2023 Volume 30 Issue 2 Pages 170-181
2023 Volume 30 Issue 2 Pages 170-181
Aim: Perilipins (PLINs), peripheral lipid droplet (LD) proteins, play important roles in lipid accumulation and maturation in adipocytes. The relationship between PLIN family proteins and macrophage polarization in atherosclerosis has not been elucidated.
Methods: The experiments used tissues from human arteries of 65 patients who had undergone a carotid endarterectomy, and cultured macrophages generated from healthy human peripheral blood mononuclear cells.
Results: Plaque immunohistochemistry demonstrated co-expression of PLIN1 and PLIN2 in both symptomatic (n=31) and asymptomatic patients (n=34). PLIN2 mRNA expression increased 3.38-fold in the symptomatic group compared with those from asymptomatic. PLIN1 was not expressed on small LDs at a shorter incubation but was on large LDs at longer incubation with oxidized LDL and VLDL, while PLIN2 was observed after 24 h and increased with a longer incubation in cultured M1 macrophage. In M2 macrophages, PLIN1 was seen as early as 24 h following incubation with VLDL, and LD size increased with longer incubation. PLIN1 overexpression increased the size of LDs in M1 macrophages, even after a short incubation, and reduced the RNA expression of TNFA, MMP2, ABCA1, and ABCG1 versus the M1 control. Conversely, silencing of PLIN1 in M2 macrophages had the opposite effects on LD size and RNA expression.
Conclusion: There was a relationship between macrophage polarity, cytosolic LD size, and PLIN1/PLIN2 expression levels. PLIN2 was mainly expressed in arterial plaques in symptomatic stroke patients, and associated with the inflammatory phenotype of human macrophages, while PLIN1 expression is closely associated with plaque stability and the anti-inflammatory phenotype.
Macrophages store lipids and develop into foam cells in atherosclerotic plaques1, 2). We have shown that the polarity of infiltrating macrophages (predominantly pro- or anti-inflammatory) in human atherosclerotic plaques is associated with plaque vulnerability and the incidence of stroke3). Plaques in patients with a history of symptomatic stroke are more unstable and contain larger numbers and a higher proportion of pro-inflammatory M1 macrophages than those in asymptomatic patients. In the latter, anti-inflammatory M2 macrophages predominate.
Macrophages store lipids in cytosolic lipid droplets (LDs)4). Intracellular lipid storage in macrophages or other non-adipose tissues plays an important role in the development of atherosclerosis or insulin resistance5). Two major lipoproteins, oxidized low-density lipoprotein (oxLDL) and remnants of very low-density lipoprotein (VLDL), contribute to macrophage foam cell formation6, 7). The accumulation of cholesterol esters in macrophages is induced by oxLDL particles after they are taken up by scavenger receptors. VLDLs are rich in triglyceride and are transported into macrophages by the VLDL receptor, which is not present in mice, but is present in human and rabbit macrophages8). Foam cell LDs have been reported to be surrounded by molecules of the LD protein perilipin 2 (PLIN2; adipophilin; ADRP)9).
In adipose tissue, immature LDs are coated with PLIN2, but this is replaced by perilipin 1 (PLIN1; perilipin A) during maturation10-13). PLIN1 plays a central role in adipocyte LD formation and maturation, which involves the coordination of a number of proteins, such as hormone-sensitive lipase11, 13-15). Low expression and impaired function of PLIN1 in humans are associated with adipose atrophy, abnormal glucose and cholesterol metabolism, hypertriglyceridemia, hyperglycemia, and hyperinsulinemia16, 17). Furthermore, PLIN1 mutants cannot bind their normal interacting proteins, which results in greater basal lipolysis18). Finally, in transgenic mice fed a high-fat diet, the overexpression of PLIN1 improves glucose and insulin homeostasis19, 20). Therefore, it has been suggested that PLIN1 is required for the stable and efficient storage of lipids in LDs and for the prevention of lipotoxicity by the sequestration of harmful lipids in LDs. However, the expression and function of PLIN1 in macrophages are poorly understood.
