Abstract
Aims: Pharmacological blockade of mineralocorticoid receptors (MRs) is a potential therapeutic approach to reduce cardiovascular complications since MRs play a crucial role in cardiovascular regulation. Recent studies suggest that MR antagonists affect several extrarenal tissues, including vessel function. We investigated the effect of a novel nonsteroidal selective MR blocker, esaxerenone, on diabetes-induced vascular dysfunction.
Methods: Diabetes was induced by a single dose of streptozotocin in 8-week-old male C57BL/6 mice. Esaxerenone (3 mg/kg/day) or a vehicle was administered by gavage to diabetic mice for 3 weeks. Metabolic parameters, plasma aldosterone levels, and parameters related to renal function were measured. Endothelium-dependent or -independent vascular responses of the aortic segments were analyzed with acetylcholine or sodium nitroprusside, respectively. Human umbilical vein endothelial cells (HUVECs) were used for the in vitro study.
Results: Induction of diabetes elevated plasma aldosterone level (P<0.05) and impaired endothelium-dependent vascular relaxation (P<0.05). The administration of esaxerenone ameliorated the endothelial dysfunction (P<0.01) without the alteration of metabolic parameters, blood pressure, and renal function. Esaxerenone improved the eNOSSer1177 phosphorylation in the aorta obtained from diabetic mice (P<0.05) compared with that in the vehicle-treated group. Furthermore, a major MR agonist, aldosterone, decreased eNOSSer1177 phosphorylation and increased eNOSThr495 phosphorylation in HUVECs, which recovered with esaxerenone. Esaxerenone ameliorated the endothelium-dependent vascular relaxation caused by aldosterone in the aortic segments obtained from C57BL/6 mice (P<0.001).
Conclusion: Esaxerenone attenuates the development of diabetes-induced endothelial dysfunction in mice. These results suggest that esaxerenone has potential vascular protective effects in individuals with diabetes.
See editorial vol. 30: 321-322
Abbreviation list: Ach: acetylcholine, ANOVA: analysis of variance, BUN: blood urea nitrogen, CVD: cardiovascular diseases, eNOS: endothelial nitric oxide synthase, HUVEC: human umbilical vein endothelial cells, MR: mineralocorticoid receptors, MRAs: mineralocorticoid receptor antagonists, NO: nitric oxide, SEM: standard error of the mean, SNP: sodium nitroprusside, STZ: streptozotocin.
Introduction
Despite accumulating knowledge and advancing therapeutics, cardiovascular disease is still responsible for a large proportion of mortality worldwide1). The pathophysiological role of aldosterone in cardiovascular disease has been demonstrated2). Volume expansion and/or a hypertensive effect via mineralocorticoid receptors (MRs) expressed in the kidney is its potent underlying mechanism3). Moreover, elevated plasma aldosterone levels correlate with increased mortality. Pharmacological blockade of MRs significantly reduced morbidity, improved survival in patients with heart failure, and decreased hospitalization in postmyocardial infarction in several clinical trials4-6). Extrarenal effects of mineralocorticoid receptor antagonists (MRAs) have recently gained attention. Several studies have been conducted to clarify the effects of MRAs on other tissues/organs, such as the heart, vessels, and metabolic organs7).
Endothelium dysfunction is a major underlying pathophysiology of cardiovascular complications in diabetic patients8, 9). Recent clinical studies have shown that MRs play a crucial role in cardiovascular regulation, particularly in the development of vascular dysfunction in diabetic patients10, 11). Moreover, MRAs reverse this vascular complication in diabetic individuals12-15). Preclinical studies also show that the diabetic condition impairs endothelium-dependent vasodilation, and MRAs prevent it by increasing eNOSSer1177 phosphorylation and reducing oxidative stress in mice aorta16-21).
