Abstract
Aim: In this study, we aim to examine the clinical meaning of low-density lipoprotein cholesterol (LDL-C) <70 mg/dL as assessed by Friedewald equation [LDL-C (F)] and Martin method [LDL-C (M)] and non-high-density lipoprotein cholesterol (HDL-C) <100 mg/dL on the occurrence of new lesions among Japanese patients with stable angina who underwent percutaneous coronary intervention (PCI) and were prescribed with strong statins.
Methods: Among the 537 consecutive stable angina patients who had underwent PCI and had been prescribed with strong statins, the association between the occurrence of new lesions with myocardial ischemia at the 9-month follow-up coronary angiography and ≤ 2 years after PCI and baseline characteristics were assessed.
Results: New lesions appeared 9 months and ≤ 2 years after PCI in 31 and 90 patients, respectively. Multivariate logistic regression analysis revealed diabetes mellitus (DM) was significantly associated with the occurrence of new lesions ≤ 2 years after PCI [odds ratio (OR) 1.71, 95 % confidence interval (CI) 1.06–2.83, p=0.031], and only non-HDL-C ≥ 100 mg/dL was associated with the occurrence of new lesions both at 9 months and ≤ 2 years after PCI [OR 1.80, 95 % CI 1.10–3.00, p=0.021 and OR 1.85, 95 % CI 1.13–3.07, p=0.016].
Conclusions: Non-HDL-C ≥ 100 mg/dL was determined to be the independent risk factor for the occurrence of new lesions 9 months and ≤ 2 years after PCI among stable angina patients with strong statins. Residual risk after PCI should be considered by assessing not only DM but also non-HDL-C beyond the scope of LDL-C-lowering therapy with strong statins.
Introduction
Coronary artery disease (CAD) has been identified as a major cause of cardiovascular deaths worldwide1). Percutaneous coronary intervention (PCI) has become a standard procedure for coronary revascularization among patients with CAD. Since the introduction of second-generation drug-eluting stents (second DES), the need for target lesion revascularization has decreased. However, despite recent clinical advances in PCI and medical therapies2), cardiovascular events have remain to be a primary problem.
Dyslipidemia is known to be significantly associated with an increased risk of CAD, and the relationship between dyslipidemia and CAD has already been well established3-5). Since clinical trials have demonstrated that decreases in serum levels of low-density lipoprotein cholesterol (LDL-C) can reduce the rate of cardiovascular events6, 7), lipid-lowering therapy represents an important strategy in primary and secondary prevention of CAD8, 9). In particular, LDL-C has been recommended as the main treatment target of lipid management in CAD patients10-12). Therefore, clinical practice guidelines recommend a target LDL-C of <70 mg/dL for high-risk patients9, 13). In Japan, LDL-C <100 mg/dL is the secondary prevention target, and <70 mg/dL is recommended for high-risk patients14), but there is still residual cardiovascular event risk even after achieving it.
LDL-C is generally measured by simply using Friedewald equation method, but this method bears disadvantage as it is not applicable to patients with triglycerides (TG) >400 mg/dL15). Martin et al. reported a novel method for estimating LDL-C that appears to provide more accurate measurements of LDL-C than values derived using Friedewald equation16). The LDL-C estimation by Martin method was calculated by applying an adjustable factor for the TG/very low-density lipoprotein cholesterol (VLDL-C) ratio based on TGs and non-high-density lipoprotein cholesterol (HDL-C) concentrations. In addition, non-HDL-C, which is easily calculated without using an estimate for measurement, is also reportedly associated with cardiovascular events as a numerical value representing remnants such as VLDL-C, which is considered as another arteriosclerotic factor alongside LDL-C.
It appears that there is yet no reports that have evaluated the effects of LDL-C as assessed using the Martin method [LDL-C (M)] or non-HDL-C on the occurrence of new lesions on coronary angiography among Japanese patients with stable angina receiving treatment with strong statins.
Aim
This study aims to investigate the clinical meaning of LDL-C <70 mg/dL as assessed using Friedewald equation (LDL-C (F)) and LDL-C (M) and non-HDL-C <100 mg/dL on the occurrence of new lesions in Japanese stable angina patients who had undergone PCI and had been prescribed with strong statins
Methods
Study Population
We evaluated a cohort study of 621 consecutive stable angina patients from a single center who had been receiving treatment with strong statins for >2 weeks on admission and who had successfully undergone elective PCI using second DES [durable polymer everolimus-eluting stent (DP-EES), Resolute zotarolimus-eluting stent (R-ZES), and biodegradable polymer biolimus-eluting stent (BP-BES)] and imaging modality (intravascular ultrasound or optical coherence tomography) for complete revascularization at Kagoshima University Hospital between January 2010 and December 2018. These patients underwent 9-month follow-up coronary angiography after PCI. All patients were administered strong statin regardless of dyslipidemia and dual antiplatelet therapy (aspirin and thienopyridine, as either ticlopidine or clopidogrel) before the PCI. ST-segment elevation myocardial infarction (STEMI) patients, non-STEMI patients, unstable angina patients, and hemodialysis patients were excluded from this study. Of the 621 patients, 23 patients were excluded because of lacking sufficient blood test items on admission or a fasting serum TG level >400 mg/dL. Furthermore, 61 patients who could not be tracked after discharge were also excluded (Fig.1). Baseline demographic data and cardiovascular risk factors such as current smoker, diabetes mellitus (DM), and hypertension were recorded at the time of enrolment.
This study was approved by the Research and Ethics Committee at Kagoshima University Hospital and was carried out in accordance with the ethical principles stated in the 1975 Declaration of Helsinki. All patients provided written informed consent prior to enrolment.
In all, 537 patients (New lesion (+) group, n=90; New lesion (−) group, n=447) fulfilled the study criteria. PCI, percutaneous coronary intervention; DES, drug-eluting stents
Measurements
In this study, blood tests and echocardiograms were conducted prior to PCI. Blood samples were drawn after 12 h of fasting. Thereafter, concentrations of hemoglobin, high-sensitivity C-reactive protein (hs-CRP), fasting plasma glucose (FPG), total cholesterol (TC), HDL-C, TG, uric acid, and creatinine were measured. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation with coefficients modified for Japanese patients, as follows: eGFR (ml/min/1.73 m2)=194×serum Cr (mg/dL)−1.094 ×age (years)−0.287 (×0.739 for female subjects)17). Meanwhile, LDL-C (F) was calculated using the Friedewald equation as follows: (TC − HDL-C − TG/5)15). LDL-C (M) was calculated as reported by Martin et al.16) using the equation TC − HDL-C − TG/adjustable factor, where the adjustable factor was determined from strata-specific median TG/VLDL-C ratios derived from a dataset of 900,605 individuals in the United States. Non-HDL-C was calculated using the equation TC − HDL-C.
