2023 Volume 30 Issue 1 Pages 66-73
2023 Volume 30 Issue 1 Pages 66-73
Aim: A previous randomized study showed that dual antiplatelet therapy (DAPT) with aspirin and cilostazol is not superior to aspirin monotherapy for patients with acute non-cardioembolic stroke; however, the reason for this remains uncertain. We focused on the unusual side effects of cilostazol, namely, tachycardia changes, and validated their influence on patients with acute non-cardioembolic stroke.
Methods: This post-hoc study extracted data from the acute aspirin plus cilostazol dual therapy study (ADS) registry, a multicenter, prospective, randomized, open-label trial. Patients were randomly allocated to the dual group (aspirin plus cilostazol) and the aspirin monotherapy group (aspirin alone). Tachycardia changes were defined as ≥ 5% heart rate increase at 48 h after admission compared with that at admission. Baseline data and outcomes were validated with four divided groups: aspirin-non-tachycardia changes (AN), aspirin-tachycardia changes (AT), dual-non-tachycardia changes (DN), and dual-tachycardia changes (DT).
Results: Finally, 1,188 patients were analyzed in this ADS post-hoc analysis (aspirin monotherapy group, 594; dual group, 594). The proportion of change in tachycardia was 19.2% in the aspirin monotherapy group and 38.2% in the dual group (p<0.001***). Although the recurrences of symptomatic stroke and transient ischemic attack were not significantly different, the neurological deterioration was significantly different among the AN, AT, DN, and DT groups (p<0.05*).
Conclusions: Tachycardia changes increase neurological deterioration even in patients with non-cardioembolic acute stroke. DAPT consisting of aspirin and cilostazol increases the proportion of tachycardia changes and is not superior to aspirin monotherapy.
Antiplatelet therapy is the central pillar of acute non-cardioembolic stroke therapy. The advantage of dual antiplatelet therapy (DAPT) consisting of aspirin and clopidogrel for patients with acute non-cardioembolic stroke has already been proven by previous large-scale clinical studies1, 2). Cilostazol, a reversible phosphodiesterase enzyme III (PDE III) inhibitor, is an antiplatelet drug with pleiotropic effects such as the improvement of endothelial function, neovascularization, and anti-inflammatory effects3). Thus, aspirin and cilostazol DAPT for patients with acute non-cardioembolic stroke was also expected to be more beneficial than aspirin monotherapy, and an acute aspirin plus cilostazol dual therapy study (ADS) was conducted. Contrary to the pre-study expectation, the real ADS results showed that aspirin and cilostazol DAPT for patients with acute non-cardioembolic stroke did not reduce the neurological deterioration in the acute stroke phase or demonstrate any advantages over aspirin monotherapy4).
The reason why aspirin and cilostazol DAPT is not superior to aspirin alone for patients with acute non-cardioembolic stroke was initially not well understood because the bleeding side effect was not significantly different between the two groups4). Considering solely the antiplatelet effect, the addition of cilostazol should show an advantage. From previous randomized studies1, 2, 4), some factors pertaining to the differences between clopidogrel and cilostazol effects are speculated to be related to the results. We focused on tachycardia changes, a side effect of cilostazol, induced in some patients5). These changes did not generally occur with clopidogrel. Thus, we performed ADS post-hoc analysis focusing on the effects of tachycardia changes on patients with acute non-cardioembolic stroke.
This post-hoc study extracted the data from the ADS registry, a multicenter, prospective, randomized, open-label trial. The purpose of the ADS trial was comparing the efficacy and safety of acute aspirin plus cilostazol dual therapy for acute mild stroke within 48 h of symptoms onset. The ADS trial was conducted between May 2011 and June 2017, involving 34 centers in Japan. The institutional review boards at all participating institutions approved the study, which was registered with the Japan Clinical Trials Registry (http://umin.ac.jp/ctr/index/htm), under the number UMIN000004950. The study was organized by a coordinating center at Kawasaki Medical School from May 2011 until June 2014 and then by Nippon Medical School from July 2014 until June 2017. The principal investigator (K.K.) had complete access to all data and was responsible for preparing the manuscript. Clinical data were registered in an online database managed by the Shibasho Corporation (Osaka, Japan). Patients were randomly allocated to the dual group (aspirin plus cilostazol) and the aspirin monotherapy group (aspirin alone). The inclusion criteria were as follows: age ≥ 18 years, non-cardioembolic stroke, presented within 48 h of symptoms onset, neurological deficits with National Institutes of Health Stroke Scale (NIHSS) scores <20, and a pre-morbid modified Rankin Scale (mRS) score between 0 and 2. The exclusion criteria were as follows: cardioembolic stroke with a high-risk source defined by the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) criteria6), the use of antiplatelet agents, cilostazol, aspirin ≥ 200 mg, clopidogrel, ticlopidine, or any anticoagulants before stroke onset, and having undergone or planning to undergo thrombectomy.