PLIN1 was initially identified in adipocytes21), but was later shown to also be expressed in macrophages, and specifically in ruptured plaques in mice22, 23). Studies of ApoE-knockout mice have helped elucidate the relationship between PLIN family members in macrophages and the pathogenesis of atherosclerotic plaques. PLIN2 inactivation protects against atherosclerosis24). In human THP-1 cells, it has been suggested that PLIN2 coexists with PLIN1 and regulates intracellular lipid accumulation and cholesterol efflux through the regulation of neutral cholesteryl ester hydrolase9). PLIN1 overexpression in macrophages protects against the progression of atheroma in ApoE-knockout mice, in the absence of changes in fat mass or plasma lipid profile, presumably through effects on the stability and/or inflammatory phenotype of the macrophages25). However, the relationship between macrophage polarity and LD protein expression, and its involvement in the pathophysiology of atherosclerosis, have not been investigated.
In the present study, we first demonstrated the expression of LD proteins in macrophages in human atherosclerotic plaques. Then, to determine the role of LD proteins, including PLIN1, in human macrophages and the relationship between PLIN1 and macrophage inflammatory polarity, we measured LD protein expression in atherosclerotic plaques from patients who had undergone carotid endarterectomy (CEA) and in macrophages derived from healthy human peripheral blood mononuclear cells.
We studied patients who underwent CEA at Hokkaido University Hospital. The present trial was registered with the University Hospital Medical Information Network (UMIN) Center (UMIN 000008661) before enrolment commenced. The study protocol was approved by the Ethics Review Board of Hokkaido Hospital (approval 09-Aug 2012), and was performed according to Good Clinical Practice and the principles of the Declaration of Helsinki. Written informed consent was obtained prior to surgery.
The clinical indication for CEA was high-grade (>70%) stenosis in asymptomatic patients with stable plaques, or intermediate-grade (>50%) stenosis in patients with any ischemic symptoms resulting from the presence of unstable plaque in the internal carotid artery, according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) guidelines, identified by ultrasonography, computed tomography, or magnetic resonance imaging. The excised atherosclerotic lesions were cut into pieces for subsequent RNA and protein expression analysis. We enrolled 65 patients (52 men and 13 women; mean age 68.9 years old), who were allocated to two groups: an asymptomatic group (n=34) and a symptomatic group (n=31). The clinical characteristics of the participants and the definitions of stable and unstable plaques have been provided previously3).
Cell CultureMonocytes were harvested from healthy human peripheral blood samples using Magnetic Activated Cell Sorting (Miltenyi Biotec, Bergisch Gladbach, Germany) and differentiated into macrophages. Monocyte differentiation was induced by 6 days of culture in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA), supplemented with gentamicin (40 µg/mL), L-glutamine (2 mmol/L) (Sigma-Aldrich, St. Louis, MI, USA), and 10% pooled human serum (Abcys, Talloires-Montmin, France). Differentiated M1 and M2 macrophages were obtained from these monocytes using previously described methods26). Briefly, M1 macrophage differentiation was induced by the addition of tumor necrosis factor (TNF)α (10 ng/ml) and M2 macrophage differentiation was induced by the addition of a low concentration of recombinant human interleukin (IL)-4 (15 ng/ml) (Promocell, Heidelberg, Germany) for 6 days. The differentiation to M1/M2 macrophages were confirmed by immunostaining of CD11c/CD163 (Supplemental Fig.1). Then, the macrophages were washed and incubated for 24 h or 7 days with oxLDL or VLDL (Meridian Life Science, Inc., TN, USA), after which they were lysed for protein expression analysis or fixed in formaldehyde for histological analysis.Thirty macrophages were randomly selected in each group and the mean diameter of the largest 10 LDs in each macrophage was calculated.

Green, anti-CD11c; red, anti-CD163; blue, DAPI. Scale bar=10 µm.