Definitions
Body mass index was calculated as body weight divided by height squared (kg/m2). Hypertension was defined based on the following criteria: systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or the use of antihypertensive medications. DM was defined based on the following criteria: FPG >126 mg/dL, HbA1c ≥ 6.5 % (in accordance with the National Glycohemoglobin Standardization Program), or the use of antihyperglycemic medications. Dyslipidemia was defined as LDL-C (F) ≥ 140 mg/dL, TG ≥ 150 mg/dL, HDL-C< 40 mg/dL, or the use of antidyslipidemic medications. Current smokers were defined as those who were actively smoking at the time of admission.
As per the findings of the PATROL trial18), it was determined that the efficacy and safety of atorvastatin (10 mg/day), rosuvastatin (2.5 mg/day), and pitavastatin (2 mg/day) are the same in Japanese. Therefore, strong statins were defined as doses of atorvastatin (10 mg/day) or rosuvastatin (2.5 mg/day) or pitavastatin (2 mg/day) or more than these doses in this study.
The clinical practice guideline of Japan Atherosclerosis Society has recommended that LDL-C <70 mg/dL is the secondary prevention target for familial hypercholesterolemia, acute coronary syndrome, or DM with high-risk factors for CAD recurrence14). In this study, we used the cut-off level of LDL-C <70 mg/dL, as DM patients with chronic kidney disease, metabolic syndrome, or overlap of major risk factors or who are smokers were 58 %, and all patients underwent PCI. In addition, current recommendations set the goal of non-HDL-C as 30 mg/dL higher than the corresponding LDL-C goals19-20). Based on the cut-off levels of 70 mg/dL, 70 mg/dL and 100 mg/dL for LDL-C (F), LDL-C (M), and non-HDL-C, respectively, patients were then divided.
The new lesions at 9-month follow-up coronary angiography were defined as the lesion showing ≥ 75 % stenosis on angiography; moreover, there had myocardial ischemia estimated by fractional flow reserve or myocardial perfusion single-photon emission computed tomography. Furthermore, the occurrence of new lesions ≤ 2 years after PCI was defined as the incidences of clinically driven non-target lesion revascularization after PCI including new lesions on 9-month follow-up coronary angiography.
All patients were followed up at our hospital or by their physician; they continued to take strong statins during follow-up. In this study, we examined the relationship between the occurrence of new lesions leading to myocardial ischemia and the baseline characteristics of patients.
Statistical Analysis
Quantitative data are presented as mean±standard deviation for data showing normal distributions, while median and interquartile range (IQR) was utilized for data showing non-normal distributions. Fisher’s exact test was then used to compare the incidence of categorical variables, with the latter expressed as percentages. Continuous variables were compared between the New lesion (+) and New lesion (−) groups using Student’s t-test (normal distribution) or the Wilcoxon rank-sum test (non-normal distribution). Logistic regression analysis was also performed to analyze the relationship between the New lesion (+) group and background factors, with the results expressed as odds ratios (OR) and 95 % confidence intervals (CIs). Independent associations between the New lesion (+) group and baseline characteristics were assessed by multivariate logistic regression modeling using relevant factors. Variables with p<0.05 on univariate analysis were used in the multivariate analysis model. Values of p<0.05 were considered indicative of statistical significance. Statistical analyses were performed using SAS software (JMP version 14.0).
Results
Baseline Characteristics
Median follow-up duration was 1160 days. Baseline patient clinical characteristics have been provided in Table 1. The median values of TC, LDL-C (F), LDL-C (M), HDL-C, non-HDL-C, and TG were 155 (136–181) mg/dL, 80 (63–100) mg/dL, 82 (67–105) mg/dL, 47 (40–58) mg/dL, 103 (86–128) mg/dL, and 109 (82–155) mg/dL, respectively.
Table 1.
Baseline characteristics of study patients at PCI
| Variables |
Overall (n = 537)
|
| Age, years |
69 [64–76] |
| Sex: male, n (%)
|
392 (73) |
| Body mass index, kg/m2
|
23.8 [21.5–26.2] |
| Risk factors, n (%)
|
|
| Hypertension |
438 (82) |
| Diabetes mellitus |
313 (58) |
| Dyslipidemia |
408 (76) |
| Current smoker |
63 (12) |
| History of smoking |
81 (15) |
| Medication, n (%)
|
|
| Oral anticoagulation |
65 (12) |
| Calcium-channel blocker |
229 (43) |
| ACEI |
101 (19) |
| ARB |
225 (42) |
| β-blocker |
185 (34) |
| Ezetimibe |
40 (7) |
| Nitrates |
50 (9) |
| Proton pump inhibitor |
262 (49) |
| Laboratory data |
|
| Hemoglobin, g/dL |
12.9±0.1 |
| hs-CRP, mg/L |
1.2 [0.4–3.2] |
| TC, mg/dL |
155 [136–181] |
| LDL-C (F) ≥ 70 mg/dL, n (%)
|
348 (65) |
| LDL-C (M) ≥ 70 mg/dL, n (%)
|
372 (69) |
| HDL-C <40 mg/dL, n (%)
|
133 (25) |
| Non-HDL-C ≥ 100 mg/dL, n (%)
|
304 (57) |
| TG ≥ 150 mg/dL, n (%)
|
141 (26) |
| Uric acid, mg/dL |
5.9 [4.9–6.8] |
| FPG, mg/dL |
110 [96–133] |
| eGFR, mL/min/1.73 m2
|
61.4 [47.5–75.2] |
| LVEF, 50% |
58.3 [48.7–66.2] |
Values are shown as number and percentage or median with interquartile range. Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; LVEF, left ventricular ejection fraction; LDL-C, low-density lipoprotein cholesterol; PCI, percutaneous coronary intervention; TC, Total cholesterol; TG, Triglyceride.
The comparison of baseline characteristics of study patients according to the occurrence of new lesions 9 months and ≤ 2 years after PCI is shown in Table 2. Thirty-one patients (6 %) were determined to have new lesions at 9-month follow-up coronary angiography after PCI. Furthermore, of the 537 patients recruited, 90 patients (17 %) showed new lesions and were taken as the New lesion (+) group, with the remaining 447 patients (83 %) taken as having no new lesions [New lesion (−) group] ≤ 2 years after PCI. There were 45 (8 %) patients with in-stent restenosis.
Table 2.