Patients in the dual group were treated with cilostazol (200 mg/day) and aspirin (80–200 mg/day) for 14 days, whereas those in the aspirin monotherapy group were treated with only aspirin (80–200 mg/day) for 14 days. Because neurological status frequently deteriorated within the acute phase and long-term dual therapy is likely to cause significant hemorrhagic complications, the period of dual therapy was defined as 2 weeks (1 week shorter than the CHANCE1) or POINT2) studies). After 2 weeks, cilostazol (200 mg/day) was continued for 3 months after stroke onset. Concomitant warfarin, clopidogrel, ticlopidine, sarpogrelate, and urokinase were excluded.
2.2. Definition of Tachycardia ChangesThe purpose of this post-hoc analysis study was to validate the effects of tachycardia changes on patients with acute non-cardioembolic stroke treated with antiplatelet drugs. For the present study, we defined tachycardia changes as a ≥ 5% increase in heart rate at 48 h after admission compared with that at admission (≥ 5% increase definition was referred to other studies focusing on tachycardia influence in acute stroke7, 8)). In addition, to validate the tachycardia effect with degree, we added the sub-analysis to the dual-tachycardia changed group divided into the mild tachycardia group and the severe tachycardia group.
2.3. PatientsWe only included patients with acute non-cardioembolic ischemic stroke who underwent a 14-day assessment. Therefore, patients who did not undergo a 14-day assessment were excluded. In addition, for the purpose of this study, cases with inadequate heart rate data at 48 h after admission were excluded.
2.4. OutcomeA primary efficacy outcome was defined as any one of the following occurring within 14 days of onset: neurological deterioration, symptomatic stroke recurrence, and transient ischemic attack (TIA), which is the same as the primary outcome of ADS. When the clinical symptoms deteriorated or new neurological symptoms appeared, we recorded the NIHSS score at that time. In addition, neurological deterioration was defined as positive when NIHSS scores increased by two or more points within 14 days after admission. As a secondary outcome, the NIHSS score at 7 and 14 days after admission, antiplatelet drug withdrawal within 14 days of onset, intracranial bleeding, duration of hospital stay, and discharge mRS were validated.
2.5. Statistical AnalysisFirst, the tachycardia change proportions were calculated in both the aspirin monotherapy and the dual (aspirin plus cilostazol) groups and were validated using the chi-square test. Next, baseline data and outcomes were validated among four groups: aspirin non-tachycardia changes (AN), aspirin-tachycardia changes (AT), dual-non-tachycardia changes (DN), and dual-tachycardia changes (DT). All statistical analyses were performed using the JMP 16 statistical software (SAS Institute Inc., Cary, NC, USA). The data are expressed as median±interquartile range and were analyzed using the chi-square test or nonparametric Kruskal–Wallis test. Statistical significance was set at p<0.05. According to the statistically significant data, for post-hoc analysis clearing differences among the four groups, the corresponding analysis was performed using the chi-square test, and the Steel–Dwass test was performed using the Kruskal–Wallis test.
Between February 2011 and March 2017, 1,208 patients were enrolled in the ADS trial. Seven patients withdrew their consent after the study started, and 10 patients were lost to follow-up at 14 days. Three patients in the aspirin monotherapy group were excluded as their heart rates were not measured at 48 h after admission. Finally, 1,188 patients were analyzed in this ADS post-hoc analysis, which consisted of 594 patients in the aspirin monotherapy group and 594 patients in the dual group.