Recombinant adenovirus expressing PLIN1 was generated as described previously11-13, 25). M1 macrophages were infected using this adenovirus and then oxLDL / VLDL was added for 24 h. siRNA (Santa Cruz Biotechnology, Dallas, TX, US) was used to knock down PLIN1 expression in M2 macrophages, then oxLDL / VLDL or 5 µg/mL of T0901317 (synthetic LXRα agonist; Abcam, Cambridge, UK) were added for 24 h. These cells were then compared with macrophages that had not been treated with adenovirus or siRNA targeting PLIN1.
Immunohistochemistry and ImmunofluorescenceRabbit polyclonal antibodies targeting PLIN1 or PLIN2 were generated as described previously27). Mouse monoclonal anti-human CD68 (1:100, Dako Cytomation, Glostrup, Denmark), mouse monoclonal anti-CD11c (1:100, Dako), mouse monoclonal anti-human CD163 (1:100, Leica Biosystems, Wetzlar, Germany), and anti-alpha smooth muscle actin (α-SMA) (1:100, Abcam, Cambridge, UK) antibodies were purchased. Histological specimens and cells were blocked with 10% goat non-immune serum and were then incubated with a primary antibody overnight at 4℃. They were then stained using a DAB substrate kit (Nichirei Bioscience, Tokyo, Japan). For immunofluorescence, cells were cultured on coverslips placed in dishes and then incubated with fluorescently-labeled antibodies and Alexa Fluor 594-conjugated goat anti-rabbit IgG (Thermo Fisher Scientific, Waltham, MA, US) for 60 min, BODIPY lipid probes for triglyceride (Molecular Probes, Eugene, OR, US) for 60 min, and DAPI (Thermo Fisher Scientific). Images were acquired using a BZ-9000 microscope (Keyence, Osaka, Japan).
RNA Extraction and AnalysisRNA was extracted from plaques and macrophages using TRI reagent (Qiagen, Hilden, Germany) and purified using an RNeasy Total RNA Isolation Kit (Qiagen), then cDNA was generated from 0.5 µg of RNA (High-capacity RNA-to-cDNA kit, Applied Biosystems, Warrington, UK). Real-time PCR was performed using SYBR® Green PCR Master Mix (Applied Biosystems) and primers designed using Primer Express (Applied Biosystems). Relative gene expression was determined using the comparative critical threshold method and target gene expression was normalized to that of 18S.
Lysate Preparation and Immunoblot AnalysisPlaque samples were manually homogenized in 10 mM Tris/HCl (pH 7.4)/1 mM EDTA buffer. Equal amounts of protein, quantified using a BCA assay kit, were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. Primary antibodies targeting PLIN1 (1:500), PLIN2 (1:500), CD68 (1:1000), CD11c (1:100), or CD168 (1:500) were applied, followed by anti-rabbit (PLIN1 and PLIN2) or anti-mouse IgG (CD68, CD11c, and CD163) secondary antibodies. To ensure equal loading, membranes were stripped and re-probed with goat anti-human β-actin antibody (1:2,000; Santa Cruz). Specific bands were visualized using an Amersham ECL Advance Western Blotting Detection Kit (GE Healthcare, Little Chalfont, UK) and images were obtained using a CCD-camera system (LAS-4000UVmini; Fujifilm, Tokyo, Japan).
Statistical AnalysisContinuous data are presented as means with standard deviations or medians with minima and maxima, as appropriate, and categorical data as counts and percentages. Statistical comparisons were performed using the unpaired Student’s t-test or Mann–Whitney U test. Data were analyzed using JMP for Windows software (version 14.1.1, SAS Institute Inc. Cary, NC, US) and P<0.05 was considered to represent statistical significance.