Comparison of baseline characteristics of study patients according to occurrence of new lesions 9-month and ≤ 2 years after PCI
| Variables |
New lesion (+) 9-month |
New lesion (+) ≤ 2 years |
|
New lesion (+) group
(n = 31)
|
New lesion (-) group
(n = 506)
|
p value
|
New lesion (+) group
(n = 90)
|
New lesion (-) group
(n = 447)
|
p value
|
| Age, years |
67 [60–76] |
70 [64–77] |
0.58 |
66 [59–75] |
70 [64–77] |
0.006 |
| Sex: male, n (%)
|
22 (71) |
370 (73) |
0.83 |
67 (74) |
325 (73) |
0.73 |
| Body mass index, kg/m2
|
24.3 [20.9–25.9] |
23.8 [21.5–26.1] |
0.78 |
23.8 [21.6–26.3] |
23.9 [21.5–26.1] |
0.52 |
| Risk factors, n (%)
|
|
|
|
|
|
|
| Hypertension |
26 (84) |
412 (81) |
0.71 |
73 (81) |
365 (82) |
0.90 |
| Diabetes mellitus |
19 (61) |
294 (58) |
0.85 |
62 (69) |
251 (56) |
0.025 |
| Dyslipidemia |
22 (71) |
386 (76) |
0.52 |
68 (76) |
340 (76) |
0.92 |
| Current smoker |
2 (6) |
61 (12) |
0.36 |
13 (14) |
50 (11) |
0.36 |
| History of smoking |
18 (58) |
63 (12) |
<0.001 |
18 (20) |
63 (14) |
0.20 |
| Medication, n (%)
|
|
|
|
|
|
|
| Oral anticoagulation |
6 (19) |
59 (12) |
0.25 |
13 (14) |
52 (12) |
0.46 |
| Calcium-channel blocker |
18 (58) |
211 (42) |
0.98 |
42 (47) |
187 (42) |
0.40 |
| ACEI |
4 (13) |
97 (19) |
0.48 |
14 (16) |
87 (19) |
0.39 |
| ARB |
18 (58) |
207 (41) |
0.064 |
39 (43) |
186 (42) |
0.76 |
| β-blocker |
20 (66) |
165 (33) |
<0.001 |
31 (34) |
154 (34) |
0.99 |
| Ezetimibe |
1 (3) |
39 (8) |
0.72 |
6 (7) |
34 (8) |
0.99 |
| Nitrates |
4 (13) |
46 (9) |
0.52 |
7 (8) |
43 (10) |
0.69 |
| Proton pump inhibitor |
18 (58) |
257 (51) |
0.46 |
48 (53) |
214 (48) |
0.34 |
| Laboratory data |
|
|
|
|
|
|
| Hemoglobin, g/dL |
12.9±1.8 |
13.0±2.0 |
0.47 |
13.2±1.8 |
12.9±2.0 |
0.179 |
| hs-CRP, mg/L |
1.6 [0.6–2.4] |
1.2 [0.4–3.3] |
0.65 |
1.7 [0.6–3.7] |
1.1 [0.4–3.1] |
0.159 |
| TC, mg/dL |
174 [141–196] |
154 [135–178] |
0.028 |
168 [140–197] |
153 [135–176] |
0.001 |
| LDL-C (F) ≥ 70 mg/dL, n (%)
|
25 (81) |
323 (64) |
0.040 |
68 (76) |
280 (63) |
0.019 |
| LDL-C (M) ≥ 70 mg/dL, n (%)
|
26 (84) |
346 (68) |
0.048 |
71 (79) |
301 (67) |
0.030 |
| HDL-C <40 mg/dL, n (%)
|
7 (23) |
126 (25) |
0.68 |
25 (28) |
108 (24) |
0.47 |
| Non-HDL-C ≥ 100 mg/dL, n (%)
|
23 (74) |
281 (56) |
0.030 |
63 (70) |
241 (54) |
0.005 |
| TG ≥ 150 mg/dL, n (%)
|
8 (26) |
133 (26) |
0.99 |
25 (28) |
116 (26) |
0.69 |
| Uric acid, mg/dL |
6.2 [4.7–6.6] |
5.9 [5.0–6.8] |
0.70 |
6.1 [4.8–6.7] |
5.9 [5.0–6.8] |
0.99 |
| FPG, mg/dL |
106 [91–129] |
111 [96–134] |
0.108 |
116 [95–135] |
109 [96–133] |
0.31 |
| eGFR, mL/min/1.73 m2
|
65.4 [56.5–76.9] |
61.1 [47.2–74.7] |
0.135 |
64.9 [50.9–77.8] |
59.7 [47.2–74.1] |
0.065 |
| LVEF, % |
59 [50–66] |
58[49–66] |
0.94 |
57.7 [50.8–66.2] |
58.3 [47.9–66.2] |
0.76 |
Abbreviations are as in Table 1
The rate of history of smoking and the use of β-blockers were significantly higher in the New lesion (+) 9-month group than that in the New lesion (−) 9-month group (58 % vs. 12 %; p<0.001 and 66 % vs. 33 %; p<0.001). Frequencies of LDL-C (F) ≥ 70 mg/dL, LDL-C (M) ≥ 70 mg/dL, and non-HDL-C ≥ 100 mg/dL were significantly higher in the New lesion (+) 9-month group compared with the New lesion (−) 9-month group [LDL-C (F) ≥ 70 mg/dL, 81 % vs. 64 %, p=0.040; LDL-C (M) ≥ 70 mg/dL, 84 % vs. 68 %, p=0.048; non-HDL-C ≥ 100 mg/dL, 74 % vs. 55 %, p=0.030, respectively]
The New lesion (+) ≤ 2 years group was significantly younger compared to the New lesion (−) ≤ 2 years group (median, 66 years, IQR 59–75 years vs. median, 70 years, IQR 64–77 years; p=0.006). Frequency of DM was determined to be significantly higher in the New lesion (+) ≤ 2 years group compared with the New lesion (−) ≤ 2 years group (69 % vs. 56 %; p=0.025). Furthermore, frequencies of LDL-C (F) ≥ 70 mg/dL, LDL-C (M) ≥ 70 mg/dL, and non-HDL-C ≥ 100 mg/dL were found to be significantly higher in the New lesion (+) ≤ 2 years group than those in the New lesion (−) ≤ 2 years group [LDL-C (F) ≥ 70 mg/dL, 76 % vs. 63 %, p=0.019; LDL-C (M) ≥ 70 mg/dL, 79 % vs. 67 %, p=0.030; non-HDL-C ≥ 100 mg/dL, 70 % vs. 54 %, p=0.005, respectively].