3.2. Proportion of Tachycardia Changes in the Aspirin Monotherapy and Dual GroupsTable 1 shows the proportion of tachycardia changes in the aspirin monotherapy and dual groups. The tachycardia change (+) proportion was 19.2% in the aspirin monotherapy group and 38.2% in the dual group, which was significantly different (p<0.001***). There were 480 patients in the AN group, 114 patients in the AT group, 367 patients in the DN group, and 227 patients in the DT group.
| Aspirin monotherapy group (n = 594) | Dual group (n = 594) | p-value | |
|---|---|---|---|
| Tachycardia change (-) | 80.8% (n = 480) | 61.8% (n = 367) | <0.001***a |
| Tachycardia change (+) | 19.2% (n = 114) | 38.2% (n = 227) |
*Tachycardia change is defined as more than 5% increase in heart rate at 48 h after admission compared to that at admission.
a Chi-squared test was performed.
*p<0.05, **p<0.001, ***p<0.001
Table 2 shows the clinical backgrounds of the AN, AT, DN, and DT groups. Age, systolic blood pressure (sBP), diastolic blood pressure (dBP), cardiothoracic ratio (CTR), white blood cell count, and estimated glomerular filtration rate (eGFR) were significantly different among the four groups (p<0.05*, p<0.001***), whereas other baseline characteristics, history, and laboratory data were not significantly different.
| Aspirin monotherapy group (n = 594) | Dual group (n = 594) | p-value | |||
|---|---|---|---|---|---|
| AN group (n = 480) | AT group (n = 114) | DN group (n = 367) | DT group (n = 227) | ||
| Baseline characteristics | |||||
| Age (y), [median (IQR)] | 68 (60-77) | 73 (65-78) | 68 (59-76) | 71 (62-77) | <0.001***a |
| Male (%) | 67 | 62 | 69 | 63 | 0.43 |
| Female (%) | 33 | 38 | 31 | 37 | |
| BMI, [median (IQR)] | 24 (22–26) | 24 (21–26) | 24 (22–26) | 23 (22–26) | 0.77 |
| Smoking (%) Non-smoker | 50 | 59 | 49 | 56 | 0.06 |
| Past smoker | 14 | 19 | 15 | 16 | |
| Current smoker | 36 | 22 | 36 | 29 | |
| Drinking (%) | 45 | 45 | 48 | 44 | 0.79 |
|
Systolic blood pressure (mmHg), [median (IQR)] |
162 (146-183) | 157 (143-169) | 162 (145-181) | 162 (148-180) | 0.13 |
|
Diastolic blood pressure (mmHg), [median (IQR)] |
90 (80-102) | 86 (79-98) | 91 (80-103) | 88 (78-100) | <0.05*a |
| Pre-stroke mRS [median (IQR)] | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.06 |
|
Admission NIHSS scale [median, (IQR)] |
2 (1–4) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 0.98 |
|
Onset to admission time (min) [median, (IQR)] |
668 (265–1227) | 745 (367–1320) | 616 (265–1200) | 630 (300–1240) | 0.27 |
| Past history (+) (%) | |||||
| Hypertension | 78 | 77 | 75 | 78 | 1.02 |
| Diabetes mellitus | 31 | 34 | 31 | 33 | 0.90 |
| Hyperlipidemia | 49 | 47 | 47 | 45 | 0.83 |
| Ischemic stroke | 9 | 15 | 8 | 10 | 0.22 |
| Hemorrhage stroke | 1 | 3 | 2 | 1 | 0.50 |
| Heart failure | 4 | 4 | 4 | 7 | 0.43 |
| Chronic kidney disease | 4 | 6 | 3 | 3 | 0.29 |
| Laboratory data [median, (IQR)] | |||||
| CTR (%) | 52 (47–55) | 52 (50–56) | 51 (47–55) | 52 (48–56) | <0.05*a |
| WBC (/μL) | 6600 (5400–8200) | 6500 (5400–8230) | 6900 (5550–8500) | 6400 (5150–7700) | <0.05*a |
| LDL (mg/dL) | 120 (97–145) | 115 (94–139) | 124 (95–149) | 125 (100–150) | 0.27 |
| hsCRP (mg/dL) | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | 0.86 |
| D-dimer (μg/dl) | 0.7 (0.5–1.1) | 0.7 (0.5–1.0) | 0.7 (0.5–1.2) | 0.8 (0.5–1.2) | 0.50 |
| eGFR (mL/min/1.73 m2) | 73 (62–88) | 71 (56–87) | 76 (63–90) | 70 (59–82) | <0.01**a |
| HbA1c (%) | 5.8 (5.5–6.6) | 5.8 (5.3–6.5) | 5.8 (5.4–6.5) | 5.8 (5.4–6.6) | 0.77 |
| BNP (pg/mL) | 27 (13–58) | 28 (18–49) | 24 (13–54) | 27 (13–48) | 0.57 |
BMI: body mass index, BNP: brain natriuretic peptide, CTR: cardiothoracic ratio, eGFR: estimate glomerular filtration rate, HbA1c: hemoglobin A1c, hsCRP: high-sensitive C-reactive protein, IQR: interquartile range, mRS: modified Rankin Scale, NIHSS: National Institutes of Health Stroke Scale, LDL: low-density lipoprotein cholesterol, WBC: white blood cell count
a Nonparametric Kruskal–Wallis test was performed.
