respectively. By contrast, during the surgeries, median FXIa levels increased from 3.69 pmol/L pre-operatively to 9.41 pmol/L mid-operatively (P=4·10−4) and remained significantly elevated 24 h thereafter, with 9.38 pmol/L (P=0.001). Peak levels of F1+2 were comparable in the VTE+, VTE−, and surgery cohort (235, 268, and 253 pmol/L), whereas peak TAT levels were higher in the surgery cohort (53.1, 33.9, and 147.6 pmol/L).
Conclusions: Under in vivo conditions, the activation of FXI requires specific local features that are present at the wounded site including potential cofactors of thrombin.
Activated factor XI (FXIa) is the active serine protease of the zymogen factor XI (FXI), a homodimeric 160 kDa protein that circulates in human plasma at concentrations of 3–7 µg/mL (15–45 nM) 1- 3) . The original cascade/waterfall model of coagulation proposed that FXI is converted to FXIa by activated factor XII (FXIIa) in the intrinsic pathway of coagulation, and the formed FXIa then promotes clotting by activating factor IX 4, 5) . Although this mechanism of contact activation is used in diagnostic blood coagulation assays (e.g., activated partial thromboplastin time, aPTT), the absence of abnormal bleeding associated with hereditary deficiencies of factor XII (FXII) indicates that this is not an essential mechanism for physiological hemostasis 6, 7) . However, there is growing evidence from animal models that the FXIIa-mediated activation of FXI contributes to the development of thrombosis 8- 11) .
Unlike FXII deficiency, FXI deficiency (hemophilia C) is associated with a lifelong, albeit relatively mild, bleeding disorder, supporting the premise that FXI contributes to hemostasis independent from FXIIa 12, 13) . It has been shown in vitro that thrombin can convert FXI to FXIa in the presence of dextran sulfate 14) , polyphosphate 15- 17) , and other negatively charged substances through a template mechanism in which FXIa and thrombin bind on a polyanion in proximity to each other to achieve maximum rates of FXI activation 3) . Based on these results, a positive feedback mechanism that amplifies thrombin formation after FXIa has been generated via trace amounts of thrombin is assumed 14) . Furthermore, FXIa indirectly protects the clot from lysis through the activation of thrombin-activatable fibrinolysis inhibitor via thrombin by amplifying thrombin generation 18, 19) . However, the identity of the protease that activates FXI in vivo has not been established so far 3) .
Recent evidence that suggests that the contact pathway may play a significant role in the development of thrombosis via thrombus stabilization and growth promotion has led to the investigation of FXI and FXIa as potential new drug targets to achieve safer anticoagulation 20, 21) . Novel pharmacologic strategies that target FXI and FXIa include antisense oligonucleotides and monoclonal antibodies, which act by blocking the activation or activity of the coagulation factor, and aptamers and small molecules that block the active site or induce allosteric modulation of the protein 22, 23) .
In this study, the role of the thrombin–FXIa feedback loop is further investigated by studying the relationship between thrombin generation and the amount of circulating FXIa in two different clinical scenarios of hemostasis activation. In the first clinical model, systemic thrombin formation was induced via the administration of low-dose recombinant activated factor VII (rFVIIa) to healthy volunteers and patients with thrombophilic risk factors. We have recently shown, using this stimulated hemostasis activity pattern evaluation (SHAPE) approach, different patterns of procoagulant and anticoagulant responses depending on the presence of hereditary thrombophilia risk factors and the patients’ history of venous thromboembolism (VTE) 24, 25) . The second cohort comprised patients undergoing elective orthopedic surgeries. The surgical trauma induces localized but high-level thrombin formation as has been recently demonstrated 26) . In both clinical models, FXIa plasma levels were measured using a newly established enzyme capture assay (ECA) and correlated with the in vivo thrombin generation kinetics.
Human FXIa, corn trypsin inhibitor (CTI), the monoclonal anti-human FXI antibody AHXI-5061, and the polyclonal sheep anti-human FXI antibody PAHFXI-SAP were obtained from Haematologic Technologies (Essex Junction, VT, USA). The horseradish peroxidase-conjugated polyclonal rabbit anti-sheep antibody PO163 HRO was purchased from Dako (Glostrup, Denmark). Human α-thrombin was purchased from CellSystems (St. Katharinen, Germany). Argatroban (Argatra®) was obtained from Mitsubishi Pharma (Düsseldorf, Germany). The fluorogenic thrombin (Boc-Asp(OBzl)-Pro-Arg-AMC) and FXIa (Boc-Glu(OBzl)-Ala-Arg-AMC) peptide substrates were obtained from Bachem (Bubendorf, Switzerland). rFVIIa (NovoSeven®) was purchased from Novo Nordisk (Bagsværd, Denmark). Benzamidine, dextran sulfate sodium salt, and general chemicals were purchased from Sigma-Aldrich (Munich, Germany). Biotinylated aptamers were synthesized and purified from Microsynth (Balgach, Switzerland). Platelet-rich plasma (PRP) for in vitro thrombin generation experiments and platelet-poor normal pooled plasma (NPP) were prepared by mixing equal volumes of citrated plasma obtained from healthy donors. Citrate tubes (10.5 mmol/L final concentration, Sarstedt) additionally contained CTI (20 µg/mL final concentration) for thrombin generation experiments. PRP and platelet-poor plasma (PPP) were obtained via centrifugation (1,500×g, 2 min, and 2,600×g, 10 min, respectively) of citrated whole blood. PPP was used to dilute PRP to obtain 150,000 platelets/µL.