Influence of Baseline Characteristics on New Lesions
Univariate logistic regression analysis was performed to examine the impact of background factors on the occurrence of new lesions 9 months and ≤ 2 years after PCI (Table 3). These analyses revealed that history of smoking, usage of β-blocker, and non-HDL-C ≥ 100 mg/dL were associated with the New lesion (+) 9-month group [history of smoking: OR 9.71, 95 % CI 4.57–21.20, p<0.001; using of β-blocker: OR 3.75, 95 % CI 1.78–8.29, p<0.001; non-HDL-C ≥ 100 mg/dL: OR 2.30, 95 % CI 1.05–5.58, p=0.047]. Furthermore, age, DM, LDL-C (F) ≥ 70 mg/dL, LDL-C (M) ≥ 70 mg/dL, and non-HDL-C ≥ 100 mg/dL were determined to be associated with the New lesion (+) ≤ 2 years group [age: OR 0.97, 95 % CI 0.95–0.99, p=0.020; DM: OR 1.73, 95 % CI 1.08–2.84, p=0.027; LDL-C (F) ≥ 70 mg/dL: OR 1.84, 95 % CI 1.11–3.15, p=0.021; LDL-C (M) ≥ 70 mg/dL: OR 1.81, 95 % CI 1.07–3.19, p=0.032; non-HDL-C ≥ 100 mg/dL: OR 1.99, 95 % CI 1.24–3.29, p=0.006] (Table 3).
Table 3.
Univariate logistic analysis for the occurrence of new lesions 9-month and ≤ 2 years after PCI
|
New lesion (+) 9-month N (%) = 31 (6) |
New lesion (+) ≤ 2 years N (%) = 90 (17) |
| OR |
95%CI |
p value
|
OR |
95%CI |
p value
|
| Age, years |
0.98 |
0.95–1.02 |
0.39 |
0.97 |
0.95–0.99 |
0.020 |
| Sex: male |
1.11 |
0.48–2.40 |
0.79 |
1.09 |
0.66–1.86 |
0.73 |
| Body mass index, kg/m2
|
0.99 |
0.90–1.09 |
0.94 |
1.04 |
0.98–1.10 |
0.20 |
| Risk factors |
|
|
|
|
|
|
| Hypertension |
1.18 |
0.48–3.52 |
0.73 |
0.96 |
0.55–1.77 |
0.90 |
| Diabetes mellitus |
1.14 |
0.55–2.47 |
0.73 |
1.73 |
1.08–2.84 |
0.027 |
| Dyslipidemia |
1.31 |
0.56–2.84 |
0.50 |
1.02 |
0.58–1.71 |
0.92 |
| Current smoker |
1.99 |
0.58–12.51 |
0.35 |
1.36 |
0.68–2.55 |
0.36 |
| History of smoking |
9.71 |
4.57–21.20 |
<0.001 |
1.52 |
0.83–2.67 |
0.16 |
| Medication |
|
|
|
|
|
|
| Oral anticoagulation |
1.81 |
0.65–4.35 |
0.21 |
1.28 |
0.64–2.40 |
0.45 |
| Calcium-channel blocker |
1.93 |
0.93–4.11 |
0.08 |
1.22 |
0.77–1.92 |
0.40 |
| ACEI |
0.62 |
0.18–1.64 |
0.39 |
0.76 |
0.40–1.37 |
0.39 |
| ARB |
2.00 |
0.96–4.25 |
0.06 |
1.07 |
0.68–1.69 |
0.76 |
| β-blocker |
3.75 |
1.78–8.29 |
<0.001 |
1.00 |
0.61–1.60 |
0.99 |
| Ezetimibe |
0.39 |
0.02–1.94 |
0.37 |
0.86 |
0.32–1.98 |
0.75 |
| Nitrates |
0.67 |
0.25–2.36 |
0.48 |
0.79 |
0.32–1.71 |
0.58 |
| Proton pump inhibitor |
0.74 |
0.35–1.54 |
0.43 |
0.80 |
0.50–1.26 |
0.35 |
| Laboratory data |
|
|
|
|
|
|
| Hemoglobin, g/dL |
0.98 |
0.81–1.18 |
0.81 |
1.08 |
0.96–1.22 |
0.192 |
| hs-CRP, mg/L |
0.98 |
0.92–1.01 |
0.37 |
0.99 |
0.98–1.01 |
0.51 |
| TC, mg/dL |
1.01 |
0.99–1.02 |
0.054 |
1.01 |
1.00–1.02 |
0.003 |
| LDL-C (F) ≥ 70 mg/dL |
2.36 |
1.01–6.44 |
0.064 |
1.84 |
1.11–3.15 |
0.021 |
| LDL-C (M) ≥ 70 mg/dL |
2.40 |
0.98–7.21 |
0.078 |
1.81 |
1.07–3.19 |
0.032 |
| HDL-C <40 mg/dL |
1.13 |
0.50–2.91 |
0.77 |
1.21 |
0.72–1.99 |
0.47 |
| Non-HDL-C ≥ 100 mg/dL |
2.30 |
1.05–5.58 |
0.047 |
1.99 |
1.24–3.29 |
0.006 |
| TG ≥ 150 mg/dL |
0.95 |
0.39–2.10 |
0.91 |
1.11 |
0.66–1.83 |
0.69 |
| Uric acid, mg/dL |
0.91 |
0.71–1.17 |
0.49 |
0.99 |
0.84–1.16 |
0.89 |
| FPG, mg/dL |
0.99 |
0.98–1.00 |
0.20 |
1.00 |
0.99–1.00 |
0.26 |
| eGFR, mL/min/1.73 m2
|
1.01 |
0.99–1.02 |
0.21 |
1.01 |
0.99–1.02 |
0.102 |
| LVEF, % |
1.00 |
0.98–1.03 |
0.90 |
1.01 |
0.98–1.02 |
0.56 |
Abbreviations: CI, confidence interval; OR, odds ratio. Other abbreviations are as in Table 1.
We then performed multivariate logistic regression analysis (Tables 4, 5). Non-HDL-C ≥ 100 mg/dL was found to be an independent risk factor for the occurrence of new lesions 9 months after PCI (non-HDL-C ≥ 100 mg/dL: OR 1.80, 95 % CI 1.10–3.0, p=0.021) (Table 4). In Model 1 including LDL-C (F) ≥ 70 mg/dL and Model 2 including LDL-C (M) ≥ 70 mg/dL, DM was the only independent risk factor for the occurrence of new lesions (DM: Model 1, OR 1.70, 95 % CI 1.06–2.81, p=0.032, Model 2, OR 1.70, 95 % CI 1.05–2.79, p=0.034). On the other hand, in Model 3 including non-HDL-C ≥ 100 mg/dL, both DM and non-HDL-C ≥ 100 mg/dL were independent risk factors for the occurrence of new lesions ≤ 2 years after PCI (DM: OR 1.71, 95 % CI 1.06–2.83, p=0.031; non-HDL-C ≥ 100 mg/dL: OR 1.85, 95 %CI 1.13–3.07, p=0.016) (Table 5).