*p<0.05, **p<0.001, ***p<0.001
Table 3 shows the outcome results of the ADS post-hoc analysis. According to the primary outcome, there were significant differences among the AN, AT, DN, and DT groups (p<0.05*). The AN and DN groups were combined into one category, whereas the AT and DT groups were combined into another with the corresponding analysis (Fig.1). Although the symptomatic stroke recurrence and TIA were not significantly different, neurological deterioration was significantly different among the four groups (p<0.05*).
| Aspirin monotherapy group (n = 594) | Dual group (n = 594) | p-value | |||
|---|---|---|---|---|---|
| AN group (n = 480) | AT group (n = 114) | DN group (n = 367) | DT group (n = 227) | ||
| Primary outcome ( = one of the event; neurological deterioration, symptomatic stroke recurrence, or TIA) | 9.8% | 14.9% | 8.5% | 15.4% | <0.05*a |
| Neurological deterioration | 9.0% | 13.2% | 7.9% | 15.0% | <0.05*a |
| Symptomatic stroke recurrence | 1.5% | 0.9% | 1.1% | 0.9% | 0.89 |
| TIA | 0.0% | 0.9% | 0.3% | 0.0% | 0.18 |
| NIHSS scale at day 7 [median (IQR)] | 1 (0–3) | 1 (0–3) | 1 (0–3) | 1 (0–3) | 0.79 |
| NIHSS scale at day 14 [median (IQR)] | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.31 |
|
Systolic blood pressure at 48h (mmHg), [median (IQR)] |
146 (130-160) | 142 (129-160) | 139 (126-156) | 139 (125-155) | <0.01**b |
|
Diastolic blood pressure at 48h (mmHg), [median (IQR)] |
80 (70-90) | 78 (69-90) | 76 (67-85) | 75 (66-84) | <0.001***b |
|
Antiplatelet drug withdrawal within 14 days of onset |
6.1% | 14.0% | 12.3% | 15.4% | <0.001***a |
| Intracranial bleeding | 0.2% | 0.0% | 0.0% | 0.9% | 0.19 |
| Hospital stay (days) [median (IQR)] | 15.6 (10.4–24.2) | 17.0 (10.9–27.6) | 14.4 (10.2–20.5) | 14.6 (10.6–22.6) | <0.05*b |
| Discharge mRS [median (IQR)] | 1 (1–2) | 1 (1–3) | 1 (0–2) | 1 (1–2) | <0.05*b |
| mRS 0–2 proportion (%) | 76.0 | 70.2 | 81.7 | 76.7 | <0.05*a |
IQR: interquartile range, mRS: modified Rankin Scale, NIHSS: National Institutes of Health Stroke Scale, TIA: transient ischemic attack
a Chi-squared test was performed.
a Nonparametric Kruskal–Wallis test was performed.