FXIa ECAThe FXIa ECA was conducted in the microtiter plate format using white Maxisorp Fluoronunc microtiter modules (Nunc A/S, Roskilde, Denmark). Wells were sealed during incubation times with adhesive polyester film (Platemax®, Axygen, Union City, CA, USA) and stored in the dark. For washing, wells were generally rinsed three times with 300 µL of washing buffer (phosphate-buffered saline (PBS), 0.05% Tween 20, pH 7.4) using an automated plate washer (ELx50, Biotek, Bad Friedrichshall, Germany). Wells were initially coated with 4 µg/mL of the mouse monoclonal anti-FXI-antibody AHXI-5061 in a coating buffer (30 mmol/L Na2CO2, 200 mmol/L NaHCO3, pH 9.0). After incubation at 4℃ overnight, wells were washed, subsequently blocked (PBS, 20 mg/mL (bovine serum albumin (BSA), pH 7.4), and emptied plates stored at −20℃ until used. For running the assay, samples and calibrators were added to the wells (100 µL). Plasma-based calibration curves covering a concentration range from 0 to 20 ng/mL FXIa (0–125 pmol/L) were processed in parallel. After incubation for 2 h at room temperature, samples and calibrators were removed from the wells using an eight-channel pipette and fresh tips for each column to prevent carry-over contamination during automated washing. Then, 250 µL of PBS washing buffer was manually added to the wells, and the modules were washed using the automated procedure. Subsequently, 100 µL of the fluorogenic FXIa substrate solution (100 µmol/L) was added to the wells, and baseline fluorescence intensities were measured using a plate fluorescence reader (FLx-800, Bio-Tek, Bad Friedrichshall, Germany). Changes in fluorescence over time were taken as the measure of FXIa captured in the wells. Data obtained from the calibrators were interpolated using a four-parameter curve fit and utilized to calculate the FXIa concentration in the samples. All samples were assayed in triplicate. If not otherwise stated, plasma samples were tested at a dilution of 1:10, at which the inactive zymogen FXI did not affect FXIa measurement. PBS containing 1 mg/mL BSA, 100 mmol/L benzamidine, and 25 mmol/L sodium citrate was used as a dilution buffer. The supplemental data describe thoroughly the development of the basic assay configuration, preanalytical stability, and assay performance studies.
In Vitro Monitoring of FXIa GenerationFXI deficient plasma was obtained from seven hemophilia C patients. Supplemental Table 1 lists the patient characteristics and underlying mutations for FXI deficiency. Experiments with thrombin were conducted using plasma that had been defibrinated by reptilase (1:10) for 1 h at 37℃. A solution that contains thrombin and dextran sulfate was added (1:5) to achieve final concentrations of thrombin of 5 µg/mL (136 nmol/L) or 500 ng/mL (13.6 nmol/L) and of dextran sulfate of 5 µg/mL in the reaction mixture in order to initiate thrombin-induced FXIa generation. FXIa generation via contact activation was initiated in citrated plasma by adding aPTT reagent (Actin FS, Siemens Healthcare Diagnostics, Marburg, Germany) in a dilution of 1:5 without recalcification, thereby excluding a contribution of thrombin to FXIa generation. At pre-defined intervals, aliquots were removed from the reaction mixture, and FXIa generation was stopped by mixing them with equal volumes of PBS (pH 7.4) containing 1 mg/mL BSA, 200 mmol/L benzamidine, and 50 mmol/L sodium citrate. This mixture was further diluted 1:4 in dilution buffer to achieve the 1:10 dilution of citrated plasma required for FXIa measurement.
| No. | FXI, % | Age, years | Sex | F11 gene mutation | Protein change |
|---|---|---|---|---|---|
| 1 | 8 | 28 | M | c.[1443delT];[901T> C] | p.Ile481Metfs*4;Phe301Leu |
| 2 | 52 | 55 | M | c.[976C> T];[ = ] | p.Arg326Cys |
| 3 | 63 | 27 | F | c.[41C> T];[ = ] | p.Thr14Ile |
| 4 | 51 | 46 | M | c.[943G> A];[ = ] | p.Glu315Lys |
| 5 | 36 | 9 | F | c.[943G> A];[ = ] | p.Glu315Lys |
| 6 | 45 | 9 | F | c.[943G> A];[ = ] | p.Glu315Lys |
| 7 | 39 | 59 | F | c.[1489C> T];[ = ] | p.Arg497* |
M, male; F, female.
For western blot analysis, reaction mixtures of FXI, thrombin, and dextran sulfate were incubated at 37℃ in PBS buffer for 10 min before adding benzamidine (100 mmol/L final concentration). Samples were subjected to reduced sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted onto a polyvinylidene difluoride membrane. FXI and FXIa were detected using the polyclonal sheep anti-human FXI antibody PAHFXI-SAP and horseradish peroxidase-conjugated secondary antibody PO163 HRO in combination with a chemiluminescence-based detection kit (Millipore Immobilon, Merck, Darmstadt, Germany).
In Vitro Thrombin GenerationAll in vitro thrombin generation experiments were conducted in PRP, and measurements were conducted as described elsewhere 27, 28) : Thrombin generation was monitored using the calibrated automated thrombogram (CAT) assay using standard reagents (Thrombinoscope, Maastricht, NL), with the modification of using rFVIIa instead of tissue factor (TF) to initiate thrombin generation. Thrombin generation curves were described in terms of lag time (time until the first measurement of a thrombin concentration above baseline), time-to-peak thrombin concentration, peak thrombin concentration, and area under the curve (AUC) (endogenous thrombin potential, ETP).
Study Population and Blood SamplingThe VTE- subgroup included healthy blood donors (Factor V Leiden (FVL) and prothrombin (FII) 20210G>A non-carriers) and carriers of the FVL or FII 20210G>A mutations, who were prospectively recruited from our blood donation service and the thrombophilia outpatient clinic of our institution, respectively. The subjects in the VTE+ subgroup were also recruited from the thrombophilia outpatient clinic. Table 1 shows the details on recruitment, inclusion/exclusion criteria, laboratory screening tests, and drop-outs. After an overnight fast, a bolus of 15 µg/kg rFVIIa was injected intravenously. Blood samples were obtained immediately before and 10 min, 30 min, and 1, 2, 3, 5, and 8 h after rFVIIa administration from a new venipuncture of an antecubital vein using 21-gauge winged infusion sets (Sarstedt, Nümbrecht, Germany).
|
Subgroup (subjects in per protocol population) |
VTE- (n = 15) | VTE- (n = 30) | VTE+ (n = 34) |
|---|---|---|---|
| Source of recruitment | Healthy blood donors | Patients of thrombophilia outpatient clinic | |
| Inclusion criteria | Body mass index 18-27 kg/m² and adequate peripheral vein status | ||
| History of VTE† | |||
| Exclusion criteria | Antiplatelet or anticoagulant drugs within 2 weeks preceding blood sampling, arterial cardiovascular or malignant diseases, acute or chronic infections, pregnancy and breast feeding | ||
| Screening tests required to be negative | Transaminases, γ-glutamyl transferase, urea, creatinine in serum, tests for deficiencies of antithrombin, protein C, protein S‡ | ||
| Drop-outs, n§ | 8 | 3 | 0 |
VTE-, no history of venous thromboembolism; VTE+, history of venous thromboembolism.
