2023 Volume 30 Issue 8 Pages 1010-1021
2023 Volume 30 Issue 8 Pages 1010-1021
Aims: The role of cilostazol after intracranial or extracranial artery stent implantation is still unclear. Therefore, we designed this trial to explore the efficacy and safety of cilostazol in this particular population.
Methods: In this retrospective study, patients were divided into the cilostazol or clopidogrel group by the antiplatelet therapy received after artery stent implantation. The primary efficacy endpoint was ischemic stroke. Bleeding events and other antiplatelet drug-related adverse reactions (ADRs) were also recorded. Neurological function prognosis was evaluated by the modified Rankin Scale (mRS) after discharge.
Results: A total of 156 patients were enrolled; 56 underwent intracranial artery stenting, 95 underwent extracranial artery stenting, and 5 underwent intracranial combined with extracranial artery stenting. Any stroke and bleeding events in the hospital of the two groups were comparable (P=0.38, P=0.34, respectively). The incidence of stroke recurrence tended to be lower in the cilostazol group, although not significant (cilostazol vs. clopidogrel, 1.35% vs. 4.88%, P=0.25). There was a significant difference of any bleeding events between the two groups (cilostazol vs. clopidogrel, 5.41% vs. 20.73%, P=0.02). During follow-up, we did not observe an apparent increase of ADRs in the cilostazol group (cilostazol vs. clopidogrel, palpitation 4.05% vs. 2.44%, P=0.58; gastrointestinal discomfort events 8.11% vs. 12.20%, P=0.80). There were no differences between the two groups of neurological function prognosis (P=0.29).
Conclusions: Cilostazol-based dual antiplatelet therapy could be recommended as an effective and safe therapy regimen among patients undergoing intracranial or extracranial artery stent implantation.
Registration number: ChiCTR-ROC-16010050
Antiplatelet agents are considered as the cornerstone of the long-term management of patients undergoing various neurointerventional procedures1-5). Therefore, the rational application of antiplatelet agents plays an important role in preventing stroke recurrence and reducing the occurrence of vascular events such as stent thrombosis or restenosis2, 6-8).
As recommended in guidelines of stroke in most countries, the combination of agents can improve the effectiveness of stroke prevention9, 10). The combination of clopidogrel and aspirin is the most used antiplatelet therapy for the secondary prevention of stroke7, 11). However, several previous studies had reported an increased risk of bleeding with the combination of aspirin and clopidogrel12-14), especially in the Chinese population12).
Antiplatelet drug-related bleeding has been a concern for poor prognosis15). Therefore, optimizing antiplatelet therapy is critical for patients at high risk of bleeding. As previously reported, the bleeding risk of cilostazol was lower than those of other antiplatelet drugs16, 17). Several clinical trials had shown that the addition of cilostazol to a regimen with another antiplatelet agent can decrease the recurrence of stroke without increasing the risk of bleeding16, 18-20). It is well known that, in addition to its antiplatelet effect, cilostazol has various effects such as vascular protection, antiproliferation, alleviation of ischemia–reperfusion injury, and antiatherosclerosis16, 21-23). Therefore, the combination of traditional antiplatelet agents with cilostazol is a regimen that can be recommended for patients undergoing arterial stenting. However, the efficacy and safety of the cilostazol-based dual antiplatelet therapy (DAPT; cilostazol plus aspirin) compared with clopidogrel-based DAPT (clopidogrel plus aspirin) in patients undergoing intracranial or extracranial artery stenting implantation is still unclear.
Here, we designed this retrospective study to explore the efficacy and safety of cilostazol-based DAPT in this special population to improve the clinical outcomes of patients undergoing intracranial or extracranial artery stenting implantation.
This retrospective study was designed to compare the efficacy and safety of cilostazol plus aspirin with clopidogrel plus aspirin as secondary prevention after intracranial or extracranial artery stent implantation, evaluated by at least 12 months of follow-up. The demographic characteristics and clinical data of enrolled patients at hospital from January 2019 to January 2021 were consecutively collected through an electronic medical record system. Patients were evaluated by a professional neurointerventional team to confirm that they met the intervention indications. The stents were implanted at the site of stenosis, and the type of stent was determined after discussion by neurointerventionists based on the stenosis. The study protocol complied with the Declaration of Helsinki and was approved by the ethical committee of our institution. The study was registered in the Chinese Clinical Trial Register (ChiCTR-ROC-16010050).
Patients who were eligible for the study were (1) >18 years old, (2) underwent intracranial or extracranial artery stenting in the chronic phase of the disease, and (3) assigned dual antiplatelet agents for 3 months and then changed to aspirin 100 mg once daily for long-term therapy. Major exclusion criteria were the following: (1) using other anticoagulant or antiplatelet drugs affecting clotting during the study drug administration, (2) having any other hemorrhagic or platelet diseases, (3) suffering from untreatable tumor and other fatal diseases, resulting in a survival time of less than 1 year, and (4) unable to cooperate with follow-up.