Spironolactone and eplerenone are traditionally available MRAs; however, the clinical use of these MRAs is limited because of their relatively low MR selectivity and steroidal structure22). Recently, esaxerenone, a new nonsteroidal MR blocker with higher potency and selectivity to MR, was introduced in Japan23). Several clinical and preclinical studies have reported the great antihypertensive and renoprotective effects of esaxerenone compared with spironolactone or eplerenone. Pharmacological studies clarified that esaxerenone has more than 1,000-fold affinity to MRs over other NR3C nuclear receptors due to its flipped side chain constructure. Moreover, the binding site of esaxerenone is larger and intruded into the protein core. Therefore, the suppressive effect is more potent and longer lasting than those of spironolactone and eplerenone24-28). However, the effect of esaxerenone on vascular function in diabetes has not been fully investigated. Thus, here, we investigated whether esaxerenone ameliorates diabetes-induced endothelial dysfunction in diabetic mice.
2.Methods
2.1. Animals and Drug Administration
C57BL/6J wild-type mice were obtained from Japan SLC, Inc. Esaxerenone was supplied by Daiichi Sankyo Co., Ltd., Japan. Eight-week-old male mice were injected with a single dose of streptozotocin (STZ, 150 mg/kg, Santa Cruz) or vehicle intraperitoneally to examine the effect on diabetes-induced endothelial dysfunction. Three days after the injection, diabetic mice were randomly divided into esaxerenone (3 mg/kg/day) or vehicle (carboxymethyl cellulose) groups and treated by oral gavage once daily for three weeks. The ex vivo vascular reactivity assay used aortic segments obtained from 8-week-old male C57BL/6J mice. Mice were maintained under controlled temperature (23℃±1℃) with a 12-h artificial light and dark cycle. All experimental procedures conformed with the guidelines for animal experimentation of the Tokushima University. The Animal Care and Use Committee of Tokushima University reviewed and approved the protocol under #T2020-127.
2.2. Measurement of Plasma Aldosterone Levels and Metabolic Parameters
Blood pressure was measured by a tail-cuff system in conscious mice (Softron). At the time of sacrifice, blood was collected from the heart. Plasma was separated by centrifugation (9,000 rpm for 15 min) at 4℃ and stored until further analyses at −80℃. Plasma lipid levels (total cholesterol, high density lipoprotein cholesterol, and triglyceride), blood urea nitrogen (BUN), creatinine, and glycoalbumin were measured at the Sanritsu Zelkova examination center (Japan). Plasma aldosterone level was measured by using a commercial available kit according to the manufacturer’s recommendations (R&D Systems, Inc., USA).
2.3. Vascular Reactivity Assay
Vascular reactivity was analyzed as previously documented29). After three weeks of esaxerenone administration, the descending thoracic aorta was isolated and cut into 2-mm rings with special care. The aortic segments were mounted between two parallel wires in the organ bath filled with modified Krebs–Henseleit buffer (118.4 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, and 11.1 mM glucose) that was aerated (95% O2 and 5% CO2) and warmed (37℃). Changes in isometric tension were recorded on a polygraph (LabChart). After 60 min of stabilization, the aortic segments were exposed to 31.4 mM KCl. Endothelial relaxation was then assessed with acetylcholine (Ach; 10−9–10−4 M) in the aortic segments, previously contracted by phenylephrine (60% of maximum). Endothelium-independent relaxation was examined with increasing concentrations of sodium nitroprusside (SNP; 10−9–10−4 M). An ex vivo experiment was performed with the same protocol. The aortic rings used in the ex vivo experiment were treated with 1,000 nM aldosterone (Sigma–Aldrich) with or without 30-min pretreatment with 10 nM esaxerenone.
2.4. Cell Culture Experiments
Human umbilical vein endothelial cells (HUVECs) were purchased from Life Technologies and cultured in EGM-2 (Lonza). HUVECs (passages 4–6) were stimulated with aldosterone for 3 h in EBM-2 (Lonza) containing 2% charcoal/dextran-treated fetal bovine serum (Cytiva) with or without 4-h pretreatment with 10 nM esaxerenone.