*p<0.05, **p<0.01, ***p<0.001
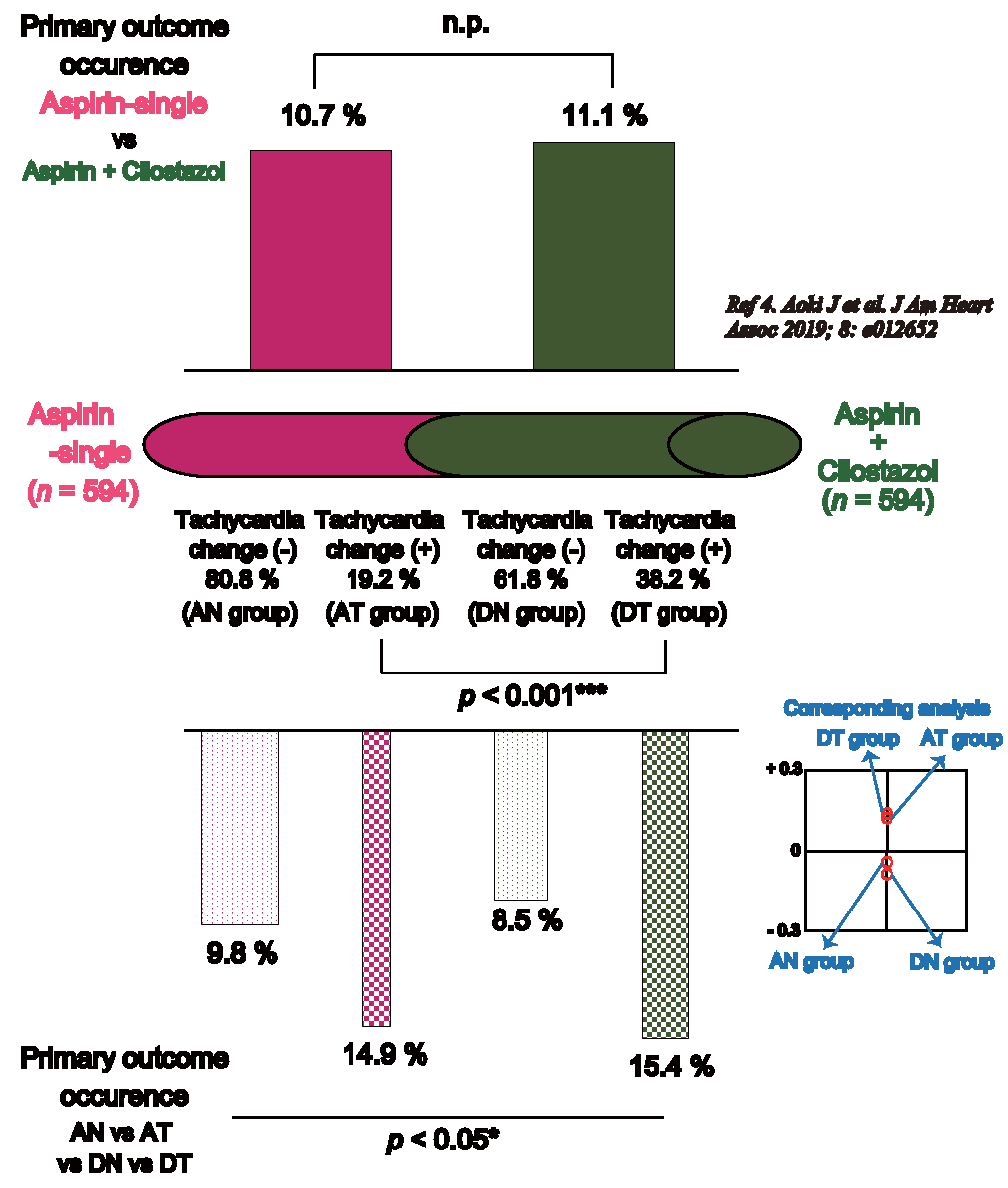
The complete results of the ADS post-hoc analysis regarding tachycardia changes
According to the secondary outcome, there were no significant differences in the NIHSS score at day 7, NIHSS score at day 14, or intracranial bleeding rate among the AN, AT, DN, and DT groups (Table 3). By contrast, the sBP and dBP at 48 h after admission, the antiplatelet drug withdrawal rate within 14 days of onset, the duration of hospital stay, and the discharge mRS were significantly different among the four groups (p<0.05*, p<0.01**, p<0.001***). Following the Steel–Dwass test, both the sBP and dBP at 48 h after admission of the AN group were significantly higher than those of the DN group or of the DT group. The duration of hospital stay of the DN group was significantly shorter than that of the AT group (14.4 vs. 17.0 days; p<0.05*). The mRS 0–2 proportions were 76.0% in the AN group, 70.2% in the AT group, 81.7% in the DN group, and 76.7% in the DT group, showing a significant difference (p<0.05*).