We divided patients into two groups, namely, the cilostazol group (aspirin 100 mg once daily plus cilostazol 100 mg twice daily) and the clopidogrel group (aspirin 100 mg once daily plus clopidogrel 75 mg once daily), according to the type of DAPT that they received in the hospital. The DAPT regimen was started at least 1 week before the implantation and lasted for at least 3 months. Since our patients came from different medical groups and different doctors had different medication habits, there were no internal rules for selecting medicines.
Follow-UpAll patients were observed for at least 12 months. During the follow-up period, outcomes were recorded through our hospital’s electronic medical record system or telephone interview. Any events related to the primary and secondary outcomes were evaluated by a doctor and checked by another doctor.
The primary efficacy outcome was recurrence of ischemic stroke. Secondary efficacy outcomes included the following: any ischemic events, all-cause death, any revascularization procedure, and change in the modified Rankin Scale (mRS) scores at 12 months; delta scores (mRS Δs) were calculated using the following formula: patient’s mRS score at admission − the mRS score at 12 months of follow-up (positive score=improvement). Bleeding events as defined by the Bleeding Academic Research Consortium were considered as primary safety outcomes14). Any patient-reported adverse reactions related to antiplatelet drugs, such as gastrointestinal discomfort, palpitations, and headache, were also analyzed as safety outcomes.
Statistical AnalysisDifferences in background characteristics between the two groups were analyzed through the independent sample t tests or Wilcoxon rank sum test for continuous variables and the chi-square test or Fisher’s exact test for binary variables. The mRS score was analyzed dependent on the different purposes. When analyzing the mRS categories at admission, nonparametric methods (independent samples Mann–Whitney U test) were used to compare the baseline difference. Binary logistics regression was used to analyze the changes in mRS scores after 12 months of follow-up, defining delta scores (mRS Δs) ≥ 1 as improved.
The cumulative incidence curves of two groups were displayed by the survival curves and compared using the log-rank test. The Cox proportional hazard models were used to calculate hazard ratio (HRs), 95% confidence interval (CI), and P value.
Of the 177 patients who received DAPT with aspirin and cilostazol or clopidogrel after intracranial or extracranial artery stent implantation, 156 patients were finally included (Fig.1).

Note: *patients change drug for it’s expensive price or inconvenience to buy
The baseline demographic and clinical characteristics of the 156 patients are presented in Table 1, including 74 patients who received cilostazol-based DAPT and 82 patients treated with clopidogrel-based DAPT. All except two patients (one from each group) had symptomatic stenosis. The background characteristics were comparable between the two groups, except for median age and body mass index (BMI). Patients in the clopidogrel group were significantly older (median age, cilostazol group vs. clopidogrel group, 63 [54, 70] vs. 68 [62, 74] years, P=0.003), and being overweight was more common in the cilostazol group (BMI, cilostazol group vs. clopidogrel group, 25.26±2.69 vs. 23.96±2.58 kg/m2, P=0.002).
| Cilostazol group (N = 74) | Clopidogrel group (N = 82) | P value | |
|---|---|---|---|
| Sex, male, n (%) | 61 (82.43) | 67 (81.71) | 0.906 |
| Age, y, median (IQR) | 63 (54-70) | 68 (62-74) | 0.003* |
| Body mass index (kg/m2) | 25.26±2.69 | 23.96±2.58 | 0.002* |
| Current cigarette smoking, n (%) | 29 (39.19) | 33 (40.24) | 0.893 |
| Hypertension, n (%) | 58 (78.38) | 66 (80.49) | 0.745 |
| Diabetes mellitus, n (%) | 28 (37.84) | 27 (32.93) | 0.521 |
| History of stent placement, n (%) | 6 (8.11) | 2 (2.44) | 0.151 a |
| Hemorrhage history, n (%) | 7 (9.46) | 6 (7.32) | 0.629 |
| History of myocardial infarction, n (%) | 1 (1.35) | 3 (3.66) | 0.622 a |
| History of coronary heart disease, n (%) | 6 (8.11) | 14 (17.07) | 0.094 |
| Creatinine (μmol/L, IQR ) | 64.50 (57.00-77.00) | 67.00 (57.75-81.00) | 0.346 |
| PLT (×109/L, IQR) | 193.50 (161.50-240.25) | 198.00 (160.50-242.25) | 0.798 |
| LDL (mmol/L, IQR) | 1.86 (1.29-2.22) | 1.86 (1.49-2.44) | 0.488 |
| Admission’s mRS score (IQR) | 2 (2-3) | 2 (1-3) | 0.053 |
| Follow-up duration (month, IQR) | 13 (12-16.25) | 18 (12-29) | <0.01* |
Abbreviations: IQR, interquartile range; LDL, low-density lipoprotein; PLT, platelet count; mRS, modified Rankin Scale; Note: *The comparisons were statistically significant; a The comparisons were accomplished by the Fisher’s exact test.
