2022 Volume 29 Issue 9 Pages 1273-1274
2022 Volume 29 Issue 9 Pages 1273-1274
See article vol. 29: 1307-1318
In this issue of Journal of Atherosclerosis and Thrombosis (J Atheroscler Thromb), Aoyama J et al.1) reported the mechanisms of neutrophils to promote atherosclerosis in which how neutrophils adhere to endothelial cells. They followed their own research2) in which they revealed that high-fat diet increases CXCL1, which activates peptidylarginine deiminase 4 (PAD4) and causes hypercitrullination of histone H3 in neutrophils. In this research, precise mechanisms of how neutrophils adhere to endothelial cells were not revealed. In the current study1), they investigated this unanswered question, and, surprisingly, the mechanism is independent of histone citrullination, but PAD4 citrullinates protein disulfide isomerase A1 (PDIA1) and further activates β2-integrin and promotes F-actin polymerization to induce neutrophil adhesion to endothelial cells.
It has been long studied how inflammation induces atherosclerosis. The role of macrophages, monocytes, and neutrophils are studied, and since, Warnatsch et al. reported that cholesterol crystals directly activate neutrophils and promote the release of neutrophil extracellular traps (NET), neutrophil–NET axis has been thought to be a pivotal key in developing atherosclerosis3). Further, the recent COVID-19 pandemic increased the attention on NET. Not only that cytokine storm is related to NET4) and prognosis of infected patients, but also NET-related vascular damages and atherosclerosis might cause long-lasting organ damages in COVID-19 survivors5). One of the key molecular mechanisms of NET is the citrullination of histone H3. Citrullinated histone induces intracellular chromatin decondensation, and it is one of the essential components of NET. NET traps bacteria and other foreign organisms, protecting us from infection and inflammation; conversely, it is believed to induce atherosclerosis; therefore, targeting NET or PAD, which is an enzyme for citrullination of histone, would be a potential therapy for atherosclerosis. This idea contains a double-edged sword risk; blocking NET or PAD is beneficial for the vasculature but notoriously increases the risk of severe infection.
Aoyama et al. reported that activation of PAD4 under a high-fat diet induces hypercitrullination of histone; also, PDIA1 is a substrate for PAD4 to be citrullinated by broad screening using LC-MS/MS. They mimicked the hypercholesterol diet by using CXCL1 to stimulate neutrophils in vitro. PAD4 is known to be activated by ROS as well. Thus, it is assumed that hypercholesterolemia and other conditions that increase ROS, such as aging, diabetes, and hypertension, are risks for developing atherosclerosis activate PAD4 and PDIA1. They showed that the knockdown or inhibition of PDIA1 successfully decreased the adhesion of neutrophils to endothelial cells by their original and very sophisticated method. Moreover, they added insight into the molecule downstream of PDIA1 to reveal αMβ2-integrin and F-actin (Fig.1). It suggests that inhibiting PDIA1 could be a promising preventing target to developing atherosclerosis without compromising the immune system. PDIA1 is an enzyme that can regulate protein folding and various proteins, of which those that contain disulfide bonds are the substrates. Indeed, red wine has the potency to inhibit PDI6), and in this case, the down streams are coagulation pathways. We should pay attention to off-target effects and evaluate values when opening new therapeutic doors, especially when targeting enzyme activity.
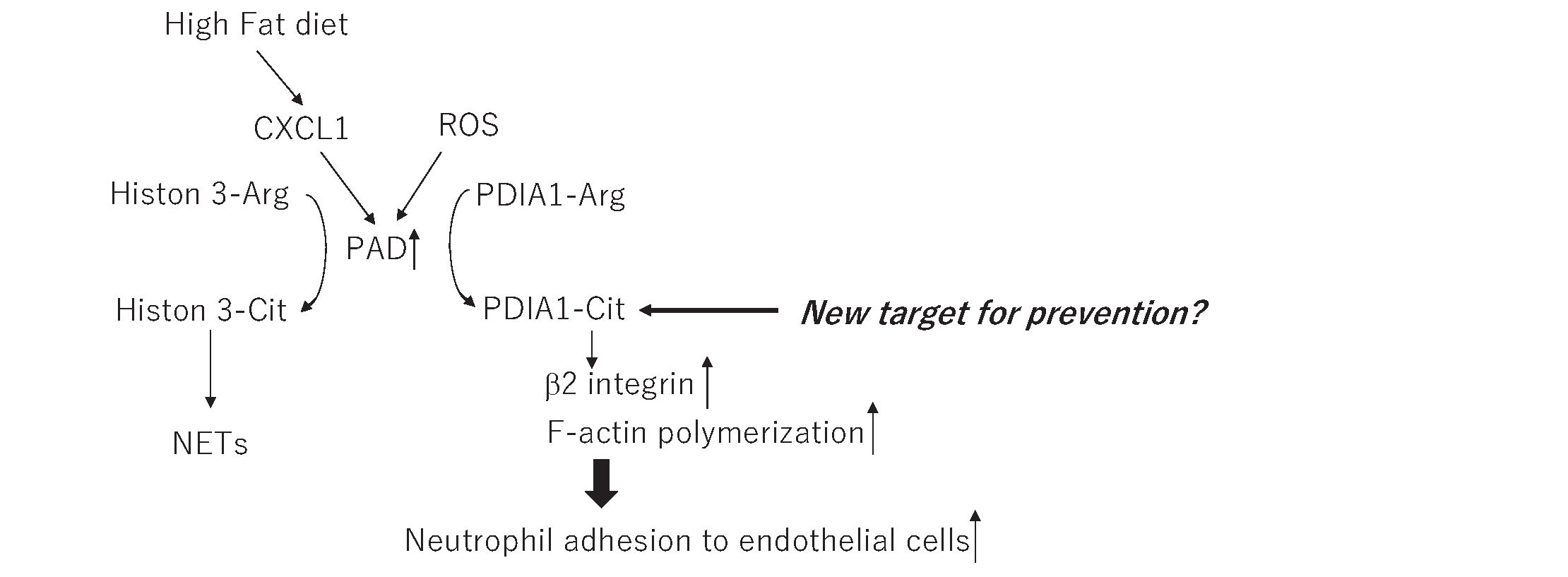
PDIA1 can be a new target to prevent atherosclerosis