Both PLIN1 and PLIN2 were expressed in human arterial plaque specimens obtained from patients undergoing CEA. Immunostaining and Western blotting revealed that PLIN2 expression was higher in plaques from the symptomatic group, whereas PLIN1 was expressed at similar levels in plaques from both groups (Fig.1A, 1B). The expression of PLIN1 was coexisted with the those of CD68, which indicated the presence of macrophages (Fig.1C). The mRNA expression of PLIN2 was 3.38-fold higher in plaques from symptomatic than asymptomatic patients (Fig.2). There were no significant differences in the mRNA expression of LD hydrolase proteins (adipose triglyceride lipase, ATGL; hormone-sensitive lipase, HSL; and neutral cholesterol ester hydrolase 1, NCEH1).

Specimens were obtained from patients undergoing carotid endarterectomy. The patients were categorized as either asymptomatic (A) or symptomatic (B) on the basis of an evaluation of their history and a clinical examination. Representative images of atherosclerotic carotid lesions, immunostained for PLIN1, PLIN2, CD68, or alpha-SMA, or to which non-immune antibodies were applied, are shown. The area within the dotted square on the section to which non-immune antibody was applied is the area to which the other antibodies were applied. *, vessel lumen. # and dotted line, incision line. Scale bar=100 µm. (C) A representative image of a plaque from the asymptomatic group immunostained with anti-PLIN1. Green, anti-CD68 (macrophages); red, anti-PLIN1; blue, DAPI. Scale bar=10 µm.

The relative mRNA expression of PLIN1, PLIN2, and hydrolases (adipose triglyceride lipase, ATGL; hormone-sensitive lipase, HSL; and neutral cholesterol ester hydrolase 1, NCEH1) was assessed using real-time PCR. The expression of mRNA species in plaques from asymptomatic patients was designated as 1.0. Data are presented as means±SDs (n=34 for the asymptomatic group and n=31 for the symptomatic group), *p<0.05. Gray bar, asymptomatic group; black bar, symptomatic group.
In cultured human macrophages, both PLIN1 and PLIN2 were expressed on the surface of LDs. Small LDs were coated with PLIN2, whereas large LDs were coated with PLIN1 (Fig.3, Supplemental Fig.2, and Supplemental Table 1). The LDs were larger in M2 macrophages than in M1 macrophages after the 7-day incubation (Fig.3A and B). CD11c, a pro-inflammatory macrophage marker, was expressed in M1 macrophages, but not in M2 macrophages, before their incubation with oxLDL or VLDL (Fig.3C and Supplemental Fig.3), whereas CD163 showed much higher expression in M2 macrophages before the incubation. CD11c expression gradually increased after the incubation with oxLDL or VLDL, whereas CD163 rapidly disappeared after the M1 macrophages were incubated with the lipoproteins.
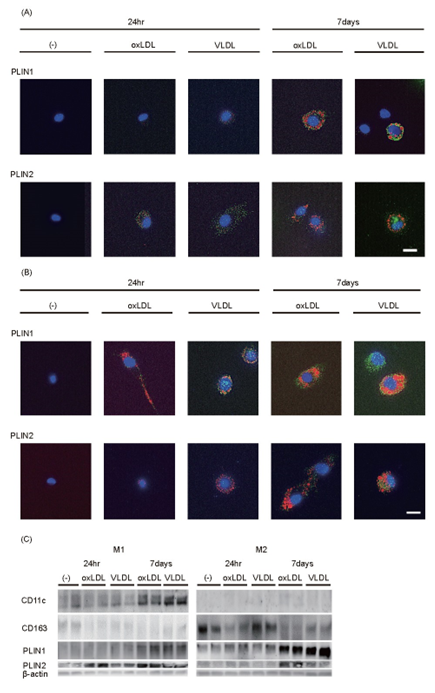
Monocytes harvested from human peripheral blood were differentiated into M1 (A) or M2 (B) macrophages, as described in the Methods, and then incubated with oxLDL or VLDL for the indicated time periods (24 hr or 7 days). Representative images of cultured human M1 (A) and M2 (B) macrophages immunostained with anti-PLIN1 (upper images) and anti-PLIN2 (lower images) are shown. Green, PLIN1 or PLIN2; red, cholesterol ester or triglyceride; blue, DAPI. Scale bar=100 µm. (C) Western blots for proteins from M1 and M2 macrophages incubated with oxLDL or VLDL. Cell lysates were prepared at the indicated time points of the incubation with oxLDL or VLDL and immunoblots for CD11c, a M1 macrophage marker, and CD163, a M2 macrophage marker, were generated.

