2022 Volume 29 Issue 7 Pages 1108-1116
2022 Volume 29 Issue 7 Pages 1108-1116
Hypertriglyceridemia (HTG) is a state of increased serum triglyceride (TG) affected by multigenetic and multifactorial causes. Serum TG concentration can be markedly elevated if exposed to precipitating factors, such as estrogen hormone and pregnancy. We report the case of a patient with severe HTG who suffered from recurrent pancreatitis during the second trimester of pregnancy conceived with in vitro fertilization-embryo transfer (IVF-ET) and was successfully controlled by multiple sessions of plasmapheresis.
A 24-year-old pregnant woman was admitted because of a sudden onset of severe abdominal pain at 26 weeks of gestation conceived by IVF-ET. She has experienced recurrent pancreatitis despite low-fat diet and dyslipidemia medications allowed in pregnancy. At admission, serum amylase and lipase were elevated to 347 and 627 U/L, respectively, along with fasting TG to 4809 mg/dL. A clinical diagnosis of HTG-induced acute pancreatitis was made, and plasmapheresis was performed. After plasmapheresis, serum TG, amylase, and lipase levels decreased to 556 mg/dL, 60 U/L, and 69 U/L, respectively, along with subsequent pain relief. The patient underwent a total of nine sessions of plasmapheresis to retain serum TG lower than 1,000 mg/dL during pregnancy, with no further recurrence of acute pancreatitis. After delivery, the serum TG level was maintained below 500 mg/dL with a combination treatment of fenofibrate, statin, and ezetimibe.
Although severe HTG is usually asymptomatic, if exposed to precipitating factors, it can cause acute pancreatitis, a fatal complication. Early application of plasmapheresis may be a useful option in HTG-induced acute pancreatitis intractable to medical treatment; however, its indications, risks, and benefits should be carefully evaluated.
Hypertriglyceridemia (HTG) is defined as increased fasting plasma triglyceride (TG) level affected by multigenetic and multifactorial causes1). HTG is a known risk factor of acute pancreatitis, and the risk increases along with the increase of TG level, especially when it exceeds 500 mg/dL2, 3). HTG can be aggravated when exposed to risk factors, including pregnancy, obesity, diabetes, hormone replacement therapy, and drugs4).
TG level elevation was related to estrogen level changes5-7). Although the mechanism is unknown, oral contraceptive or in vitro fertilization-embryo transfer (IVF-ET) is also known to increase TG level and cause acute pancreatitis8-11). Regarding pregnancy, studies have investigated clinical courses related to HTG and HTG-induced acute pancreatitis12-14). In consideration of IVF-ET, currently reported cases are focused on HTG-induced acute pancreatitis before the second trimester of pregnancy.
In this study, we report the first case of a 24-year-old patient with HTG-induced acute pancreatitis during the second trimester of IVF-ET pregnancy, which was successfully controlled by multiple sessions of plasmapheresis.
A 24-year-old, nonsmoking, nonalcoholic, Korean pregnant woman at 26 weeks of gestation conceived with the first attempt of IVF-ET was transferred to further manage recurrent pancreatitis. She had missed abortion two years before without any complication indicating HTG.
At gestational age (GA) 17 weeks, the patient visited another hospital emergency department because of severe epigastric pain. With a high blood TG level of 7,358 mg/dL, she was suspected of familial hypertriglyceridemia (FHTG). The patient had neither previous history of childhood hyperlipidemia nor suspected FHTG history. The TG level before pregnancy was not checked. The laboratory test revealed increased amount of apolipoprotein (Apo) A-II (ApoAII) (42.30 mg/dL; normal range, 25.1–34.5 mg/dL), C-II (>10 mg/dL; normal range, 1.6–4.2 mg/dL), and C-III (>22 mg/dL; normal range, 5.5–9.5 mg/dL) and decreased ApoE (<0.2 mg/dL; normal range, 2.7–4.5 mg/dL). The genotype of ApoE was identified as ε2/ε3. ApoAI and B laid within normal limits. Lipoprotein electrophoresis was performed at a TG level of 809 mg/dL, which was after the patient was treated with insulin, omega-3, fibrate, and a low-fat diet. The additional analysis data when TG level exceeded 1,000 mg/dL was not obtained because of the patient’s financial shortage. Lipoprotein analysis showed an increase in the pre-β band without the presence of chylomicron and β band at TG level of 809 mg/dL (Fig.1). During the initial admission, she was managed with fasting, fluid hydration, short-term insulin injection, and gemfibrozil 600 mg twice daily with omega-3 fatty acid 4 g once daily. However, the therapeutic effect was insufficient, and the frequency of acute pancreatitis attacks increased until she was transferred to our hospital. She was shortly prescribed with bile acid sequestrant (cholestyramine) during GA 25–26 weeks, which was discontinued because of severe nausea.