†Additional inclusion criteria in the VTE+ group were, that the VTE occurred at least 6 months ago and in the absence of situational high-risk
factors (surgery, trauma, immobilization, pregnancy, puerperium).
‡Healthy blood donors were additionally screened for the factor V Leiden and prothrombin 20210G> A mutation, and were required to be non-
carriers, while the mutation carrier status of the outpatients was already known at inclusion.
§Three VTE- healthy volunteers and 3 VTE- factor V Leiden or prothrombin 20210G> A mutation carriers were excluded due to abnormalities in the laboratory screening, 5 VTE- healthy volunteers withdrew consent before administration of recombinant activated factor VII (rFVIIa). All remaining 79 included subjects completed administration of rFVIIa and follow-up blood sampling.
The surgery cohort included patients undergoing elective orthopedic surgery and has been described in a previous report 26) . Blood samples were obtained 2 h before and 24 h after surgery from a new venipuncture of an antecubital vein using 21-gauge winged infusion sets (Sarstedt, Nümbrecht, Germany). During the surgeries, samples were obtained from a 21-gauge indwelling venous cannula (B. Braun, Melsungen, Germany), maintained patent by continuous infusion of physiological sodium chloride solution, at the incision and final suture of the skin and at the middle of the surgeries. All patients received 40 mg enoxaparin subcutaneously 4–8 h post-operatively.
In both cohorts, blood was drawn into citrate tubes (10.5 mmol/L final concentration, Sarstedt) that additionally contained argatroban (100 µmol/L final concentration) for thrombin measurement and benzamidine (50 mmol/L final concentration) for FXIa measurement. Plasma samples were obtained by centrifugation (2,600×g, 10 min) and subsequently stored at less than −40℃.
In Vivo Coagulation MonitoringFXIa in plasma was quantified by ECA as described above. Thrombin was measured using an oligonucleotide-based enzyme capture assay (OECA) that has been initially described by Müller et al. 29) and applied in other studies 25, 26, 30- 32) . Briefly, Maxisorp Fluoronunc microtiter modules were initially coated with 10 µg/mL of BSA (100 µl/well). After incubation at 4℃ overnight, wells were washed, and a solution of 10 µg/mL streptavidin was added and incubated for 1 h at room temperature. Subsequently, wells were washed, blocked with 2% BSA for 2 h at room temperature, and emptied plates stored at −20℃ until used. For running the thrombin–OECA, 1 pmol 3’-biotinylated aptamers HD1-22 was located in the wells (100 µl/well). After washing, plasma samples and calibrators, which covered a 1/2-log10 concentration range from 0 to 10 ng/mL (0–272 pmol/L) thrombin, were added to the designated wells. After incubation and washing, the fluorogenic thrombin substrate was added. Changes in fluorescence over time were taken as the measure of thrombin captured in the wells. Data obtained from the calibrators were interpolated by a four-parameter curve fit and used to calculate the thrombin concentration in the samples. All samples were assayed in triplicate.
The STACLOT VIIa-rTF assay (Stago, Asnières sur Seine, France) was used to determine the plasma concentration of activated factor VII (FVIIa). Plasma levels of prothrombin fragment 1+2 (F1+2) and thrombin–antithrombin complex (TAT) were determined using the Enzygnost F1+2 (monoclonal) assay and the TAT microassay, respectively (both Siemens Healthcare Diagnostics Products, Marburg, Germany). The activity levels of prothrombin, factor VII (FVII), FXI, protein C (PC), antithrombin (AT), and the plasma concentration of d-dimer were determined using the BCS XP system (Siemens Healthcare Diagnostics, Eschborn, Germany) together using corresponding reagents and controls.
In the VTE− subjects of the SHAPE cohort, who were recruited from healthy blood donors ( Table 1) , only FXIa and thrombin were newly measured, whereas the measurement results of the other laboratory parameters had been obtained and reported previously 25) . In all other subjects of the SHAPE cohort, who were recruited from thrombophilia outpatients (VTE− and VTE+), all laboratory data were newly obtained. In the surgery cohort, all laboratory measurement results had been obtained and reported previously 26) , except for the FXIa concentration in plasma, which was newly measured.
StatisticsIn vitro data are generally presented as mean and standard deviation. Data obtained from the study population are presented as the median and interquartile range (IQR). Depending on the distribution of data, an analysis of variance or a Kruskal–Wallis test was conducted, followed by an unpaired Student t-test or Mann–Whitney test, to compare the data obtained in the two subgroups of probands with and without a history of VTE in the SHAPE cohort. The Wilcoxon signed-rank test was used to compare values measured at different sampling time points in both the surgery cohort and the SHAPE cohort. The Bonferroni method was applied to correct for multiple testing. H.R. analyzed the data. All coauthors had access to primary data.
Ethics StatementThe Institutional Review Board and Ethics committee of the University Hospital of Bonn approved the present study. All study participants provided written informed consent in accordance with the Declaration of Helsinki.