Table 4.
Predictive value of the occurrence of new lesions 9-month after PCI as determined by multivariate logistic analysis
|
OR |
95%CI |
p value
|
| Age, years |
0.98 |
0.96–1.00 |
0.085 |
| History of smoking |
1.35 |
0.72–2.43 |
0.33 |
| β-blocker |
0.98 |
0.59–1.60 |
0.95 |
| Non-HDL-C ≥ 100 |
1.80 |
1.10–3.00 |
0.021 |
Abbreviations are as in Tables 1, 2 and 3.
Table 5.
Predictive value of the occurrence of new lesions ≤ 2 years after PCI as determined by multivariate logistic analysis modeling
|
Model 1 |
Model 2 |
Model 3 |
| OR |
95%CI |
p value
|
OR |
95%CI |
p value
|
OR |
95%CI |
p value
|
| Age, years |
0.98 |
0.96–1.00 |
0.086 |
0.98 |
0.96–1.00 |
0.075 |
0.98 |
0.96–1.00 |
0.086 |
| Sex: male |
1.02 |
0.61–1.76 |
0.94 |
1.05 |
0.61–1.77 |
0.92 |
1.01 |
0.60–1.75 |
0.96 |
| Diabetes mellitus |
1.70 |
1.06–2.81 |
0.032 |
1.70 |
1.05–2.79 |
0.034 |
1.71 |
1.06–2.83 |
0.031 |
| LDL-C (F) ≥ 70 |
1.67 |
0.99–2.89 |
0.060 |
- |
- |
- |
- |
- |
- |
| LDL-C (M) ≥ 70 |
- |
- |
- |
1.62 |
0.94–2.89 |
0.091 |
- |
- |
- |
| Non-HDL-C ≥ 100 |
- |
- |
- |
- |
- |
- |
1.85 |
1.13–3.07 |
0.016 |
Abbreviations are as in Tables 1, 2 and 3.
Model 1, adjusted for age, sex (male), diabetes mellitus and LDL-C (F) ≥ 70 mg/dL Model 2, adjusted for age, sex (male), diabetes mellitus and LDL-C (M) ≥ 70 mg/dL Model 3, adjusted for age, sex (male), diabetes mellitus and non-HDL-C ≥ 100 mg/dL
Predictive Values of LDL-C (F), LDL-C (M), and Non-HDL-C for the Occurrence of New Lesions
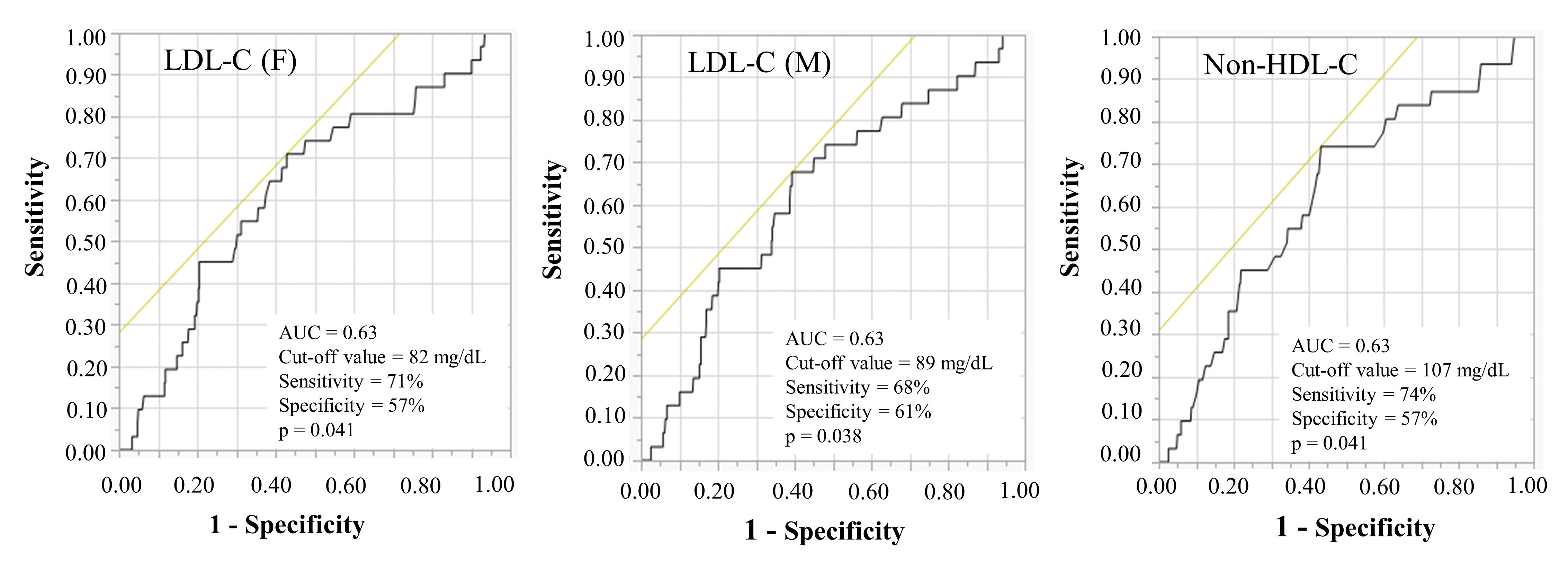
These results show that DM and non-HDL-C ≥ 100 mg/dL were potent independent risk factors of the occurrence of new lesions ≤ 2 years after PCI. We further performed a receiver operating characteristic (ROC) curve analysis in order to evaluate the discriminatory capacity of LDL-C (F), LDL-C (M), and non-HDL-C levels in predicting the occurrence of new lesions 9 months (Fig.2) and ≤ 2 years after PCI (Fig.3).
AUC, area under the curve; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PCI, percutaneous coronary intervention.
The ROC cut-off values for the LDL-C (F), LDL-C (M), and non-HDL-C levels in predicting the occurrence of new lesions at 9 months were 82 mg/dL, 89 mg/dL, and 107 mg/dL, respectively [area under the curve (AUC)=0.63, p=0.041, AUC=0.63, p=0.038 and AUC=0.63, p=0.041]. Comparisons of ROC curves to predict the occurrence of new lesions 9 months after PCI showed that there were no significant differences in the AUC for the LDL-C (F), LDL-C (M), and non-HDL-C levels (p=0.39) (Fig.2).