When it comes to the localization of stents of the 156 enrolled patients, 56 patients underwent intracranial artery stenting, 95 patients underwent extracranial artery stenting, and 5 patients underwent intracranial combined with extracranial artery stenting (Table 2). The types of stents are shown in Table 2.
| Cilostazol group (N= 74) | Clopidogrel group (N= 82) | P value | |
|---|---|---|---|
| Localization of stents, n (%) | 0.13 a | ||
| Intracranial stents | 32 (43.24) | 24 (29.27) | |
| Internal carotid artery | 3 (4.05) | 8 (9.76) | |
| Middle cerebral artery | 19 (25.68) | 12 (14.63) | |
| Vertebral artery | 7 (9.46) | 3 (3.66) | |
| Basilar artery | 3 (4.05) | 3 (3.66) | |
| Extracranial artery stents | 39 (52.70) | 56 (68.29) | |
| Carotid artery | 21 (28.38) | 29 (35.37) | |
| Subclavian artery | 9 (12.16) | 11 (13.41) | |
| Vertebral artery | 19 (25.68) | 25 (30.49) | |
| Combination intracranial and Extracranial artery stents | 3 (4.05) | 2 (2.44) | |
| Types of stents , n (%) | 0.63 | ||
| Self-Expanding stent | 36 (48.65) | 38 (46.34) | |
| Balloon-expandable stent | 25 (33.78) | 33 (40.24) | |
| Combination Self-Expanding and balloon-expandable stents | 13 (17.57) | 11 (13.41) |
Note: a The comparisons were accomplished by the Fisher’s exact test. Two patients had stents in two or more parts of the Intracranial artery. Nineteen patients had stents in two or more parts of the extracranial artery.
None of the included patients had serious adverse events before surgery. Adverse events after surgery during hospitalization were similar between the two groups. The cumulative incidence curves of in hospital adverse events are shown in Fig.2. The incidence of any stroke and bleeding events did not differ significantly between the two groups (Table 3, Fig.2).
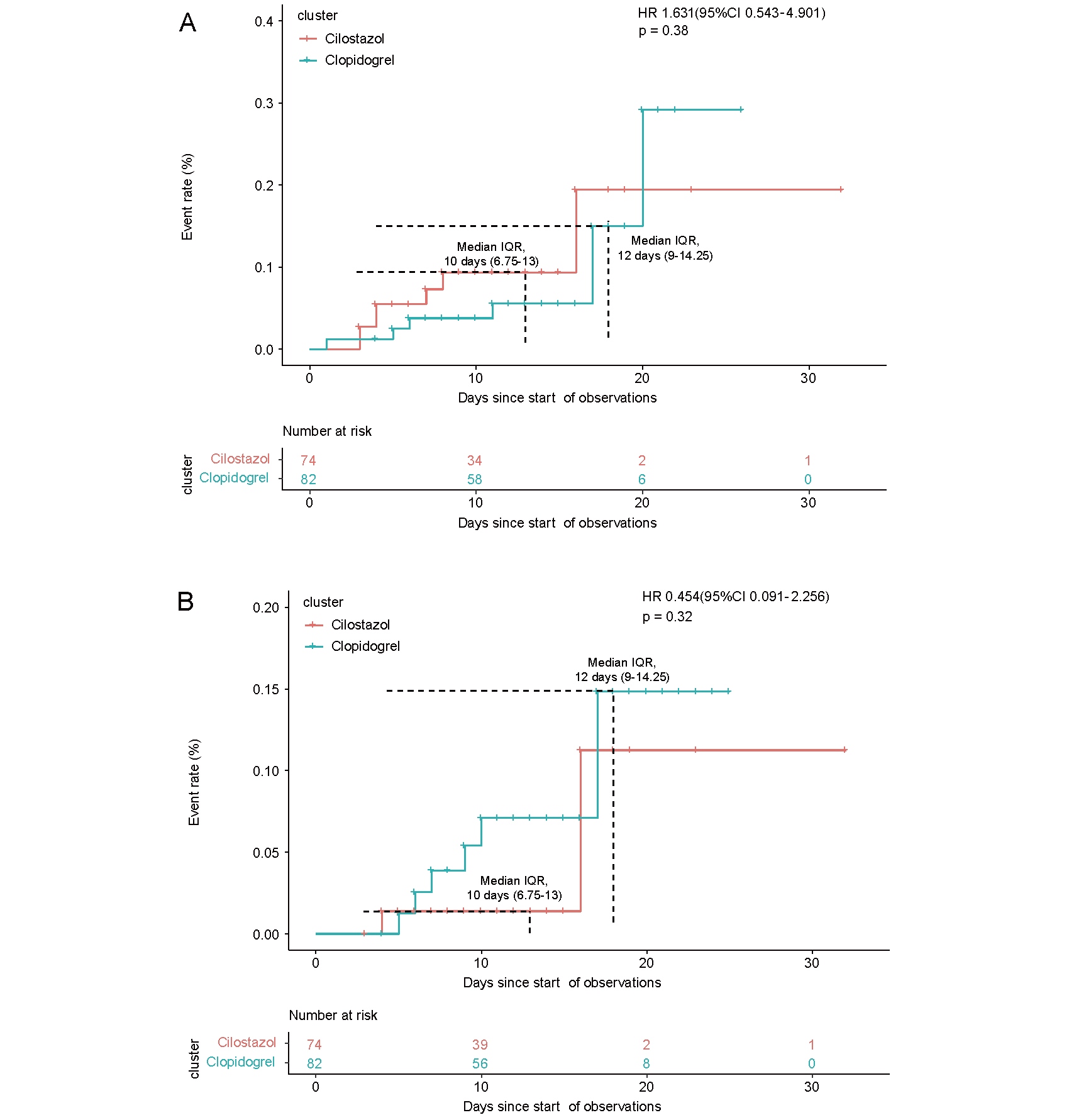
Kaplan–Meier curves for the time to the first event of any stoke (A) and bleeding events (B) in the hospital
| Cilostazol group (N= 74) | Clopidogrel group (N= 82) | HR (95%CI) | P value | |
|---|---|---|---|---|