In a first series of experiments, we evaluated the ability of the FXIa ECA to monitor FXIa generation induced by thrombin or through the contact system in vitro. Western blot analysis revealed that thrombin requires a cofactor such as dextran sulfate to become an effective FXI activator ( Fig.1A) . Evaluation of different concentrations of the cofactor dextran sulfate of up to 100 µg/mL demonstrated that the highest amount of FXIa was generated, if FXI, thrombin, and dextran sulfate were applied in equal concentrations of 100 ng/mL in the reaction mixture ( Fig.1B) . The application of both higher and lower concentrations of dextran sulfate induced the generation of smaller amounts of FXIa in plasma. We applied thrombin and dextran sulfate at final concentrations of 5 µg/mL each to induce FXIa generation in citrated plasma assuming an FXI concentration in normal human plasma of approximately 5 µg/mL. Control experiments were conducted with defibrinated NPP that contained the contact phase inhibitor CTI at a concentration of 100 µg/mL to exclude contact activation of FXI in this design. Vice versa the thrombin inhibitor argatroban was added at a final concentration of 190 µmol/L to specifically inhibit thrombin-induced FXI activation. FXIa formation was completely abolished in the presence of argatroban whereas the inhibition of contact activation had no effect on FXIa generation over time ( Fig.1B-C) . Fig.1D shows typical FXIa generation curves, induced by different concentrations of thrombin in the presence of dextran sulfate. The AUC was 5.7±0.9 nmol·min·L−1 when a thrombin concentration of 5 µg/mL (136 nmol/L) and a concentration of dextran sulfate of 5 µg/mL were applied. With 270.5±45.9 nmol·min·L−1, an approximately 50-fold greater AUC was obtained by the contact activation of FXI ( Fig.1E) . Plasma samples of seven patients with hereditary FXI deficiency and FXI levels ranging between 8% and 63% were analyzed to study the dose–response relationship between FXI and FXIa formation. Fig.1F shows that there was a strong linear correlation between AUC and FXI level for thrombin-induced FXI activation (r=0.982) and contact activation (r=0.968).

(A) Western blot analysis of reaction mixtures containing FXI, thrombin (FIIa), and dextran sulfate (DS, 40 µg/mL final concentration each). (B) Dependence of thrombin-induced activation of FXI on dextran sulfate. Dextran sulfate was used at indicated concentrations; thrombin and FXI, at a final concentration of 100 ng/mL each. Columns and error bars show mean and standard deviation (SD), respectively. (C) FXIa generation in defibrinated NPP (98% FXI activity) was induced by thrombin (5 µg/mL=136 nM final concentration) and dextran sulfate (5 µg/mL final concentration). Benzamidine (100 mmol/L final concentration) was added to the reaction mixture at the indicated time points. NPP contained CTI or argatroban where indicated. FXIa concentration was determined by ECA. Columns and error bars show mean and SD, respectively. (D) FXIa generation in defibrinated NPP was induced by thrombin at final concentrations of 136 nM (
 , red line) or 13.6 nM (
, red line) or 13.6 nM (
 , green line) and dextran sulfate (DS, 5 µg/mL final concentration), benzamidine was added at the indicated time points. Data points and error bars show mean and SD, respectively. (E) FXIa generation in NPP was induced by adding aPTT reagent (Actin FS, 1:5) to NPP. Data points and error bars show mean and SD, respectively. (F) FXIa generation in plasma from patients with hereditary FXI deficiency. FXIa generation was induced by aPTT reagent (
, green line) and dextran sulfate (DS, 5 µg/mL final concentration), benzamidine was added at the indicated time points. Data points and error bars show mean and SD, respectively. (E) FXIa generation in NPP was induced by adding aPTT reagent (Actin FS, 1:5) to NPP. Data points and error bars show mean and SD, respectively. (F) FXIa generation in plasma from patients with hereditary FXI deficiency. FXIa generation was induced by aPTT reagent (
 , blue line) or thrombin and dextran sulfate (
, blue line) or thrombin and dextran sulfate (
 , red line). Data points show relative changes of the area under the curve (AUC) in relation to FXI plasma levels. Linear regression curves and Pearson correlation coefficients are shown.
, red line). Data points show relative changes of the area under the curve (AUC) in relation to FXI plasma levels. Linear regression curves and Pearson correlation coefficients are shown.
In Vitro Thrombin Generation Induced by rFVIIa
In vitro thrombin generation was induced by different concentrations of rFVIIa, ranging from 0.25 to 32 µg/mL, and monitored via CAT to study if a rFVIIa dose of 15 µg/kg induced the formation of a sufficiently high amount of thrombin to potentially generate FXIa in vivo. The concentration of rFVIIa of 0.25 µg/mL corresponded to plasma levels of rFVIIa that were observed in probands 10 min after the administration of a rFVIIa dose of 15 µg/kg 24, 25) . With increasing doses of rFVIIa, the mean lag time shortened from 44 min for 250 ng/mL rFVIIa to 8 min for 32 µg/mL rFVIIa ( Fig.2A) , the peak thrombin concentration increased from 85 to 203 nmol/L ( Fig.2B) , the ETP increased from 1,144 to 1,842 nmol·min·L−1 ( Fig.2C) , and the time to peak shortened from 65 to 16 min ( Fig.2D) . Thus, applying a rFVIIa dose of 15 µg/kg, a peak thrombin concentration that had been shown to induce the generation of quantifiable amounts of FXIa in the presence of dextran sulfate in vitro was achieved ( Fig.1D) .
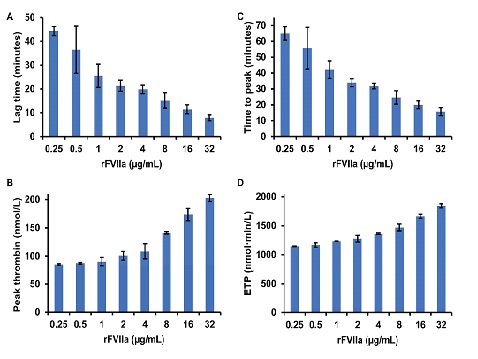
(A) Lag time, (B) peak thrombin concentrations, (C) time to peak thrombin concentration, and (D) endogenous thrombin potential (ETP) were measured in normal pooled platelet-rich plasma (PRP, 150,000 platelets/µL) by calibrated automated thrombography (CAT) using rFVIIa at the indicated final concentrations to initiate thrombin generation. Citrated whole blood, from which PRP was obtained, contained corn trypsin inhibitor (CTI) at a final concentration of 20 µg/mL. Columns and error bars show the mean and standard deviation of three independent experiments.