2.5. Western Blot Analysis
Cells and tissues were lysed with RIPA buffer (Wako Pure Chemical Industries, Ltd.) containing a protease inhibitor cocktail (Takara Bio Inc.) and phosphatase inhibitors (Roche) on ice. Proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and then transferred onto polyvinylidene difluoride membranes (Hybond-P; GE Healthcare). After blocking with 5% bovine serum albumin or 5% skimmed milk, the membranes were incubated overnight at 4℃ with primary antibody against either phosphorylated-eNOSSer1177, phosphorylated-eNOSThr495, eNOS (BD Biosciences), phosphorylated-AktSer473, Akt (Cell Signaling Technology), or β-actin (Sigma). After five washings with TBS-T buffer, each membrane was incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. The signal was detected using a luminescent image analyzer (LAS-1000, Fuji Film) with ECL-plus reagent (GE Healthcare). The ratio of phosphorylated-eNOSSer1177 to phosphorylated-eNOSThr495 was calculated as another marker for endothelial function30).
2.6. Statistical Analysis
All numerical values are expressed as means±standard error of the mean (SEM). An unpaired Student’s t-test analyzed the parameter comparisons between the two groups. Differences between multiple groups were performed by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. Comparison of dose-response curves was performed by two-factor repeated measures ANOVA, followed by Dunnett’s post hoc test for comparison between groups, and P-value <0.05 was considered significant.
3.Results
3-1. Effect of Esaxerenone on Metabolic Parameters
Diabetes induction by STZ significantly increased the blood glucose level, which was accompanied with elevation of plasma glycoalbumin level. Induction of diabetes by STZ also elevated the plasma lipid levels as in previous studies31). The induction of diabetes increased the plasma aldosterone level, which was further enhanced by esaxerenone treatment. In this study, esaxerenone did not lower blood pressure in diabetic mice. Furthermore, esaxerenone did not affect blood lipid levels, glucose levels, renal functions as determined by creatinine and BUN levels, and body weight in diabetic mice. These data are summarized in Table 1.
Table 1.
Effect of esaxerenone on metabolic parameters after 3 weeks of treatment
|
CTRL |
STZ |
Esax |
P-value
|
| Body weight, g |
25.7±0.5†††
|
22.7±0.7 |
22.1±0.3 |
<0.001 |
| Blood glucose, mg/dl |
136.4±3.9†††
|
563.0±42.8 |
658.3±51.8 |
<0.001 |
| Systolic blood pressure, mmHg |
105.1±1.9 |
109.2±4.1 |
106.6±2.1 |
0.59 |
| Diastolic blood pressure, mmHg |
69.6±2.3 |
63.5±4.1 |
66.1±2.3 |
0.37 |
| Total-cholesterol, mg/dl |
105.6±2.7††
|
161.8±15.7 |
144.4±11.1 |
0.004 |
| Triglycerides, mg/dl |
145.5±14.2††
|
408.0±65.1 |
353.9±61.2 |
0.003 |
| HDL-cholesterol, mg/dl |
59.7±2.0†
|
77.5±7.7 |
77.5±4.8 |
0.04 |
| Aldosterone, pg/ml |
293.7±42.2†
|
696.2±108.0 |
1244.6±179.9††
|
<0.001 |
| BUN, mg/dl |
29.4±1.0 |
34.7±2.2 |
34.6±3.4 |
0.20 |
| Creatinine, mg/dl |
0.15±0.004††
|
0.11±0.008 |
0.13±0.006 |
0.004 |
| Glycoalbumin, mg/dl |
3.1±0.2†††
|
14.6±0.9 |
15.0±0.5 |
<0.001 |
| Food intake, g/day/head |
2.8±0.3††
|
5.5±0.4 |
6.0±0.6 |
0.004 |
CTRL: non-diabetic control, Esax: esaxerenone, HDL: high density lipoprotein, STZ: streptozotocin. All values are mean±SEM. †; P<0.05, ††; P<0.01, †††; P<0.001 vs. STZ group