Histogram of the mean diameter (mm) of lipid droplets in M1 macrophages (A) and M2 macrophages (B)
| 24hr None | 24hr oxLDL | 24hr VLDL | 7days oxLDL | 7days VLDL | |
|---|---|---|---|---|---|
| M1 | - | 0.68±0.43 | 0.75±0.24 | 2.39±1.01*** | 2.68±0.88*** |
| M2 | - | 1.13±0.38††† | 1.15±0.59†† | 3.11±0.73***†† | 4.22±1.20***†††‡‡ |
**P<0.01, ***P<0.001 for 24 hr vs. 7 days (paired t-test); ††P<0.01, †††P<0.001 for M1 vs. M2 (Student’s t-test); ‡P<0.01 for oxLDL vs. VLDL (Student’s t-test). None: without any lipoprotein.

Western blots for proteins from M1 and M2 macrophages incubated without oxLDL or VLDL for CD11c, CD163, PLIN1, PLIN2, and β-actin
In M1 macrophages, PLIN2 was expressed after the 24-h incubation with oxLDL or VLDL, whereas PLIN1 expression only became apparent after 7 days of incubation of the macrophages. In M2 macrophages, PLIN2 expression was higher after the long incubation, especially if it was with oxLDL. PLIN1 was expressed at an early stage, but its expression was much higher after the long incubation with oxLDL or VLDL, which was consistent with the presence of large LDs in M2 macrophages at this time point (Fig.3B and C). In contrast, PLIN1 was not expressed and the LDs were very small after the short-term incubation of M1 macrophages (Fig.3A and C). Large LDs developed, which were surrounded by PLIN1, shortly after PLIN1 overexpression was induced by adenoviral infection in M1 macrophages (Fig.4A and B). Following a 24-h incubation with oxLDL, VLDL (Fig.4C and 4D), or T0901317 (Fig.4D), PLIN1-overexpressing M1 macrophages showed significantly lower mRNA expression of TNFA, MMP2, ABCA1, and ABCG1 than control M1 macrophages that were similarly treated.

PLIN1 was adenovirally overexpressed (O/E), as described in the Methods. (A) Western blot demonstrating PLIN1-O/E. (B) Representative immunostaining for lipid droplets in PLIN1-O/E M1 macrophages following a 24-h incubation with oxLDL and VLDL. Green, PLIN1/PLIN2; red, LDs; blue, DAPI. Scale bar=10 µm. (C) Relative mRNA expression of M1/M2 polarizing genes (TNFA and IL4) and matrix metalloproteinases (MMP2 and MMP9) following a 24-h incubation of M1 macrophages that were or were not overexpressing PLIN1 with oxLDL and VLDL. (D) Relative mRNA expression of and lipid efflux proteins (ABCA1 and ABCG1) following a 24-h incubation of M1 macrophages that were or were not overexpressing PLIN1 with oxLDL, VLDL, or synthetic LXRα agonist TO901317 (T). The expression of each mRNA species in control M1 macrophages that had not been incubated with lipoproteins for 24-h was designated as 1.0. Data are means±SDs for experiments performed in triplicate. *p<0.05.
When PLIN1 expression in M2 macrophages was knocked down using siRNA (Fig.5A), the opposite pattern was induced with respect to LD size and mRNA expression: the formation of large LDs was suppressed (Fig.5B). Fig.5C showed that the mRNA expression of TNFA, MMP2, and MMP9 was significantly increased by PLIN1-knockdown and 24-h incubation with oxLDL or VLDL. The incubation with oxLDL and TO901317 increased the expression of ABCA1 and ABCG1, and the expression of these mRNAs was further increased by PLIN1-knockdown (Fig.5D).