Increased pre-β band without the presence of chylomicron and β band at triglyceride level of 809 mg/dL (Gestational age 17 weeks 6 days). Ref: reference
After nine weeks (GA 26 weeks), the patient suddenly had severe and sharp pain at the epigastric area to the left upper quadrant. She also had dyspnea, chest discomfort, nausea, and vomiting but no fever. Her body weight and height were 72 kg and 160.2 cm, respectively. There was neither xanthelasma nor tendon xanthomas. The patient denied any consumption of alcohol/tobacco. Abdomen ultrasonography was performed instead of computerized tomography scan because of concern of radiation, and no biliary obstruction was observed. The evaluation of the pancreas was limited because of the increased size of the uterus and bowel gas. The serum level of amylase and lipase were elevated to 347 U/L (normal range, 28–100 U/L) and 627 U/L (normal range, 7–60 U/L), along with an increase in fasting TG concentration to 4,809 mg/dL (normal range, 0–200 mg/dL) and total cholesterol to 1,120 mg/dL (normal range, 0–240 mg/dL). With severe pain, the patient’s respiratory rate increased to 30/minute, with a decrease in SpO2 to 94% and a pulse rate of 140 beats/minute. Oxygen supply via nasal prong was applied to relieve the symptom.
As soon as the HTG-induced acute pancreatitis was suspected, fasting, fluid hydration, and immediate plasmapheresis were performed. After the first plasmapheresis, fasting TG concentration decreased to 882 mg/dL, total cholesterol to 281 mg/dL, amylase to 81 U/L, and lipase to 113 U/L (Fig.2). One of the concerns in determining the appropriate number of plasmapheresis was minimizing the number of invasive procedures, such as internal jugular catheter insertion, needed for plasmapheresis. After the first plasmapheresis (P#1), although TG level was below 1,000 mg/dL, we assumed TG level would rise soon and wanted to lower the level as much as possible with the inserted internal jugular catheter. In addition, the TG concentration continuously increased after each plasmapheresis session, necessitating multiple plasmapheresis sessions (Fig.3(a)). The symptoms resolved after the third plasmapheresis session. The patient was discharged to an outpatient clinic. The serum amylase and lipase levels decreased to 60 and 69 U/L, respectively, after three plasmapheresis sessions.