In addition, the ROC cut-off values for the LDL-C (F), LDL-C (M), and non-HDL-C levels in predicting the occurrence of new lesions ≤ 2 years were 88 mg/dL, 107 mg/dL, and 129 mg/dL, respectively [AUC=0.62, p=0.001, AUC=0.62, p<0.001 and AUC=0.63, p<0.001]. Comparisons of ROC curves for predicting the occurrence of new lesions ≤ 2 years after PCI also showed that there were no significant differences in the AUC for the LDL-C (F), LDL-C (M), and non-HDL-C levels (p=0.39) (Fig.3).
Discussion
This current study has demonstrated that non-HDL-C ≥ 100 mg/dL, but not LDL-C (F) ≥ 70 mg/dL or LDL-C (M) ≥ 70 mg/dL, was an independent risk factor for the occurrence of new lesions 9 months and ≤ 2 years after PCI for stable angina patients on strong statins.
PCI using second DES has become the standard procedure for coronary revascularization among patients with CAD, and many clinical trials using statins to lower LDL-C have shown substantial reductions in major cardiovascular events. However, residual risk is present even in patients with well-controlled LDL-C levels. Five-year outcomes of the j-Cypher Registry showed that the cumulative incidences of non-target lesion revascularization after PCI at 1 and 5 years were 16.1 % and 31.2 %, respectively21). In a study by Stone et al., it was found that among patients presenting with acute coronary syndrome who underwent PCI, major adverse cardiovascular events occurring during 3-year follow-up were equally attributable to the recurrence at the site of culprit lesions (12.9 %) and non-culprit lesions (11.6 %)22). PCI has been deemed suitable for revascularization of culprit lesions; however, it is ineffective for suppressing new lesions. Suppressing the occurrence of new lesions after PCI is thus a significant issue for the prognosis of patients with CAD. Recently, the A Prospective Natural-History Study has demonstrated that predictors of non-culprit lesion-related major adverse cardiovascular events included insulin-requiring DM, baseline plaque burden ≥ 70 %, minimal luminal area ≤ 4.0 mm2, and the presence of thin-cap fibroatheroma22).
DM patients show approximately two to four times the risk of developing CAD compared to patients without DM23, 24). In addition, DM patients often suffer from a higher risk of adverse outcomes following PCI25-28). Thus, identification of factors that may increase CAD risk in DM patients following PCI is clinically important. DM patients can have an increased prevalence of lipid abnormalities, and dyslipidemia in DM patients is characterized by hypercholesterolemia, hypertriglyceridemia, low HDL-C levels, and small dense LDL particles29). DM is thus one of the substantial residual risks for CAD despite statin therapy30). These results seem to support our result that DM was strongly associated with the occurrence of new lesions after PCI despite strong statin therapy.
Lipid-lowering therapy has been identified to be vital in the primary and secondary prevention of CAD8, 9). Many clinical trials using statins have revealed that lowering LDL-C was significantly associated with substantial reductions in major cardiovascular events31-34). The Friedewald equation using a fixed TG/VLDL-C ratio of 5 is the standard method for estimating LDL-C (F) levels, but this equation is not recommended for use in patients with TG >400 mg/dL, and underestimation of LDL-C levels has been reported at both low LDL-C levels and high TG levels15). Martin et al. have recently reported a novel method for estimating LDL-C using an adjustable factor instead of a fixed factor of 5 16). Furthermore, we have demonstrated that in Japanese CAD patients receiving strong statins, LDL-C (M) was more accurate compared to LDL-C (F), particularly in patients with LDL-C <70 mg/dL35). In addition, we reported that LDL-C (M) was more useful than LDL-C (F) among Japanese patients with type 2 DM36). Thus, LDL-C (M) seems to be more useful for predicting for major cardiovascular events than LDL-C (F). However, both LDL-C (F) and LDL-C (M) levels are estimates from calculation formulas and thus do not evaluate remnant cholesterol, which remains a risk factor for arteriosclerosis along with LDL-C.
In patients with mild to moderate hypertriglyceridemia and associated cardiometabolic disorders such as DM, obesity, and metabolic syndrome, discordance can occur due to small dense LDL, and LDL-C may not accurately reflect LDL particle concentrations or their effects on CAD37, 38). Furthermore, dyslipidemia with DM is often characterized by high serum TG concentrations, low HDL-C concentrations, and increased concentrations of small dense LDL-C particles. Non-HDL-C can be simply and accurately calculated as non-HDL-C=TC − HDL-C, regardless of a fasting or non-fasting state, and represents the cholesterol mass contained in all atherogenic lipoproteins, including LDL and VLDL, intermediate-density lipoprotein, chylomicrons, and their TG-rich lipoprotein remnants. Several lines of evidence suggest that VLDL could contribute to the pathogenesis of atherosclerosis through increased expression of proinflammatory cytokines and induction of endothelial cell apoptosis39-43). The residual risk beyond the scope of LDL-C-lowering therapies may reflect the aspects of atherogenesis not captured by LDL-C, including the effects of VLDLs. We, therefore, considered non-HDL-C as a superior marker to LDL-C, given the inclusion of all lipoproteins that are considered to be causes of arteriosclerosis, including VLDL-C. Several recent reports have also suggested non-HDL-C as a more important target for predicting cardiovascular events than LDL-C44-46). These results seem to support our result that non-HDL-C was strongly associated with the occurrence of new lesions after PCI despite strong statin therapy.
Furthermore, our study has demonstrated that there were no significant differences in ROC curves for predicting the occurrence of new lesions at 9 months and ≤ 2 years after PCI in the LDL-C (F), LDL-C (M), and non-HDL-C levels. However, the cut-off values for the LDL-C (F) and LDL-C (M) for predicting the occurrence of new lesions 9 months and ≤ 2 years after PCI were quite different. It may be derived from the difference in calculation formulas between LDL-C (F) and LDL-C (M).
In addition, a significant residual risk was also noted for the occurrence of new lesions after PCI despite aggressive lipid-lowering therapy. In Japan, LDL-C <100 mg/dL is the secondary prevention target, while <70 mg/dL is recommended for high-risk patients14), but there is still residual cardiovascular event risk even after achieving it. Furthermore, many trials about the efficacy of lipid-lowering therapy have shown that the cardiovascular benefits of statins which have “pleiotropic effects” such as contributing to plaque stabilization may go beyond their influence on LDL-C levels.
In this study, non-HDL-C ≥ 100 mg/dL was determined to be independently associated with the occurrence of new lesions both at 9 months and ≤ 2 years after PCI in stable angina patients prescribed with strong statins. Non-HDL-C ≥ 100 mg/dL was the important risk factor as well as LDL-C (F) ≥ 70 mg/dL, or LDL-C (M) ≥ 70 mg /dL. The assessment using the non-HDL-C which can be simply and accurately calculated, regardless of a fasting or non-fasting state, and represents the cholesterol mass contained in all atherogenic lipoproteins, was thought to be more important than LDL-C. Therefore, the priority of non-HDL-C assessment to predict occurrence of new lesions after PCI in stable angina patients prescribed with strong statins should be ahead of LDL-C.