The SHAPE cohort comprised 79 subjects, thereof 34 with (VTE+) and 45 without a history of VTE (VTE−). The surgery cohort included 35 patients without a history of VTE. Surgical procedures included nucleotomy (n=13), arthroscopic intervention (n=8), osteosynthesis (n=5), implant removal (n=5), and total hip arthroplasty (n=4), Age, body mass index (BMI), and proportion of males were higher in the surgery patients than in the SHAPE group, but they were similar in the VTE− and VTE+ subgroups of the SHAPE cohort ( Table 2) . In the SHAPE cohort, 67% of the VTE− subjects and 62% of the VTE+ subjects were carriers of either the FVL or the prothrombin 20210G>A mutation, whereas the patients in the surgery cohort were not tested for the presence of these mutations.
| Surgery, n = 35 | SHAPE | ||
|---|---|---|---|
| VTE-, n = 45 | VTE+, n = 34 | ||
| Age, years | 50±17 | 36±12 | 39±12 |
| Sex (male/female), n | 21/14 | 15/30 | 12/22 |
| BMI, kg/m2 | 26.4±2.6 | 22.5±3.0 | 24.1±2.0 |
| FVL carriers, n | ND | 17 (3†) | 9 (1) |
| FII 20210G> A carriers, n | ND | 13 (2†) | 12 |
Data for age and body mass index (BMI) are presented as mean and standard deviation. FII, prothrombin; ND, not determined; VTE-, no history of venous thromboembolism; VTE+, history of venous thromboembolism; SHAPE, stimulated hemostasis activity pattern evaluation.
†thereof homozygous carriers.
The activity levels of prothrombin, FVII, FXI, PC, and AT were within normal ranges in all study participants. Table 3 summarizes the results of hemostasis activity markers measured at baseline. Median FXIa levels in the SHAPE cohort and thrombin levels in all cohorts were below quantifiable ranges. With 3.69 pmol/L FXIa median plasma levels of FXIa slightly above the lower limit of quantification of 3.48 pmol/L in the surgery cohort. The median plasma levels of the activation markers of coagulation were below the established upper normal limits of F1+2 (340 pmol/L), TAT (41.5 pmol/L), and d-dimer (0.5 mg/L) in all cohorts.
| Cohort, subjects |
Reference data n = 15† |
Pre- vs. mid-operative plasma levels Surgery, n = 35 |
SHAPE baseline vs. peak plasma levels | |
|---|---|---|---|---|
| SHAPE VTE-, n = 45 | SHAPE VTE+, n = 34 | |||
| FXIa, pmol/L | <3.48 (<3.48 - <3.48) | 3.69 (<3.48 - 9.25) vs. | <3.48 (<3.48 - <3.48) vs. | <3.48 (<3.48 - 3.62) vs. |
| 9.41 (5.47 - 15.94)** | <3.48 (<3.48 - 4.49) | <3.48 (<3.48 - 3.67) | ||
| F1+2, pmol/L | 168 (106 – 225) | 144 (118 - 171) vs. | 171 (127 - 216) vs. | 200 (126 - 293) vs. |
| 253 (183 - 357) ** | 235 (182 - 303) ** | 268 (198 - 355) ** | ||
| Thrombin, pmol/L | <1.06 (<1.06 - <1.06) | <1.06 (<1.06 - 1.17) vs. | <1.06 (<1.06 - <1.06) vs. | <1.06 (<1.06 - <1.06) vs. |
| 9.96 (4.39 - 30.68) ** | <1.06 (<1.06 - 1.14) | <1.06 (<1.06 - 1.21) | ||
| TAT, pmol/L | <21.3 (<21.3 - <21.3) | 33.5 (24.4 - 48.1) vs. | <21.3 (<21.3 - 25.7) vs. | <21.3 (<21.3 - 29.5) vs. |
| 147.6 (78.5 - 266.8) ** | 33.9 (23.0 - 54.6) ** | 53.1 (35.1 - 74.8) ** | ||
| d-dimer, mg/L | 0.29 (0.24 – 0.34) | 0.36 (0.29 - 0.68) vs. | 0.29 (0.22 - 0.39) vs. | 0.34 (0.26 - 0.49) vs. |
| 0.58 (0.32 - 1.13) | 0.36 (0.26 - 0.48) * | 0.41 (0.31 - 0.52) * | ||
Data are presented as median and interquartile range. The Wilcoxon signed-rank test was used to compare values measured at different sampling time points, and statistically significant differences in comparison to baseline are denoted by *(P<0.05) and **(P<0.005).
VTE-, no history of venous thromboembolism; VTE+, history of venous thromboembolism; SHAPE, stimulated hemostasis activity pattern evaluation.
†Reference data were obtained from a cohort of 15 healthy volunteers, that has been described in a previous publication, in which the shown measurement results of F1+2, TAT, and d-dimer have been reported 25).
The application of rFVIIa was well tolerated by all subjects, and no adverse events of any kind were encountered during the observation period. The pharmacokinetic profiles of rFVIIa were essentially identical in study participants with and without a history of VTE with median (IQR) peak levels of FVIIa of 0.23 (0.19–0.26) µg/mL and 0.22 (0.16–0.28) µg/mL, respectively ( Supplemental Fig.6) .

Plasma levels of FVIIa were measured before (t=0) and after IV injection of 15 µg/kg rFVIIa in 45 subjects with (
 ) and 34 subjects without a history of venous thromboembolism (●).
) and 34 subjects without a history of venous thromboembolism (●).
Fig.3 shows the courses of thrombin generation rates and plasma levels of FXIa in response to low-dose rFVIIa, and Table 3 lists peak plasma levels. Median plasma levels of F1+2 increased from 171 pmol/L at baseline to peak levels of 235 pmol/L in study participants without a history of VTE and from 200 to 268 pmol/L in the VTE+ subgroup ( Fig.3A) , and median TAT levels increased from <21.3 to 33.9 pmol/L in VTE− subjects and from <21.3 to 53.1 pmol/L in the VTE+ subgroup (P<10−4 each) ( Fig.3B) . The intervals between baseline and peak levels of TAT (but not of F1+2) were significantly greater in subjects with a history of VTE than in those without (P=0.010). Despite this increase of indirect markers of thrombin formation, median plasma levels of thrombin and FXIa remained unchanged and below their respective quantifiable limits after administration of rFVIIa ( Fig.3C) . After rFVIIa administration, a slight but statistically significant increase of d-dimer levels was observed, from 0.29 to 0.36 mg/L ( P=0.021) in the VTE− subgroup and from 0.34 to 0.41 mg/L ( P=0.028) in the VTE+ subgroup ( Fig.3D) .