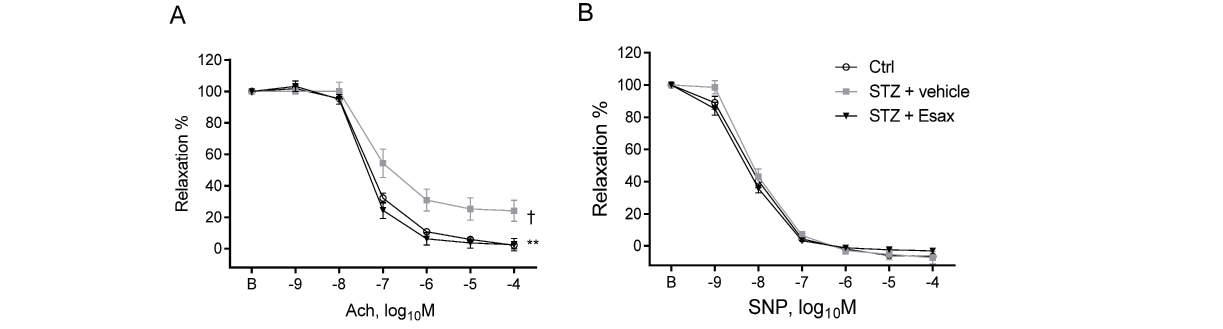
The vascular reactivity assay, which used aortic rings obtained from our mice, demonstrated that induction of diabetes by STZ impaired vasodilatation in response to Ach, suggesting impairment of endothelial function (P<0.05). However, three weeks of esaxerenone administration ameliorated endothelium-dependent vascular dysfunction (Fig.1A) (P<0.01). Conversely, in our experiment, endothelium-independent vasodilation in response to SNP did not differ among the groups (Fig.1B). eNOSSer1177 phosphorylation was reduced in diabetic mice (P<0.05), whereas esaxerenone treatment restored this response (P<0.05). In this experiment, we did not observe significant effects of esaxerenone on eNOSThr495 and Akt phosphorylation (Fig.2).
3.3. Esaxerenone Improved the Aldosterone-Induced Impairment of eNOS Phosphorylation in HUVECs
In the in vivo experiments, esaxerenone abolished the endothelial dysfunction by improving eNOSSer1177 phosphorylation. Therefore, HUVECs were used to elucidate the underlying mechanism. Aldosterone significantly reduced eNOSSer1177 phosphorylation in a dose-dependent manner and increased eNOSThr495 phosphorylation (Fig.3A). In addition, another marker for endothelial function, the eNOSSer1177/eNOSThr495 phosphorylation ratio, decreased with aldosterone treatment (P<0.001). Akt phosphorylation also decreased in the presence of aldosterone. However, the pretreatment with esaxerenone increased eNOSSer1177 phosphorylation (P<0.01) and the eNOSSer1177/eNOSThr495 phosphorylation ratio (P<0.001) and decreased the eNOSThr495 phosphorylation (P<0.01) (Fig.3B).
3.4. Esaxerenone Reduced the Aldosterone-Induced Vascular Dysfunction in the Aortic Segments
The direct effect of aldosterone on vascular relaxation was examined by vascular reactivity assay using aortic rings obtained from C57BL/6 mice. Aldosterone impaired Ach-induced vasodilatation (P<0.05) while preincubation with esaxerenone attenuated this impairment (P<0.001) (Fig.4A). Both esaxerenone and aldosterone did not affect the SNP-induced vasodilation (Fig.4B).
4.Discussion
Our results showed that MR blockade by esaxerenone ameliorated Ach-induced vascular relaxation by enhancing eNOSSer1177 phosphorylation that was impaired by the induction of diabetes by STZ without affecting blood pressure, renal function, and metabolic parameters in C57BL/6 mice. Neither the induction of diabetes nor esaxerenone affected SNP-induced vascular relaxation in our condition. In vitro experiments using HUVECs demonstrated that aldosterone decreased eNOSSer1177 phosphorylation, which was recovered in the presence of esaxerenone. These results suggest that esaxerenone ameliorates diabetes-induced endothelial dysfunction.
Vascular dysfunction is a primary contributor to CVD-associated mortality and morbidity in diabetic individuals32-34). Identical with our findings, preclinical and clinical studies have demonstrated that aldosterone plasma levels, a major agonist of MR, lead to an increase in diabetes10, 11, 35); further, MRAs ameliorated vascular dysfunction in diabetic models15, 20, 35). This supports the concept that MR antagonism with esaxerenone is a potential vasoprotective treatment in diabetes.