Our study demonstrated that the New lesion (+) ≤ 2 years group was significantly younger than the New lesion (−) ≤ 2 years group (p=0.006). However, the difference in ages between the New lesion (+) 9-month group and the New lesion (−) 9-month group was not significant (p=0.58). The reason for the difference in age effects on the new lesion occurrence between 9 months and ≤ 2 years is yet to be determined. We speculate that age is a risk factor for atherosclerosis, but risk factor other than age may be involved in short-term new lesions, and age may be involved in long-term new lesions.
Limitations
Several limitations to this study merit consideration when interpreting the results. First, this present study contained a relatively small number of patients. In the future, we need to assess the effects of non-HDL-C on new lesions over a longer period after PCI in a large-scale prospective study. Second, although we were able to demonstrate an independent association between non-HDL-C ≥ 100 mg/dL and the presence of new lesions in stable angina patients treated with strong statins who underwent PCI, we failed to examine the associations between the occurrence of new lesions and patients with TG >400 mg/dL, because of the limits to the use of Friedewald equation. Third, only patients with stable angina were recruited to this study. The impact of non-HDL-C on the presence of new lesions after PCI in patients with acute coronary syndrome should thus be investigated. Fourth, although all patients were administered strong statin, the kinds and doses of statin were different in each patient.
Conclusions
Non-HDL-C ≥ 100 mg/dL was the independent risk factor for the occurrence of new lesions both at 9 months and ≤ 2 years after PCI in stable angina patients with strong statins. Residual risk after PCI should be considered by assessing not only DM but also non-HDL-C beyond the scope of LDL-C-lowering therapy with strong statins.
Acknowledgements
We thank the staff of the Department of Cardiovascular Medicine and Hypertension at Graduate School of Medical and Dental Sciences, Kagoshima University for their assistance with data processing.
Grant Support
This work was supported in part by the Japan Society for the Promotion of Science KAKENHI (grant no. 16H070890).
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1) World Health Organization. Cardiovascular diseases: fact sheet number 310. Available at: http: //www.who.int/mediacentre/factsheets/fs310/en/ [accessed 03.02.17]
- 2) Menees DS, Peterson ED, Wang Y, Curtis JP, Messenger JC, Rumsfeld JS, Gurm HS: Door-to-balloon time and mortality among patients undergoing primary PCI. N Engl J Med, 2013; 369: 901-909
- 3) Hovingh GK, Davidson MH, Kastelein JJP, O’Connor AM: Diagnosis and treatment of familial hypercholesterolaemia. Eur Heart J, 2013; 34: 962-971
- 4) Raal FJ, Hovingh GK, and Catapano AL: Familial hypercholesterolemia treatments: guidelines and new therapies. Atherosclerosis, 2018; 277: 483-492
- 5) Stein EA: Management of dyslipidemia in the high-risk patient. Am Heart J, 2002; 144: S43-S50
- 6) Teramoto T, Kobayashi M, Tasaki H, Yagyu H, Higashikata T, Takagi Y, Uno K, Baccara-Dinet MT, Nohara A: Efficacy and safety of alirocumab in Japanese patients with heterozygous familial hypercholesterolemia or at high cardiovascular risk with hypercholesterolemia not adequately controlled with statins - ODYSSEY JAPAN randomized controlled trial. Circ J, 2016; 80: 1980-1987
- 7) Toth PP, Descamps O, Genest J, Sattar N, Preiss D, Dent R, Djedjos C, Wu Y, Geller M, Uhart M, Somaratne R, Wasserman SM; PROFICIO Investigators: Pooled safety analysis of evolocumab in over 6000 patients from double-blind and open-label extension studies. Circulation, 2017; 135: 1819-1831
- 8) Stroes E: Statins and LDL-cholesterol lowering: an overview. Curr Med Res Opin, 2005; 21: S9-S16
- 9) Grundy SM, NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, Ferranti Sd, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, and Yeboah J: 2018. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol, 2019; 73: e285-e350, doi.org/10.1016/j.jacc.2018.11.003.
- 10) Dembowski E, Davidson MH: A review of lipid management in primary and secondary prevention. J Cardiopulm Rehabil Prev, 2009; 29: 2-12
- 11) Shimizu R, Torii H, Yasuda D, Hiraoka Y, Kitada N, Hashida T, Yoshimoto A, Kita T, and Kume N: Serum lipid goal attainment in chronic kidney disease (CKD) patients under the Japan Atherosclerosis Society (JAS) 2012 guidelines. J Atheroscler Thromb, 2015; 22: 949-957
- 12) Shirai K: Obesity as the core of the metabolic syndrome and the management of coronary heart disease. Curr Med Res Opin, 2004; 20: 295-304
- 13) Authors/Task Force Members: ; Catapano AL, Graham I, De Backer G, Wiklund O, hapman MJ, Drexel H, Chapman M J, Drexel H, Hoes A W, Jennings C S, Landmesser U, Pedersen T R, Reiner Ž, Riccardi G, Taskinen M R, Tokgozoglu L, Verschuren W M M, Vlachopoulos C, Wood D A, Zamorano J L: 2016 ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European society of cardiology (ESC) and European atherosclerosis society (EAS) developed with the special contribution of the European Assocciation for cardiovascular prevention & rehabilitation (EACPR). Atherosclerosis, 2016; 253: 281-344
- 14) Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S, Committee for Epidemiology and Clinical Management of Atherosclerosis: Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984
- 15) Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 1972; 18: 499-502
- 16) Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, Jones SR: Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA, 2013; 310: 2061-2068
- 17) Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A: Collaborators developing the Japanese equation for estimated GFR: Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis, 2009; 53: 982-992
- 18) Saku K, Zhang B, Noda K, PATROL Trial Investigators: Randomized head-to-head comparison of pitavastatin, atorvastatin, and rosuvastatin for safety and efficacy (quantity and quality of LDL): the PATROL trial. Circ J, 2011; 75: 1493-1505
- 19) Anderson TJ, Gregoire J, Hegele RA, Couture P, Mancini GB J, McPherson R, Francis G A, Poirier P, Lau D C, Grover S, Genest Jr J, Carpentier A C, Dufour R, Gupta M, Ward R, Leiter L A, Lonn E, Ng D S, Pearson G J, Yates G M, Stone J A, Ur E: 2012 update of the Canadian cardiovascular society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol, 2013; 29: 151-167