Plasma levels of (A) F1+2, (B) TAT, (C) thrombin and FXIa, and (D) d-dimer were measured before (t=0), and after intravenous injection of 15 µg/kg rFVIIa. The courses of biomarker levels in probands with (
 , n=35, VTE+) and without (
, n=35, VTE+) and without (
 , n=45, VTE−) a history of VTE are shown. Data points and error bars show the median and interquartile range, respectively. The Wilcoxon signed-rank test was used to compare values measured at different sampling time points, and arrows indicate a significant increase (P<0.05) from baseline to peak values. The Mann–Whitney test was used to compare the increase of parameters in the two subgroups VTE+ and VTE−, and the associated P value is shown for TAT. The increase in TAT levels was greater in probands with a history of VTE than in asymptomatic study participants, whereas the increase of F1+2 and d-dimer did not vary significantly between VTE+ and VTE− subgroups.
, n=45, VTE−) a history of VTE are shown. Data points and error bars show the median and interquartile range, respectively. The Wilcoxon signed-rank test was used to compare values measured at different sampling time points, and arrows indicate a significant increase (P<0.05) from baseline to peak values. The Mann–Whitney test was used to compare the increase of parameters in the two subgroups VTE+ and VTE−, and the associated P value is shown for TAT. The increase in TAT levels was greater in probands with a history of VTE than in asymptomatic study participants, whereas the increase of F1+2 and d-dimer did not vary significantly between VTE+ and VTE− subgroups.
Surgical trauma-induced coagulation activation was characterized by a rapid and long-lasting increase of FXIa levels. With 7.75 (3.48–10.59) pmol/L, FXIa levels were higher at the time of incision than at baseline, but the difference was statistically not significant ( Fig.4) . During the surgeries and at the time of suture, FXIa levels increased further and were significantly higher in comparison with baseline, with 9.41 (5.47–15.94) pmol/L (P=0.001) and 10.13 (5.47–15.41) pmol/L (P=0.009), respectively. With 9.38 (5.28–9.38) pmol/L, they remained significantly elevated at 24 h after the surgeries (P=0.001). Spearman analysis revealed a significant and positive correlation between FXIa and the other hemostasis biomarkers that was strong for TAT (rs=0.794, P<10−6), moderate for F1+2 (rs=0.649, P=2·10−5) and thrombin (rs=0.604, P=2·10−4), and weak for d-dimer (rs=0.392, P=0.023) ( Fig.5) . Table 3 shows the median (IQR) plasma levels of these parameters and of FXIa during the surgeries in the overall cohort. In comparison with baseline, F1+2 increased from 144 to 253 pmol/L; thrombin, from <1.06 to 9.96 pmol/L; and TAT, from 33.5 to 147.6 pmol/L (P<10−4 each), whereas the changes in d-dimer levels did not reach statistical significance.

Plasma levels of FXIa were measured at the indicated time points in patients ( n=35) undergoing orthopedic surgery. The boxes show quartiles and the median of the data, the whiskers extend up to 1.5 times the TIQR from the box, and circles show outlying data values. The Wilcoxon signed-rank test was used to compare values measured at different sampling time points, and P values of the comparisons between baseline and the other time points are shown. For the calculation of the shown P values, the Bonferroni method was applied to correct for multiple testing and was conducted for four comparisons.
NS, not significant.

(A) F1+2, (B) thrombin, (C) TAT, and (D) d-dimer were measured in patients (n=35) undergoing orthopedic surgery. Linear regression curves and Spearman rank correlation coefficients are shown with associated P values.
The dynamics of FXIa and thrombin formation were monitored in parallel in a clinical model of low-level systemic thrombin formation and during surgical hemostasis to investigate the role of thrombin in the activation of FXI during in vivo hemostasis.
We established an ECA that allows sensitive and specific determination of FXIa to measure plasma levels of FXIa. With a lower limit of quantification of 3.48 pmol/L, the analytical sensitivity of the FXIa ECA is comparable with the sensitivity reported for a thrombin generation-based assay that uses an inhibitory anti-FXIa antibody to achieve FXIa specificity 33, 34) . In contrast to the thrombin generation-based FXIa assay, the FXIa ECA allows the addition of benzamidine to prevent inhibition of FXIa by endogenous inhibitors and thereby improves the preanalytical stability of FXIa. We conducted initial experiments in a purified system to evaluate the practicability of the FXIa ECA for monitoring of FXIa formation. Mixing of plasma-purified FXI with thrombin did not result in a time- or concentration-dependent generation of FXIa as confirmed by FXIa ECA and western blot analysis. The formation of FXIa was initiated by adding dextran sulfate to the reaction mixture. Maximal FXI activation rates were achieved at equal concentrations of thrombin, FXI, and dextran sulfate. This finding is in line with previous reports showing that thrombin requires cofactors such as negatively charged macromolecules to become an efficient FXI activator 14- 17) . However, even under optimized conditions, compared with contact phase-catalyzed FXI activation, the thrombin activation of FXI was on an approximately 50-fold lower level. The most probable explanation for this difference is that in the aPTT reagent approach, the activation of the contact phase induces a continuous and longer-lasting FXIa formation, whereas in the thrombin approach, the initially added thrombin is rapidly inactivated by endogenous inhibitors 30) and is thus not able to induce further activation of FXI.