In this study, we used esaxerenone, a recently approved MR blocker in Japan. Esaxerenone has higher MR-binding specificity and nonsteroidal structure23). Previous preclinical and clinical studies have demonstrated the superior potency of esaxerenone to that of spironolactone or eplerenone for treating hypertension23, 24). The extrarenal effects of MR antagonists have been gaining attention. Among them, several studies have shown the antiatherosclerotic effects of MRAs targeting vascular cells36, 37). Endothelium dysfunction is a major underlying pathophysiology of cardiovascular complications in diabetic patients8, 9). The prevention of endothelial dysfunction is indispensable to avoid vascular complications. Therefore, we focused on the effects of esaxerenone on vascular function in diabetes. Previous studies have already demonstrated that both spironolactone and eplerenone prevent endothelial dysfunction in diabetes15, 35). However, the use of traditionally available MRAs is limited, owing to their relatively low selectivity and steroidal structure22). In this study, esaxerenone clearly had protective effects on endothelial function in diabetic mice. Esaxerenone also restored decreased eNOSSer1177 phosphorylation caused by STZ injection in the aorta. In our in vitro experiments, aldosterone abolished the eNOSSer1177 phosphorylation and promoted eNOSThr495 phosphorylation in HUVECs. The presence of esaxerenone inhibited these effects. Further, these results suggest that esaxerenone increased nitric oxide (NO) production in this cell type, leading to the improvement of endothelial function. Previous studies have reported that aldosterone infusion promoted NAD(P)H oxidase activity, promoting oxidative stress38, 39). Moreover, aldosterone is suggested to affect NO production and bioavailability through various pathways16-18, 40-45). By contrast, previous studies have reported that MRAs reduce vascular dysfunction by promoting eNOS activity and NO bioavailability in diabetic mice14, 15). These results were confirmed by a study that used endothelium-specific MR-deleted mice35). NO is a principal vascular tone regulator synthesized primarily by eNOS in endothelial cells46). Diabetes-associated eNOS dysfunction has also been known. Therefore, esaxerenone administration could be a potential strategy for this central mechanism of diabetic vascular complications. To the best of our knowledge, this is the first study to report the protective effects of esaxerenone on diabetes-induced endothelial function. Previous studies have demonstrated amelioration of endothelial dysfunction by traditionally available MRAs; however, esaxerenone is expected to have more beneficial effects because of its higher affinity to MR and nonsteroidal structure. Further studies are needed to clarify the underlying mechanisms of esaxerenone for vascular protection.
Esaxerenone did not lower blood pressure in our study condition. Previous studies suggest that the effects of MRAs on blood pressure depend on the mouse model47). In mice given a high-salt diet or water, MRAs, including esaxerenone, lowered blood pressure; however, in mice kept under normal salt conditions, MRAs did not affect blood pressure. In fact, in diabetic mice, esaxerenone and other MRAs did not lower blood pressure15, 48, 49). The present study is consistent with the previous studies, indicating that the effects of esaxerenone on endothelium are independent of blood pressure, at least partially. The results of our in vitro studies, which demonstrated a direct effect of aldosterone and esaxerenone on HUVECs, partially support this finding.
This study has several limitations. First, we injected STZ to induce diabetes. This model is widely used for diabetic research; however, this model does not completely represent type 2 diabetes, a common pattern of diabetes. Second, the aldosterone dose used in this study was higher than in human hyperaldosteronism. However, previous studies used similar doses to show its effects on eNOS in HUVECs16, 18). Third, in this study, esaxerenone did not lower blood pressure in our diabetic mice. Esaxerenone is approved for the treatment of patients with hypertension in Japan. In future, further studies using hypertension models may be needed when we consider the clinical setting.
In conclusion, our data demonstrated that esaxerenone administration ameliorates diabetes-induced impairment of endothelial function by promoting eNOS phosphorylation in diabetic mice. Moreover, aldosterone-induced vascular dysfunction was ameliorated by esaxerenone in the aortic segments and HUVECs. Our results strengthen the concept that MR plays a crucial role in vascular dysfunction, and MR blockade by esaxerenone is a promising therapeutic approach for vascular dysfunction in diabetes.