Serum triglyceride, amylase, and lipase concentration at the admission of GA 26 weeks, after first plasmapheresis session, and four weeks after delivery, C/S: cesarean section TG: triglyceride
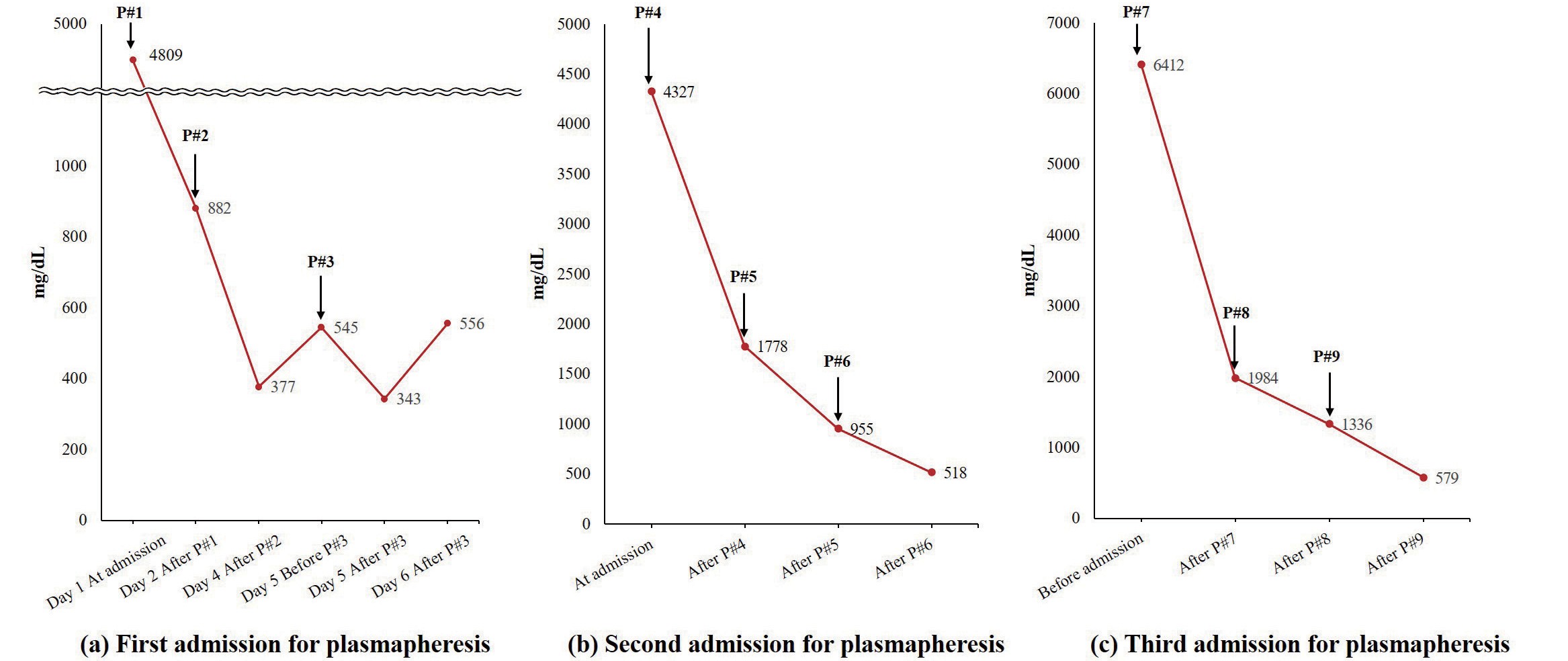
(a) At first admission for plasmapheresis, triglyceride level continuously increased after P#2, P#3. (b) During the second admission for plasmapheresis, triglyceride level decreased to l,778mg/dL after P#4, 955mg/dL after P#5, and 518mg/dL after P#6 (c) At third admission, the triglyceride was lowered below <l,000mg/dL only after P#9. P# : plasmapheresis session number
Four weeks after the plasmapheresis, TG level gradually increased to 4,327 mg/dL. Therefore, the patient was hospitalized for an additional three more sessions of plasmapheresis (P#4–P#6 in Fig.4(a)). After the fourth plasmapheresis (P#4), TG level only decreased to 1,778 mg/dL, eventually necessitating further sessions of plasmapheresis (P#5 and P#6). TG level decreased to 955 mg/dL after the fifth plasmapheresis (P#5) and 518 mg/dL after the sixth plasmapheresis (P#6) (Fig.3(b)). Three weeks after the second admission, the patient was hospitalized again for plasmapheresis due to a TG concentration of 6,412 mg/dL (P#7–P#9 in Fig.4(a)). TG level was decreased to 1,984 mg/dL after the seventh plasmapheresis (P#7), leading to the eighth plasmapheresis (P#8) (TG level decreased to 1,336 mg/dL) and ninth plasmapheresis (P#9) (TG level decreased to 579 mg/dL) (Fig.3(c)). The patient underwent a total of nine plasmapheresis sessions during the whole pregnancy, three for each admission. After plasmapheresis was performed, the patient did not experience additional acute pancreatitis during the remaining pregnancy. The patient’s diet differed between the admitted and discharged states. During the hospitalization period, the patient was provided with a low-fat diet (<15% of the total calorie; 26 g of fat per day, including 15 g of vegetable oil used for cooking). However, the patient did not follow a strict fat-restricted diet when discharged (Fig.4(a)).