PLIN1 was knocked down using siRNA (Si), as described in the Methods. (A) Western blotting to confirm PLIN1-deficiency. (B) Representative PLIN1-deficient M2 macrophages showing lipid droplets immunostained for PLIN1/PLIN2 following a 24-h incubation with oxLDL and VLDL. Green, PLIN1/PLIN2; red, LDs; blue, DAPI. Scale bar=10 µm. (C) Relative mRNA expression of M1/M2 polarizing genes (TNFA and IL4) and matrix metalloproteinases, (MMP2 and MMP9) following a 24-h incubation of PLIN1-silenced or control M2 macrophages with oxLDL and VLDL. (D) Relative mRNA expression of and lipid efflux proteins (ABCA1 and ABCG1) following a 24-h incubation of PLIN1-silenced or control M2 macrophages with oxLDL, VLDL, or TO901317 (T). The expression of mRNA species in control M2 macrophages that had or had not been incubated with lipoproteins for 24-h was designated as 1.0. Data are means±SDs for experiments performed in triplicate. *p<0.05.
In the present study, we have investigated the roles of the LD proteins, PLIN1 and PLIN2, in human macrophages using human carotid atherosclerotic plaque samples and cultured macrophages derived from circulating human monocytes. We have shown that PLIN2 is expressed at higher levels in carotid atherosclerotic plaques from symptomatic patients, whereas PLIN1 is expressed at similar levels in plaques from symptomatic and asymptomatic patients. We have also demonstrated close relationships of macrophage polarity (M1 versus M2) with the protein composition of LDs (PLIN2 versus PLIN1) and LD size in vitro. Interestingly, small LDs were more frequent in proinflammatory M1 macrophages than in anti-inflammatory M2 macrophages. PLIN2 predominated and coated small LDs in M1 macrophages from the early phase of lipid loading, whereas PLIN1 predominated and coated large LDs in M2 macrophages after a long period of lipid loading. Furthermore, we have shown that the overexpression or silencing of PLIN1 alters the phenotype of cultured macrophages. Higher PLIN1 expression tilts human macrophages toward an anti-inflammatory phenotype, which is consistent with previous findings in PLIN2-knockout and PLIN1-overexpressing mouse macrophages: these genetically modified mice were both protected against atheroma progression23, 24). These findings suggest that PLIN2 would tend to promote the progression of atherosclerotic lesions whereas PLIN1 would have protective effects.
We previously reported that there is a close relationship between the nature of human carotid plaques and macrophage phenotype. Vulnerable human plaques, which are rich in proinflammatory cytokines, contain numerous M1 macrophages, whereas stable human plaques contain numerous M2 macrophages3). Taken together, these findings suggest that PLIN1 might be responsible for more stable lipid storage in large LDs, resulting in a reduction in the inflammatory potential of macrophages. Conversely, PLIN2 was highly expressed in symptomatic plaques, the instability of which could be characterized using ultrasonography3).
PLIN1 is well known to play a central role in the stable storage of triglycerides in large LDs in adipocytes. In contrast, PLIN2 is expressed not only in adipocytes and macrophages, but also in many other cell types, where it does not facilitate stable lipid storage, but rather rapidly packages newly-synthesized triglyceride into small LDs. There are several differences between macrophages and adipocytes in their method of lipid storage. Macrophages store both cholesterol and triglyceride, whereas adipocytes store only triglyceride. In addition, PLIN1 and PLIN2 are differentially expressed in macrophages and adipocytes. Adipocytes display a coordinate shift from PLIN2 to PLIN1 expression as they mature10, 28). PLIN1 is essential for stable lipid storage in large LDs in M2 macrophages and is co-expressed with PLIN2 in macrophages, unlike in adipocytes.