We also compared the mild and the severe tachycardia groups in the DT group (Table 4). The cutoff point between the mild and severe groups set the 18.2% increase in heart rate at 48 h, which was the median value of the DT group. The severe tachycardia group (heart rate increase ≥ 18.2%) showed the worse outcomes tendency than the mild tachycardia group (heart rate increase=5%–18.2%).
| Dual tachycardia (DT) group (n = 227) | |||
|---|---|---|---|
|
Mild tachycardia group (HR increase = 5-18.2%) (n = 114) |
Severe tachycardia group (HR increase ≥ 18.2%) (n = 113) | p-value | |
| Primary outcome ( = one of the event; neurological deterioration, symptomatic stroke recurrence, or TIA) | 11.4% | 19.5% | 0.09 |
| Neurological deterioration | 10.5% | 19.5% | 0.06 |
| Symptomatic stroke recurrence | 0.9% | 0.9% | 1.00 |
| TIA | 0.0% | 0.0% | - |
| NIHSS scale at day 7 [median (IQR)] | 1 (0–2) | 1 (0–3) | 0.39 |
| NIHSS scale at day 14 [median (IQR)] | 1 (0–2) | 1 (0–2) | 0.11 |
|
Antiplatelet drug withdrawal within 14 days of onset |
14.0% | 16.8% | 0.56 |
| Intracranial bleeding | 0.9% | 0.9% | 1.00 |
| Hospital stay (days) [median (IQR)] | 15.2 (11.3–23.7) | 14.4 (9.9–20.7) | 0.26 |
| Discharge mRS [median (IQR)] | 1 (1–2) | 1 (1–3) | 0.56 |
| mRS 0–2 proportion (%) | 79.8 | 73.5 | 0.26 |
HR: heart rate, IQR: interquartile range, mRS: modified Rankin Scale, NIHSS: National Institutes of Health Stroke Scale, TIA: transient ischemic attack
a Chi-squared test was performed.
a Nonparametric Kruskal–Wallis test was performed.
The results of this ADS post-hoc analysis according to tachycardia changes are shown in Fig.1.
Our results show that 1) the proportion of tachycardia changes (≥ 5% increase in heart rate) significantly increased, nearly doubled (38.2% vs. 19.2%, p<0.001***), in the dual group compared to the aspirin monotherapy group and 2) the tachycardia changes are significantly associated with neurological deterioration—the NIHSS score increased by two or more points in both the aspirin monotherapy and dual groups (p<0.05*). The primary outcome proportions of tachycardia changes (+) or (−) were remarkably similar in both the aspirin monotherapy and dual groups (9.8% in AN and 8.5% in DN vs. 14.9% in AT and 15.4% in DT).
Tachycardia is reported as a negative prognostic factor for both ischemic and hemorrhagic stroke outcome7), and heart rate influences stroke recurrence and mortality in acute ischemic stroke with atrial fibrillation8). However, to our knowledge, validation of tachycardia or heart rate effects on patients with acute non-cardioembolic stroke in a prospective randomized trial has never been reported. For patients with cardioembolic acute stroke, tachycardia on admission is considered a negative prognostic factor9). However, it remains uncertain whether tachycardia increases stroke recurrence or independently worsens ischemic stroke outcome. A previous observational study reported that tachycardia of ≥ 120 bpm occurred in 15% of all acute ischemic stroke cases and independently predicted poor outcomes regardless of the TOAST criteria subtype10). Another study on tachycardia in acute ischemic stroke, focusing on the tachycardia burden, which is defined as the duration of heart rate over 95 per minute divided by the total monitoring time, also shows that tachycardia burden is a factor for poor 3-month functional outcome11). Our ADS results, thus, support previous findings that tachycardia is a negative prognostic factor for acute ischemic stroke. That said, these studies have some limitations such as the non-randomized nature of the trials and the heterogeneity of the participants as they comprised patients with both acute cardioembolic and non-cardioembolic stroke10, 11). Our ADS complements the prospective, multicenter, randomized trial results, and the tachycardia effect on acute non-cardioembolic stroke. Our study also revealed that both the sBP and dBP at 48 h after admission of the DN and the DT groups, dual group (aspirin plus cilostazol), were significantly decreased than those of the aspirin monotherapy group. Some studies show that the blood pressure drop is the unfavorable status in the acute ischemic stroke12, 13). Cilostazol decreases blood pressure with the vasodilator action than aspirin in the acute ischemic stroke phase and may concern with the prognosis. In contrast, the outcomes between the DN and DT groups were different, although the blood pressure changes of both groups were similar, suggesting that tachycardia status independently affects the outcomes.