(a) The course of serum triglyceride, total cholesterol, amylase, lipase during pregnancy with a change of diet, medications and multiple plasmapheresis sessions. (b) the course of serum amylase and lipase during pregnancy in specific. C/S: cesarean section, NPO: nothing per oral P#: plasmapheresis session number TG: triglyceride
Plasmapheresis was performed using a centrifugal apheresis system (Spectra Optia, Terumo BCT, Lakewood, CO, USA) via a right internal jugular catheter with the patient at left lateral decubitus position. Each session consisted of a 1.5 plasma volume exchange using 5% albumin and fresh frozen plasma (50:50) as the replacement fluid. The patient’s blood volume was estimated using the Nadler’s equation. Acid citrate dextrose-A was used for anticoagulation at a whole blood-to-anticoagulant ratio of 18:1. Additional heparin infusion was not administered during the plasma exchange. Each plasmapheresis took approximately three hours. After each session, 3–4 L of plasma was replaced, and the collected plasma was milky, indicating high TG concentration (Fig.5). Supplemental calcium was administered only when the patient developed mild hypocalcemia symptoms. Other than a perioral tingling sense, the patient and the fetuses did not experience any significant complications during and after plasmapheresis.

(a) plasmapheresis in progress (b) the patient’s blood is being separated into two different layers of red blood cells (red arrow) and milky plasma (orange arrow) (c) the bag of triglyceride rich plasma collected after the plasmapheresis
At 36 weeks of gestation, the patient underwent a cesarean section and gave birth to healthy twin males weighing 2.675 and 2.790 kg with the Apgar score of 8 at 1 minute and 9 at 5 minutes, respectively. On the day of the cesarean section, her TG levels varied from 2,772 to 1,833 mg/dL. The patient was discharged with fenofibrate and atorvastatin on postdelivery day 3. Statin was initiated after careful consideration of the risk and benefit during breastfeeding because of the patient’s rejection on omega-3 due to nausea and vomit. The patient also failed at keeping the strict fat-restricted diet after the delivery. During the regular outpatient clinic follow-up, TG level remained below 500 mg/dL, except for the period when the patient arbitrarily discontinued the whole medication.
The whole treatment is summarized in Fig.4(a). During the whole pregnancy, the patient underwent nine sessions of plasmapheresis with subsequent modification of medicines considering the status of pregnancy and breastfeeding. Insulin was injected during the first acute pancreatitis attack but was discontinued because of recurrent hypoglycemia. During insulin administration, blood glucose level decreased to 46 mg/dL despite using dextrose fluid. The patient took gemfibrozil and omega-3 since GA 17 weeks, and gemfibrozil was changed to fenofibrate at GA 25 weeks. She was shortly prescribed with cholestyramine during GA 25–26 weeks, which was discontinued because of severe nausea. The omega-3 was continued until the delivery day and was changed to statin at discharge. At four weeks postpartum, ezetimibe was added to earn the synergetic effect of ezetimibe–statin combination therapy as the low-density lipoprotein (LDL) cholesterol increased to 132 mg/dL compared to 49 mg/dL at discharge.
During the postpartum period, she arbitrarily discontinued the whole medication because of concern for the lactation and absence of subjective discomfort. As a result, serum TG levels markedly elevated to 2,076 mg/dL. Total cholesterol level also elevated to 235 mg/dL with decreased HDL cholesterol of 25 mg/dL. After resuming the treatment of statin, ezetimibe, and fenofibrate, TG level decreased to the normal range of 175 mg/dL with total cholesterol and HDL cholesterol level of 86 and 29 mg/dL, respectively. Therefore, ezetimibe was discontinued, and the statin–fibrate combination was continued until the recent follow-up.