Acknowledgements
The authors thank Shintaro Okamoto and Etsuko Uematsu (Tokushima University) for their technical assistance.
Competing Interests
The authors declare that they have no conflict of interest. Esaxerenone was supplied by Daiichi Sankyo Co., Ltd.
Sources of Funding
This work was partially supported by JSPS Kakenhi Grants (Number 19K08584 to D.F. and Number 19H03654 to M.S.), Bristol-Myers Squibb Research Grants (D.F.), The Uehara Memorial Foundation (D.F.), Takeda Science Foundation (M.S.), and the Vehicle Racing Commemorative Foundation (M.S.). The funders had no role in the study design, data collection and analysis, or preparation of the manuscript.
References
- 1) Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB and Tsao CW. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation, 2020; 141: e139-e596
- 2) Fuller PJ and Young MJ. Mechanisms of mineralocorticoid action. Hypertension, 2005; 46: 1227-1235
- 3) Pascual-Le Tallec L and Lombes M. The mineralocorticoid receptor: a journey exploring its diversity and specificity of action. Mol Endocrinol, 2005; 19: 2211-2221
- 4) Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J and Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med, 1999; 341: 709-717
- 5) Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ and Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med, 2011; 364: 11-21
- 6) Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J and Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med, 2003; 348: 1309-1321
- 7) Nguyen Dinh Cat A and Jaisser F. Extrarenal effects of aldosterone. Curr Opin Nephrol Hypertens, 2012; 21: 147-156
- 8) Raghavan S, Vassy JL, Ho YL, Song RJ, Gagnon DR, Cho K, Wilson PWF and Phillips LS. Diabetes Mellitus-Related All-Cause and Cardiovascular Mortality in a National Cohort of Adults. J Am Heart Assoc, 2019; 8: e011295
- 9) Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R and Di Carli MF. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation, 2012; 126: 1858-1868
- 10) Bender SB, McGraw AP, Jaffe IZ and Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes, 2013; 62: 313-319
- 11) Bruder-Nascimento T, da Silva MA and Tostes RC. The involvement of aldosterone on vascular insulin resistance: implications in obesity and type 2 diabetes. Diabetol Metab Syndr, 2014; 6: 90
- 12) Adel H, Taye A and Khalifa MM. Spironolactone improves endothelial dysfunction in streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol, 2014; 387: 1187-1197
- 13) Brown SM, Meuth AI, Davis JW, Rector RS and Bender SB. Mineralocorticoid receptor antagonism reverses diabetes-related coronary vasodilator dysfunction: A unique vascular transcriptomic signature. Pharmacol Res, 2018; 134: 100-108
- 14) Schafer A, Vogt C, Fraccarollo D, Widder J, Flierl U, Hildemann SK, Ertl G and Bauersachs J. Eplerenone improves vascular function and reduces platelet activation in diabetic rats. J Physiol Pharmacol, 2010; 61: 45-52
- 15) Silva MA, Bruder-Nascimento T, Cau SB, Lopes RA, Mestriner FL, Fais RS, Touyz RM and Tostes RC. Spironolactone treatment attenuates vascular dysfunction in type 2 diabetic mice by decreasing oxidative stress and restoring NO/GC signaling. Front Physiol, 2015; 6: 269
- 16) Hashikabe Y, Suzuki K, Jojima T, Uchida K and Hattori Y. Aldosterone impairs vascular endothelial cell function. J Cardiovasc Pharmacol, 2006; 47: 609-613
- 17) Kirsch T, Beese M, Wyss K, Klinge U, Haller H, Haubitz M and Fiebeler A. Aldosterone modulates endothelial permeability and endothelial nitric oxide synthase activity by rearrangement of the actin cytoskeleton. Hypertension, 2013; 61: 501-558