Cilostazol specifically inhibits PDE III, which is compartmentalized in cardiac monocytes and vascular smooth vessels due to its sarcoplasmic reticulum-anchoring moiety. Inhibition of PDE III induces a reaction cascade that increases cyclic adenosine monophosphate levels in these tissues, which, in turn, activates protein kinase A and increases heart rate5, 14). There are several possibilities based on the effects of the increase in heart rate due to cilostazol on acute ischemic stroke. The possibility that an increase in heart rate increases the risk of stroke recurrence in patients with cardioembolic stroke was considered in a previous study8). However, in our ADS, no significant difference was observed between symptomatic stroke and TIA. However, neurological deterioration showed a significant difference, suggesting the possibility that the increased heart rate itself might worsen the ischemic status of non-cardioembolic stroke. Although cardiac contractility, cardiac output, and arterial pressure increase due to dobutamine, the blood flow of the internal carotid artery decreases, and cerebral blood flow worsens15). In addition, an increase in heart rate induces increase in cardiac oxygen consumption, reducing the oxygen supply to the brain16). An interesting and important study reported a linear relationship between heart rate and oxygen uptake in thoracic level paraplegics17). This shows that an increase in heart rate influences the oxygen uptake of the central nervous system.
Factors other than tachycardia may be related to the ADS result. The dual therapy period of 2 weeks, 1 week shorter than that of previous randomized trials, may be the cause of the dual therapy underperformance. The significant difference in the antiplatelet drug withdrawal rate between the aspirin monotherapy and dual groups is also a concern. By contrast, the real ADS results pertaining to tachycardia and primary outcome differences between the two groups showed important suggestions.
Although aspirin and cilostazol DAPT was not superior to aspirin alone with respect to neurological deterioration in patients with acute non-cardioembolic stroke, this ADS post-hoc analysis suggests the potential of aspirin and cilostazol DAPT. Required days of hospital stay and the proportion of mRS results showed the benefit of aspirin and cilostazol DAPT in the absence of tachycardia. To prevent tachycardia, a combination of β-blocker in addition to aspirin and cilostazol DAPT may provide benefits for patients with acute non-cardioembolic stroke.
This study has some limitations. First, the heart rate was evaluated only twice upon admission and at 48 h after admission. Continuous or multiple heart rate evaluations were not available. Second, dividing the groups further into four subgroups weakened the statistical power of the study. Third, neurological relentless did not define based on the tissue such as MRI infarction area but on the clinical. The data according to the tissue difference between DAPT and aspirin monotherapy were absent in this study. Fourth, most patient NIHSS scores at admission in our study were low. In results, the DAPT effect to moderate to severe ischemic stroke, the higher NIHSS score, was unclear from our study results. Fifth, some confounding factors may be present according to the antiplatelet drug withdrawal of patients in our study. After randomization, the antiplatelet drug was withdrawn when the paroxysmal atrial fibrillation was detected, but the paroxysmal atrial fibrillation was significantly more detected in the dual group18).
In conclusion, our results suggest that tachycardia changes affect neurological deterioration and carry a poor functional outcome for patients with acute non-cardioembolic stroke regardless of the antiplatelet treatment regimen. Additionally, further verification of dual therapy with cilostazol without the effect of tachycardia changes is required.
The authors thank all clinical staff who assisted with the study. We would like to thank Editage (www.editage.com) for English language editing.
Dr. Fujimoto reports having received lecture fees and speaker’s fees from Nippon Boehringer Ingelheim, Co., Ltd, Bayer Yakuhin, Ltd, Pfizer Japan Inc., DAIICHI SANKYO Co., Ltd., Eisai Co., Ltd., and Bristol-Myers Squibb Co. Dr Kimura reports having received lecture fees and speaker’s fees from Otsuka Pharmaceutical Co., Bayer Yakuhin, Ltd, and Sanofi K.K.
This study received funding from Kawasaki Medical School and Nippon Medical School and an endowment from Otsuka Pharmaceutical Corporation (Tokyo, Japan) that markets cilostazol (Pletaal). Otsuka Pharmaceutical Corporation was blinded to the study design, data collection, and data analysis. This was stated on the informed consent form approved by each institutional review board, and the conflict of interest was appropriately managed at all participating institutions.