We measured plasma levels of FXIa after the induction of systemic thrombin formation through the administration of low-dose rFVIIa to study if in vivo thrombin formation can trigger FXIa activation. In in vitro thrombin generation experiments, the rFVIIa dose of 15 µg/kg, which was applied in the SHAPE approach, was shown to induce the generation of thrombin amounts that were sufficient to in turn induce the generation of quantifiable amounts of FXIa in the presence of the cofactor dextran sulfate. Although the SHAPE approach induced thrombin formation, as indicated by an increase of the thrombin markers F1+2 and TAT, an increase of the plasma concentration of free thrombin above the lower limit of quantification was not observed. This can be explained by the rapid inhibition of formed thrombin, predominantly by complex formation with antithrombin, as indicated by the observed increase of TAT levels. The marker of thrombin formation F1+2 and that of thrombin inactivation TAT have significantly longer plasma half-lives than that of free thrombin, with 2 h for F1+2 and 44 min for TAT 35) , which additionally facilitates their detection. FXIa levels also remained below quantifiable limits. Even in the cohort of patients with a history of VTE, in whom the thrombin marker response to rFVIIa was significantly greater than in VTE− subjects, FXIa levels remained below this threshold. These data suggest that the higher thrombin response rates in VTE+ patients do not depend on the presence of significant amounts of FXIa.
The situation was different in the surgery cohort of our study. The median FXIa plasma level before surgery of 3.69 pmol/L was comparable with that obtained in a previous study through thrombin generation-based measurement of FXIa in healthy controls of 2.7 (IQR: 1.6–4.2) pmol/L 33) . However, repeated measurements during and after surgery showed an increase of FXIa levels in plasma, which became statistically significant mid-operatively, reaching levels of 9.38–10.13 pmol/L. These data agree well with previous studies in which median FXIa levels of 5.7–6.8 pmol/L were measured in patients with injuries of varying severity without shock 36) , and mean FXIa levels of 10.6 pmol/L were measured in patients after penetrating trauma 37) , clinical situations comparable with surgical trauma. Besides the increase of FXIa levels, there was an increase of free thrombin and indirect markers of thrombin activation in the surgery cohort that has been described and discussed thoroughly in a previous report 26) . In plasma samples obtained mid-operatively, FXIa concentrations correlated well with those of free thrombin, F1+2, and TAT, suggesting an association between formation rates of thrombin and FXIa. This represents further in vivo evidence for the role of thrombin as the physiological activator of FXI that has been suggested based on in vitro studies 14, 15, 38) .
The difference in the FXIa response between the SHAPE cohort and the cohort of surgery patients can be explained by differences between the two cohorts including age, BMI, and female-to-male ratio that promote a higher basal level of coagulation activation in the surgery cohort. Most likely, however, the absence of quantifiable plasma levels of FXIa after administration of rFVIIa can be explained by the cofactor dependence of thrombin activation of FXI. Possible cofactors are polyphosphate released from activated platelets, RNA and DNA that are released from the damaged tissue, or, most importantly, TF that becomes exposed to blood because of the tissue damage caused by surgical procedures 15, 39- 41) . However, there are alternative explanations on how local conditions at the surgery site could explain the observation of higher FXIa levels in comparison with the rFVIIa cohort. The local concentration of thrombin at the surgical wound could be high enough to surpass a threshold for generating quantifiable amounts of FXIa. This assumption is supported by higher levels of free thrombin and TAT in the surgery cohort. Further alternative explanations could be the presence of other proteases than thrombin at the wound site that could induce activation of FXI, or inhibitors of FXIa could be present at lower levels, thus resulting in higher levels of circulating FXIa.
The activity of FXIa in plasma is controlled by a variety of plasma serpins including antithrombin, C1-inhibitor, protease nexin 1, and protein Z-dependent protease inhibitor limiting its circulatory half-life to approximately 2.5 h 2, 33) . Despite this strong inhibitory pressure, increased levels of FXIa were measured in the surgery cohort not only during but also 24 h after surgery. This indicates ongoing FXIa formation even after the formation of a wound-sealing clot. Moreover, it may indicate that FXIa contributes to the postoperative procoagulant state and supports an anticoagulant approach that targets FXIa 20, 42) .
So far, two experimental drugs that follow this anticoagulant approach were studied in randomized phase II clinical trials in the indication of postoperative prophylaxis of VTE. The efficacy and safety of FXI-ASO (ISIS 416858), an antisense oligonucleotide that specifically reduces FXI levels, were compared with those of enoxaparin in patients undergoing knee arthroplasty 22) . In this study, a regimen of 200 mg FXI-ASO was non-inferior to 40 mg of enoxaparin once daily, whereas a 300 mg regimen was superior, and FXI ASO appeared to be safe with respect to the risk of bleeding. In the FOXTROT study, osocimab, a monoclonal antibody against FXIa, was compared with enoxaparin in a study population of 813 patients undergoing knee arthroplasty 43) . In this study, postoperative osocimab showed non-inferiority to enoxaparin, whereas preoperative osocimab was superior to enoxaparin regarding the incidence of VTE. Further studies are warranted to establish the efficacy and safety of these drugs relative to standard thromboprophylaxis.
The FXIa ECA used in this study represents an alternative to existing methods of FXIa measurement in plasma. The application of this assay to monitor the FXIa response during surgery showed a significant and long-lasting increase of FXIa plasma levels, whereas rFVIIa-induced thrombin formation was not associated with an FXIa increase. These in vivo data strengthen the hypothesis that thrombin-catalyzed activation of FXI requires a cofactor, thereby supporting in vitro data, although higher amounts of thrombin being generated during surgery might partly explain the discrepant FXIa responses. Further investigation is required to determine a potential cofactor since we did not measure platelet polyphosphate or other cofactor candidates.
This work was supported by a grant from the Stiftung Hämotherapie-Forschung (Hemotherapy Research Foundation).
None.
The monoclonal anti-human factor XI antibody AHXI-5061 was incubated in the wells of microtiter modules, and unbound molecules were removed by washing, in order to evaluate different loading concentrations of the FXIa capture antibody. At different FXIa concentrations between 1 and 100 ng/mL, stable hydrolysis rates of the fluorogenic substrate were achieved at AHXI-5061 loading concentrations above 1 µg/mL ( Supplemental Fig.1) . Based on these results, captured antibody loading concentrations of 4 µg/mL were utilized in all further experiments.