- 18) Nagata D, Takahashi M, Sawai K, Tagami T, Usui T, Shimatsu A, Hirata Y and Naruse M. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension, 2006; 48: 165-171
- 19) Sanz-Rosa D, Oubiña MP, Cediel E, De las Heras N, Aragoncillo P, Balfagón G, Cachofeiro V and Lahera V. Eplerenone reduces oxidative stress and enhances eNOS in SHR: vascular functional and structural consequences. Antioxid Redox Signal, 2005; 7: 1294-1301
- 20) Thai HM, Do BQ, Tran TD, Gaballa MA and Goldman S. Aldosterone antagonism improves endothelial-dependent vasorelaxation in heart failure via upregulation of endothelial nitric oxide synthase production. J Card Fail, 2006; 12: 240-245
- 21) Toda N, Nakanishi S and Tanabe S. Aldosterone affects blood flow and vascular tone regulated by endothelium-derived NO: therapeutic implications. Br J Pharmacol, 2013; 168: 519-533
- 22) Lainscak M, Pelliccia F, Rosano G, Vitale C, Schiariti M, Greco C, Speziale G and Gaudio C. Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone. Int J Cardiol, 2015; 200: 25-29
- 23) Arai K, Homma T, Morikawa Y, Ubukata N, Tsuruoka H, Aoki K, Ishikawa H, Mizuno M and Sada T. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur J Pharmacol, 2015; 761: 226-234
- 24) Arai K, Tsuruoka H and Homma T. CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol, 2015; 769: 266-273
- 25) Kato M, Furuie H, Shimizu T, Miyazaki A, Kobayashi F and Ishizuka H. Single- and multiple-dose escalation study to assess pharmacokinetics, pharmacodynamics and safety of oral esaxerenone in healthy Japanese subjects. Br J Clin Pharmacol, 2018; 84: 1821-1829
- 26) Ito S, Itoh H, Rakugi H, Okuda Y and Yamakawa S. Efficacy and safety of esaxerenone (CS-3150) for the treatment of essential hypertension: a phase 2 randomized, placebo-controlled, double-blind study. J Hum Hypertens, 2019; 33: 542-551
- 27) Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M and Yamakawa S. Double-Blind Randomized Phase 3 Study Comparing Esaxerenone (CS-3150) and Eplerenone in Patients With Essential Hypertension (ESAX-HTN Study). Hypertension, 2020; 75: 51-58
- 28) Takahashi M, Ubukata O, Homma T, Asoh Y, Honzumi M, Hayashi N, Saito K, Tsuruoka H, Aoki K and Hanzawa H. Crystal structure of the mineralocorticoid receptor ligand-binding domain in complex with a potent and selective nonsteroidal blocker, esaxerenone (CS-3150). FEBS Lett, 2020; 594: 1615-1623
- 29) Pham PT, Fukuda D, Yagi S, Kusunose K, Yamada H, Soeki T, Shimabukuro M and Sata M. Rivaroxaban, a specific FXa inhibitor, improved endothelium-dependent relaxation of aortic segments in diabetic mice. Sci Rep, 2019; 9: 11206
- 30) Matsumoto S, Shimabukuro M, Fukuda D, Soeki T, Yamakawa K, Masuzaki H and Sata M. Azilsartan, an angiotensin II type 1 receptor blocker, restores endothelial function by reducing vascular inflammation and by increasing the phosphorylation ratio Ser(1177)/Thr(497) of endothelial nitric oxide synthase in diabetic mice. Cardiovasc Diabetol, 2014; 13: 30
- 31) Suto K, Fukuda D, Shinohara M, Ganbaatar B, Yagi S, Kusunose K, Yamada H, Soeki T, Hirata KI and Sata M. Pemafibrate, A Novel Selective Peroxisome Proliferator-Activated Receptor alpha Modulator, Reduces Plasma Eicosanoid Levels and Ameliorates Endothelial Dysfunction in Diabetic Mice. J Atheroscler Thromb, 2021; 28: 1349-1360
- 32) Wenzel P, Daiber A, Oelze M, Brandt M, Closs E, Xu J, Thum T, Bauersachs J, Ertl G, Zou MH, Förstermann U and Münzel T. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis, 2008; 198: 65-76