We attempted to measure lipolysis after the activation of protein kinase A in cultured human macrophages loaded with lipids, but this could not be detected (data not shown), probably because of the much smaller volume of lipid present in macrophages, compared with adipocytes. However, although the precise mechanism whereby PLIN1 prevents the development of a proinflammatory phenotype remains to be determined, the ability of PLIN1 to maintain stable lipid storage in macrophages is likely to be a key component of the mechanism.
PLIN1 overexpression also caused the downregulation of lipid efflux protein (ABCA1 and ABCG1) expression (Fig.4), whereas these proteins were upregulated by PLIN1 knockdown (Fig.5). In contrast, several recent studies have shown that the downregulation of ABCA1 and ABCG1 in macrophages is associated with larger atherosclerotic plaque lesions in ApoE−/− mice29-31), which suggests that their downregulation may promote a pro-atherosclerotic phenotype. Chen et al.31) proposed that pro-atherosclerotic M1 macrophages cannot effectively handle harmful lipids and that lipid removal from atherosclerotic plaques to the circulation by lipid efflux proteins would have an anti-atherosclerotic effect. These propositions are not consistent with the present findings, which may be explained by differences in the in vitro systems studied. ABCA1 and ABCG1 expression in macrophages were upregulated following incubation with lipoproteins in the present study. In addition, the greater ability to stably store lipids inside macrophage LDs conferred by PLIN1 overexpression probably reduces the necessity for lipid efflux from macrophages, which may explain the lower ABCA1 and ABCG1 expression in PLIN1-overexpressing macrophages. The stable storage of lipids in macrophage LDs is likely to be very important to prevent the development of an inflammatory phenotype. However, once the lipid content of macrophages exceeds the capacity of PLIN1 to contain it, lipid efflux may be of more significance. In the present study, we have shown that the addition of oxLDL increases the expression of ABCA1 and ABCG1, and this effect is similar to that of the administration of a synthetic LXRα agonist. However, the increase in expression caused by oxLDL may be smaller in M2 than M1 macrophages. This is analogous to the previous finding that human M2 macrophages have a lower cholesterol efflux capacity, lower expression of ABCA1, and higher phagocytic activity32). However, once macrophages are exposed to excess cholesterol over a long period of time, the balance between cholesterol uptake, storage, and efflux is disturbed, with unregulated uptake exceeding the cellular capacity for storage and efflux, even in M2 macrophages. The expression of VLDL receptor is upregulated during the formation of macrophage foam cells, and to maintain their storage capacity by removing excess cholesterol, cholesterol efflux proteins are upregulated33). In addition, it has been reported that the VLDL receptor is not downregulated by intracellular lipoproteins, unlike the LDL receptor; therefore, it may play a more significant role in an environment characterized by continuous lipid accumulation, such as atherosclerotic plaque6).
In conclusion, PLIN2 is highly expressed in human carotid atherosclerotic plaques from symptomatic patients. Furthermore, there are close relationships of macrophage polarity (M1 versus M2) with the protein composition of LDs and LD size. PLIN2 would tend to promote the progression of atherosclerotic lesions whereas PLIN1 expression is closely associated with carotid atherosclerotic plaque stability and an anti-inflammatory macrophage phenotype.
We thank Fujimori N. and Watanabe R. for their technical assistance. We also thank Mark Cleasby, Ph. D, from Edanz (https://jp.edanz.com/ac), for editing drafts of this manuscript.
All data are contained within the manuscript. Additional data underlying this article will be shared at reasonable request to the corresponding author.
Cho KY and Miyoshi H. designed research; Cho KY performed research, analyzed data, and wrote the paper; Miyoshi H, Nakamura A, Greenberg AS, and Atsumi T reviewed and edited the manuscript. All authors contributed to the discussion of results and approved the final version of this manuscript. The corresponding author is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr. Greenberg holds grants (8050-51000-105-02S, ARS/USDA, P30 DK046200-23, P30 DK048200-27S1, DK108722 01A1). The other authors declare no conflict of interest.