- 20) Contois JH, McConnell JP, Sethi AA, Csako G, Devaraj S, Hoefner DM, Warnick R, AACC Lipoproteins and Vascular Diseases Division Working Group on Best Practices: Apolipoprotein B and cardiovascular disease risk: position statement from the AACC Lipoproteins and vascular diseases division working group on Best practices. Clin Chem, 2009; 55: 407-419
- 21) Kimura T, Morimoto T, Nakagawa Y, Kawai K, Miyazaki S, Muramatsu T, Shiode N, Namura M, Sone T, Oshima S, Nishikawa H, Hiasa Y, Hayashi Y, Nobuyoshi M, Mitudo K, j-Cypher Registry Investigators: Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-Cypher Registry. Circulation, 2012; 125: 584-591
- 22) Stone GW, Maehara A, Lansky AJ, Bruyne Bd, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW, PROSPECT Investigators: A prospective natural-history study of coronary atherosclerosis. N Engl J Med, 2011; 364: 226-235
- 23) Emerging Risk Factors Collaboration; Sarwar N, Gao P, Kondapally Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CDA, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J: Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet, 2010; 375: 2215-2222
- 24) Goff DC Jr, Gerstein HC, Ginsberg HN, Cushman WC, Margolis KL, Byington RP, Buse JB, Genuth S, Probstfield JL, Simons-Morton DG, ACCORD Study Group: Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol, 2007; 99: 4i-20i
- 25) Abizaid A, Kornowski R, Mintz GS, Hong MK, Abizaid AS, Mehran R, Pichard AD, Kent KM, Satler LF, Wu H, Popma JJ, Leon MB: The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J Am Coll Cardiol, 1998: 32: 584-589
- 26) Mathew V, Gersh BJ, Williams BA, Laskey WK, Willerson JT, Tilbury RT, Davis BR, Holmes DR Jr: Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation, 2004; 109: 476-480
- 27) Luca GD, Gibson CM, Bellandi F, Noc M, Dudek D, Zeymer U, Arntz H-R, Cutlip D, Maioli M, Zorman S, Gabriel HM, Emre A, Rakowski T, Gyongyosi M, Huber K, Van’t Hof AWJ: Diabetes mellitus is associated with distal embolization, impaired myocardial perfusion, and higher mortality in patients with ST-segment elevation myocardial infarction treated with primary angioplasty and glycoprotein IIb-IIIa inhibitors. Atherosclerosis, 2009; 207: 181-185
- 28) Robertson BJ, Gascho JA, Gabbay RA, McNulty PH: Usefulness of hyperglycemia in predicting renal and myocardial injury in patients with diabetes mellitus undergoing percutaneous coronary intervention. Am J Cardiol, 2004; 94: 1027-1029
- 29) Garg A, Grundy SM: Management of dyslipidemia in NIDDM. Diabetes Care, 1990; 13: 153-169
- 30) Warraich HJ, Wong ND, Rana JS: Role for combination therapy in diabetic dyslipidemia. Curr Cardiol Rep, 2015; 17: 32
- 31) Sever PS, Dahlöf B, Poulter N R, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J, ASCOT investigators: Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet, 2003; 361: 1149-1158
- 32) Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E, for the Cholesterol and Recurrent Events Trial investigators: The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med, 1996; 335: 1001-1009
- 33) Shepherd J, Cobbe S M, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ, for the West of Scotland Coronary Prevention Study Group: Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med, 1995; 333: 1301-1307
- 34) Baigent C, Keech A, Kearney P M, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists’ (CTT) Collaborators: Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet, 2005; 366: 1267-1278
- 35) Sonoda T, Takumi T, Miyata M, Kanda D, Kosedo I, Yoshino S, Ohishi M: Validity of a novel method for estimating low-density lipoprotein cholesterol levels in cardiovascular disease patients treated with statins. J Atheroscler Thromb, 2018; 25: 643-652
- 36) Chaen H, Kinchiku S, Miyata M, Kajiya S, Uenomachi H, Yuasa T, Takasaki T, Ohishi M: Validity of a novel method for estimation of low-density lipoprotein cholesterol levels in diabetic patients. J Atheroscler Thromb, 2016; 23: 1355-1364
- 37) Cantey EP, Wilkins JT: Discordance between lipoprotein particle number and cholesterol content: an update. Curr Opin Endocrinol Diabetes Obes, 2018; 25: 130-136
- 38) Langlois MR, Chapman MJ, Cobbaert C, Mora S, Remaley AT, Ros E, Watts GF, Borén J, Baum H, Bruckert E, Catapano A, Descamps OS, von Eckardstein A , Kamstrup PR, Kolovou G, Kronenberg F, Langsted A, Pulkki K, Rifai N, Sypniewska G, Wiklund O, Nordestgaard BG, European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Joint Consensus Initiative: Quantifying Atherogenic Lipoproteins: Current and Future Challenges in the Era of Personalized Medicine and Very Low Concentrations of LDL Cholesterol. A Consensus Statement from EAS and EFLM. Clin Chem, 2018; 64: 1006-1033
- 39) Saraswathi V, Hasty AH: The role of lipolysis in mediating the proinflammatory effects of very low density lipoproteins in mouse peritoneal macrophages. J Lipid Res, 2006; 47: 1406-1415
- 40) Stollenwerk MM, Lindholm MW, Pörn-Ares MI, Larsson A, Nilsson J, Ares MP: Very low-density lipoprotein induces interleukin-1beta expression in macrophages. Biochem Biophys Res Commun, 2005; 335: 603-608
- 41) Stollenwerk MM, Schiopu A, Fredrikson GN, Dichtl W, Nilsson J, Ares MP: Very low density lipoprotein potentiates tumor necrosis factor-alpha expression in macrophages. Atherosclerosis, 2005; 179: 247-254
- 42) Reinbold M, Hufnagel B, Kewitz T, Klumpp S, Krieglstein J: Unsaturated fatty acids liberated from VLDL cause apoptosis in endothelial cells. Mol Nutr Food Res, 2008; 52: 581-588
- 43) Masuda D, Yamashita S: Postprandial Hyperlipidemia and Remnant Lipoproteins. J Atheroscler Thromb, 2017; 24: 95-109
- 44) Blaha MJ, Blumenthal RS, Brinton EA, Jacobson TA, National Lipid Association Taskforce on Non-HDL Cholesterol: The importance of non-HDL cholesterol reporting in lipid management. J Clin Lipidol, 2008; 2: 267-273
- 45) Su X, Kong Y, Peng D: Evidence for changing lipid management strategy to focus on non-high density lipoprotein cholesterol. Lipids Health Dis, 2019; 18: 134
- 46) Carr SS, Hooper AJ, Sullivan DR, Burnett JR: Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology, 2019; 51: 148-154