The monoclonal anti-human factor XI antibody AHXI-5061 was incubated in the wells of microtiter modules at the indicated concentrations and unbound molecules removed. FXIa was added at final concentrations of 100 ng/mL (●), 10 ng/mL (◯), and 1 ng/mL (▽) in buffer (PBS, 1 mg/mL BSA, pH 7.4) and incubated at room temperature. FXIa captured in the wells was measured through hydrolysis rates of the fluorogenic FXIa substrate Boc-Glu(OBzl)-Ala-Arg-AMC.
A quenching strategy using a reversible active site inhibitor is required to avoid the preanalytical inactivation of FXIa by endogenous inhibitors. The initial evaluation of different concentrations of benzamidine on FXIa activity in buffer showed that a benzamidine concentration of 100 mmol/L was high enough to temporarily block the active site of FXIa ( Supplemental Fig.2A) without significant impairment of FXIa detection ( Supplemental Fig.2B) . A final benzamidine concentration of 100 mmol/L was used in all further experiments.

A FXIa measurement in homogenous solution. The reversible protease inhibitor benzamidine was added to buffer (PBS, 1 mg/mL BSA, pH 7.4) at the indicated concentrations and mixtures spiked with FXIa to achieve final concentrations of 2 ng/mL. FXIa was measured through hydrolysis rates of the fluorogenic FXIa substrate Boc-Glu(OBzl)-Ala-Arg-AMC. B FXIa measurement using the ECA configuration. Buffer spiked with benzamidine at the indicated concentrations and FXIa at final concentrations of 2 ng/mL was incubated in the wells of microtiter modules loaded with the FXIa capture antibody AHXI-5061 at concentrations of 4 µg/mL. FXIa captured in the wells was measured through hydrolysis rates of the fluorogenic FXIa substrate.
Plasma samples with endogenous FXI levels ranging from 64% to 111% were spiked with benzamidine (100 mmol/L final concentration) and FXIa (4 ng/mL final concentration) to evaluate the influence of the zymogen FXI on FXIa measurement results. The ECA was conducted after the dilution of the preparations 1:4 and 1:10, respectively. Supplemental Fig.3 shows that recovery rates of FXIa were not influenced by endogenous FXI levels at a dilution factor of 1:10. In another experiment, the comparison of recovery rates of an FXIa serial dilution series in FXI-deficient plasma and in NPP demonstrated that FXIa was detectable at each dilution point when diluted 1:4 and 1:10. Nevertheless, only the 1:10 diluted samples showed similar results within the analyzed sample matrices ( Supplemental Fig.4) . Plasma samples were diluted 1:10 in all further experiments based on these results.

Benzamidine-primed plasma samples containing the indicated concentrations of FXI were spiked with FXIa (4 ng/mL final concentration) and diluted in dilution buffer (PBS, 1 mg/mL BSA, 100 mmol/L benzamidine, 25 mmol/L sodium citrate, pH 7.4) 1:4 (◯) and 1:10 (●), respectively. Recovery rates of FXIa were measured using the ECA.

FXIa was diluted in buffer (▼), FXI deficient plasma (◯), and NPP (●) yielding the indicated concentrations. The mixtures were further diluted 1:4 (A) or 1:10 (B) in dilution buffer (PBS, 1 mg/mL BSA, 100 mmol/L benzamidine, 25 mmol/L sodium citrate, pH 7.4) before analysis.
Correspondingly drawn whole blood was spiked with FXIa to achieve a final concentration of 10 ng/mL to evaluate the FXIa-stabilizing capacity of the benzamidine-citrate blood sampling buffer. Subsequently, the preparation was split and stored at room temperature or at 4℃. Aliquots were removed over time, centrifuged, and obtained plasma stored at −40℃ until the ECA was conducted. Testing of the FXIa quenching efficiency of the benzamidine-sodium citrate buffer in whole blood revealed that >95% of FXIa activity was preserved during 30 min of storage on ice. Storage at room temperature decreased the recovery rate to approximately 80%. In processed plasma, >95% of FXIa activity could be preserved during a storage time of 12 h on ice and 1 h at room temperature ( Supplemental Fig.5A) . When plasma samples were immediately diluted 1:10 in dilution buffer, no loss of FXIa activity was observed even after a storing time of 48 h at room temperature ( Supplemental Fig.5B) . These data demonstrated that FXIa activity was preserved in samples during processing and incubation times of the FXIa ECA.

A FXIa was added to benzamidine-primed citrate-anticoagulated whole blood (▼ stored at room temperature, ● stored at 4℃) or citrated plasma (▽ stored at room temperature, ◯ stored at 4℃). At the indicated time points, FXIa plasma levels were measured using the enzyme capture assay. B FXIa was added to benzamidine-primed citrated plasma. The mixtures were diluted 1:10 in dilution buffer (PBS, 1 mg/mL BSA, 100 mmol/L benzamidine, 25 mmol/L sodium citrate, pH 7.4) and frozen at the indicated time points (●), or diluted 1:10 in dilution buffer immediately and frozen at the indicated time points (◯).
Hexaplicates of NPP-based calibrators were measured, and the limit of detection (LOD) and the lower limit of quantification (LLOQ) were esteemed as the concentrations corresponding to changes in fluorescence of 3 and 9 times the standard deviation of the blanks, respectively, to determine the LOD and the LLOQ of FXIa. The determinations were repeated on different days and calculated mean values defined as the LOD and LLOQ of the assay. The LOD was determined as 0.931 pmol/L, whereas the LLOQ was calculated as 3.481 pmol/L. Plasma samples containing FXIa at concentrations ranging from 1 to 10 pmol/L were measured in triplicates in individual runs, repeated on three different days, to evaluate the reproducibility of the assay. Supplemental Table 2 that within- and between-run coefficients of variation did not exceed 17% even at the lowest concentrations tested.
| FXIa input concentration, pmol/L | Mean within-run CV, %±SD | Between-run CV, % |
|---|---|---|
| 10 | 5.6±2.3 | 8.5 |
| 2.5 | 12.2±8.6 | 13.8 |
| 1 | 11.0±6.7 | 17.1 |
CV, coefficient of variation; SD, standard deviation.