The level of serum amylase and lipase in specific is depicted in Fig.4(b). At the first pancreatitis attack, amylase elevated to 438 U/L and lipase to 435 U/L. During the second abdominal pain event, serum amylase and lipase were 62 and 60 U/L, respectively. Lastly, during the acute pancreatitis attack at 26 weeks, amylase elevated to 239 U/L and lipase to 220 U/L. After performing plasmapheresis, serum amylase and lipase maintained normal range and were not routinely checked as abdominal pain did not recur.
The patient provided written informed consent on March 26, 2020.
HTG is a multigenetic and multifactorial disorder1). Along with multiple genes related to TG level, precipitating factors, including pregnancy, obesity, diabetes, hormone replacement therapy, and drugs, can exaggerate TG level. HTG is thought to be associated with complications such as cardiovascular disease and acute pancreatitis15, 16).
During pregnancy, estrogen gradually increases TG concentration from the end of the first trimester to the end of pregnancy up to 2.5 folds of the normal range5-7). Although this change is usually tolerable in a healthy population, patients with genetic causes are usually more susceptible to the severe complications of HTG-induced acute pancreatitis during pregnancy12, 14, 17-20). HTG can be more complicated with IVF-ET. In previous reports, patients who underwent IVF-ET developed HTG-induced acute pancreatitis a week after the procedure and at nine weeks of GA8, 10, 21). In our case, the patient underwent IVF-ET without experiencing HTG-induced pancreatitis until GA 17 weeks. However, after GA 17 weeks, she was first diagnosed with HTG-induced acute pancreatitis with recurrent symptoms. The IVF-ET in this patient might have worked as a precipitating factor on serum TG levels. Then, it was amplified by pregnancy, resulting in multiple recurrent HTG-induced acute pancreatitis.
Previously, hyperlipoproteinemia phenotype was classified using Fredrickson classification with the use of lipoprotein electrophoresis22). However, accumulating data shows that more than 30 genes are associated with HTG1). As the differentiation between monogenic and polygenic chylomicronemia can be complex, the practical term “primary chylomicronemia,” which indicates TG exceeding 1,000 mg/dL after adequate management of secondary factors, is lately being used23). Recent studies have found that patients with severe HTG share common characteristic in the overrepresentation of common and rare pathogenic gene variants24). Such result indicates the multigenetic nature of HTG along with the monogenic causes. The previously known monogenic causes include apolipoprotein A5 (APOA5), apolipoprotein C2 (APOC2), lipase maturation factor 1 (LMF1), lipoprotein lipase (LPL), glycerol-3-phosphate dehydrogenase 1 (GPD1), and glycosylphosphatidylinositol-anchored HDL binding protein 1 (GPIHBP1)25-27). Among the monogenic causes, the heterozygous mutation of LPL, APOC2, and APOA5 showed a spectrum of TG level1). The current next-generation sequencing of 73 genes and 185 single-nucleotide polymorphisms found that 32% of the cohort has an extreme accumulation of common variants, indicating high polygenic risk28). A similar study by Matsunaga et al. also found 70 variants with low prevalence of rare variants in patients with severe HTG, supporting the multigenetic nature of HTG29). Regarding HTG-induced acute pancreatitis during pregnancy, a study by Pu et al. showed an association with APOA5 rs2075291 gene30). One of the limitations of our study was that our patient was only examined with the genotype of ApoE and no other specific genes related to HTG.