- 33) Chen B, Zhao Q, Ni R, Tang F, Shan L, Cepinskas I, Cepinskas G, Wang W, Schiller PW and Peng T. Inhibition of calpain reduces oxidative stress and attenuates endothelial dysfunction in diabetes. Cardiovasc Diabetol, 2014; 13: 88
- 34) Knapp M, Tu X and Wu R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol Sin, 2019; 40: 1-8
- 35) Schäfer N, Lohmann C, Winnik S, van Tits LJ, Miranda MX, Vergopoulos A, Ruschitzka F, Nussberger J, Berger S, Lüscher TF, Verrey F and Matter CM. Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J, 2013; 34: 3515-3524
- 36) Raz-Pasteur A, Gamliel-Lazarovich A, Coleman R and Keidar S. Eplerenone reduced lesion size in early but not advanced atherosclerosis in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol, 2012; 60: 508-512
- 37) Takai S, Jin D, Muramatsu M, Kirimura K, Sakonjo H and Miyazaki M. Eplerenone inhibits atherosclerosis in nonhuman primates. Hypertension, 2005; 46: 1135-1139
- 38) Kobayashi N, Fukushima H, Takeshima H, Koguchi W, Mamada Y, Hirata H, Machida Y, Suzuki N, Yokotsuka F, Tabei K, Kobayashi E, Fukuda N and Ishimitsu T. Effect of eplerenone on endothelial progenitor cells and oxidative stress in ischemic hindlimb. Am J Hypertens, 2010; 23: 1007-1013
- 39) Maron BA, Zhang YY, Handy DE, Beuve A, Tang SS, Loscalzo J and Leopold JA. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J Biol Chem, 2009; 284: 7665-7672
- 40) Schmidt BM, Oehmer S, Delles C, Bratke R, Schneider MP, Klingbeil A, Fleischmann EH and Schmieder RE. Rapid nongenomic effects of aldosterone on human forearm vasculature. Hypertension, 2003; 42: 156-160
- 41) Nietlispach F, Julius B, Schindler R, Bernheim A, Binkert C, Kiowski W and Brunner-La Rocca HP. Influence of acute and chronic mineralocorticoid excess on endothelial function in healthy men. Hypertension, 2007; 50: 82-88
- 42) Liu SL, Schmuck S, Chorazcyzewski JZ, Gros R and Feldman RD. Aldosterone regulates vascular reactivity: short-term effects mediated by phosphatidylinositol 3-kinase-dependent nitric oxide synthase activation. Circulation, 2003; 108: 2400-2406
- 43) Farquharson CA and Struthers AD. Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone-induced vasculopathy. Clin Sci (Lond), 2002; 103: 425-431
- 44) Farquharson CA and Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation, 2000; 101: 594-597
- 45) Tsuchiya K, Yoshimoto T and Hirata Y. Endothelial dysfunction is related to aldosterone excess and raised blood pressure. Endocr J, 2009; 56: 553-559
- 46) Malinski T, Taha Z, Grunfeld S, Patton S, Kapturczak M and Tomboulian P. Diffusion of nitric oxide in the aorta wall monitored in situ by porphyrinic microsensors. Biochem Biophys Res Commun, 1993; 193: 1076-1082
- 47) Li L, Guan Y, Kobori H, Morishita A, Kobara H, Masaki T, Nakano D and Nishiyama A. Effects of the novel nonsteroidal mineralocorticoid receptor blocker, esaxerenone (CS-3150), on blood pressure and urinary angiotensinogen in low-renin Dahl salt-sensitive hypertensive rats. Hypertens Res, 2019; 42: 769-778
- 48) Arai K, Morikawa Y, Ubukata N and Sugimoto K. Synergistic reduction in albuminuria in type 2 diabetic mice by esaxerenone (CS-3150), a novel nonsteroidal selective mineralocorticoid receptor blocker, combined with an angiotensin II receptor blocker. Hypertens Res, 2020; 43: 1204-1213
- 49) Guo C, Martinez-Vasquez D, Mendez GP, Toniolo MF, Yao TM, Oestreicher EM, Kikuchi T, Lapointe N, Pojoga L, Williams GH, Ricchiuti V and Adler GK. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology, 2006; 147: 5363-5373