ApoE is an essential ligand of lipid metabolism as it clears the remnants of TG-rich lipoproteins from circulation into the liver. Among the three alleles of ApoE, ε2 is known to have the lowest LDL receptor-binding affinity, which slows down the plasma clearance of remnants, leading to increased plasma TG and cholesterol31, 32). The previous meta-analysis showed an association between higher TG concentrations in subjects carrying the ε2 allele33). However, less than 5% of ε2/ε2 carriers develop hyperlipidemia, and patients with the ε2 allele had a higher chance of reduction in TG when administered with a statin or exercise34-36). Our patient had a ε2/ε3 genotype with a decreased ApoE protein (<0.2 mg/dL). She developed HTG-induced pancreatitis after exposure to multiple trigger factors related to pregnancy and IVF-ET. We can suspect that, for ε2 carriers, precipitating factors play a more crucial role in the actual level of TG.
The treatment of severe HTG includes low-fat diet, avoidance of carbohydrates and alcohol, and administration of omega-3 fatty acid37, 38). Regarding the low-fat diet for severe HTG (TG >1,000 mg/dL), diet containing fat below 10%–15% (<15–20 g) of the total calorie is recommended to prevent postprandial hyperchylomicronemia16, 39-41). Ezetimibe, fibrate, statin, and niacin can also be used as a combination therapy to lower TG concentration42, 43). According to the FDA pregnancy risk classification, fibrate, niacin, and ezetimibe are classified as Category C and statin as Category X. During pregnancy, fenofibrate and gemfibrozil have been cautiously used in previous case reports17, 44-46). Similarly, our patient was first prescribed with gemfibrozil and omega-3; then, gemfibrozil was changed to fenofibrate. Statin and ezetimibe were not used during the pregnancy but started after the delivery.
In previous reports, the treatment of HTG-induced acute pancreatitis during pregnancy included a low-fat diet, gemfibrozil, omega-3 fatty acid, and plasmapheresis45, 47, 48). Heparin has also been an option alone or in combination with insulin14, 49). However, the prolonged use of heparin showed rebound HTG-induced acute pancreatitis; thus, it should be used with caution50). A recent randomized controlled trial by He et al. showed that plasmapheresis could lower TG level more rapidly and efficiently but that it was not superior to low-molecular-weight heparin and insulin combination therapy in consideration of clinical outcomes and costs51).
In our case, the patient was prescribed with fibrate and omega-3 fatty acid based on previously reported cases. As for low-fat diet, we first prescribed low-fat diet containing fat below 15% of the total calorie and planned to step up to further restriction (below 10% of the total calorie). However, the patient was intolerable to even below 15% fat containing diet because of severe nausea with anorexia and rejected receiving further fat-restricted diet (below 10% of the total calorie). She also did not follow a strict fat-restricted diet when discharged. Along with anorexia, insulin administration during the first pancreatitis attack led to recurrent hypoglycemia despite using dextrose fluid, resulting in the withdrawal of insulin. Despite the medication, she repeatedly experienced HTG-induced acute pancreatitis until TG level was controlled below 1,000 mg/dL with multiple plasmapheresis sessions.
Along with low efficacy, the patient also experienced worse nausea and vomiting when taking gemfibrozil and omega-3 fatty acid, leading to intolerance. These disadvantages of oral medication and low compliance to low-fat diet enlightened the importance of plasmapheresis in this case. Earlier application of plasmapheresis might have reduced the chance of acute pancreatitis recurrence.
However, the application of plasmapheresis for HTG-induced acute pancreatitis is still a field of unestablished guideline with only low-quality or very low-quality evidence and weak recommendation. In a recent guideline of therapeutic apheresis by the American Society for Apheresis, therapeutic plasma exchange/lipoprotein apheresis is recommended in hypertriglyceridemic pancreatitis as (1) category III and grade IC recommendation in severe case and (2) category III and grade 2C in the prevention of relapse52). Thus, decision-making on applying plasmapheresis should be individualized with careful consideration23).
In summary, we report the first case of a patient with severe HTG conceived with IVF-ET pregnancy who suffered from gestational HTG-induced acute pancreatitis during the second trimester. The HTG-induced acute pancreatitis was successfully controlled by plasmapheresis with a target serum TG level below 1,000 mg/dL in this study; however, the application of plasmapheresis should be made after careful consideration of the risk and benefit of individual case.
This paper was not supported by any grants.
The authors declare no conflict of interest.