| Any stoke, n (%) | 7 (9.46) | 6 (7.32) | 1.63 (0.54-4.90) | 0.38 |
| Bleeding events, n (%) | 2 (2.70) | 6 (7.32) | 0.45 (0.09-2.26) | 0.34 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
As shown in Table 4, a total of 5 ischemic strokes occurred at the end of follow-up, including 1 (1.35%) case in the cilostazol group and 4 (4.88%) cases in the clopidogrel group. The cumulative incidence curve of ischemic stroke is shown in Fig.3B. In the Cox proportional risk regression model, there were no statistical differences in the incidence of ischemic stroke (HR, 0.28; 95% CI, 0.03–2.47; P=0.25), as shown in Table 4.
| Cilostazol group (N= 74) | Clopidogrel group (N= 82) | HR (95%CI) | P value | |
|---|---|---|---|---|
| Ischemic events, n (%) | 2 (2.70) | 7 (8.54) | 0.34 (0.07-1.62) | 0.18 |
| Ischemic stoke, n (%) | 1 (1.35) | 4 (4.88) | 0.28 (0.03-2.47) | 0.25 |
| TIA, n (%) | 1 (1.35) | 3 (3.66) | 0.44 (0.04-4.30) | 0.48 |
| Bleeding events, n (%) | 4 (5.41) | 17 (20.73) | 0.27 (0.09-0.81) | 0.02* |
| Type 1 bleeding events, n (%) | 2 (2.70) | 14 (17.07) | 0.16 (0.04-0.72) | 0.02* |
| Type 2 bleeding events, n (%) | 2 (2.70) | 3 (3.66) | 0.89 (0.15-5.52) | 0.90 |
| Palpitation, n (%) | 3 (4.05) | 2 (2.44) | 1.66 (0.28-9.95) | 0.58 |
| Gastrointestinal discomfort, n (%) | 6 (8.11) | 10 (12.20) | 0.87 (0.31-2.46) | 0.80 |
| Any revascularization procedure, n (%) | 0 (0.00) | 1 (1.22) | - | >0.999 a |
| Delta mRS (≥ 1), n (%) | 37 (50.00) | 34 (41.46) | 1.41 (0.75-2.66) c | 0.29 b |
| All-cause death, n (%) | 1 (1.35) | 1 (1.22) | 1.11 (0.07-18.06) c | 0.94 b |
Abbreviations: TIA, transient ischemic attack; mRS, modified Rankin Scale; HR, hazard ratio, CI, confidence interval.
Note: *The comparisons were statistically significant. P-Values were all calculated by Cox proportional hazard regression model, except for those marked with “a” and “b”. a The comparisons were accomplished by the Fisher’s exact test; b The comparisons were accomplished by the Binary Logistic Analysis. c The results were accomplished by the Binary Logistic Analysis as HR and CI
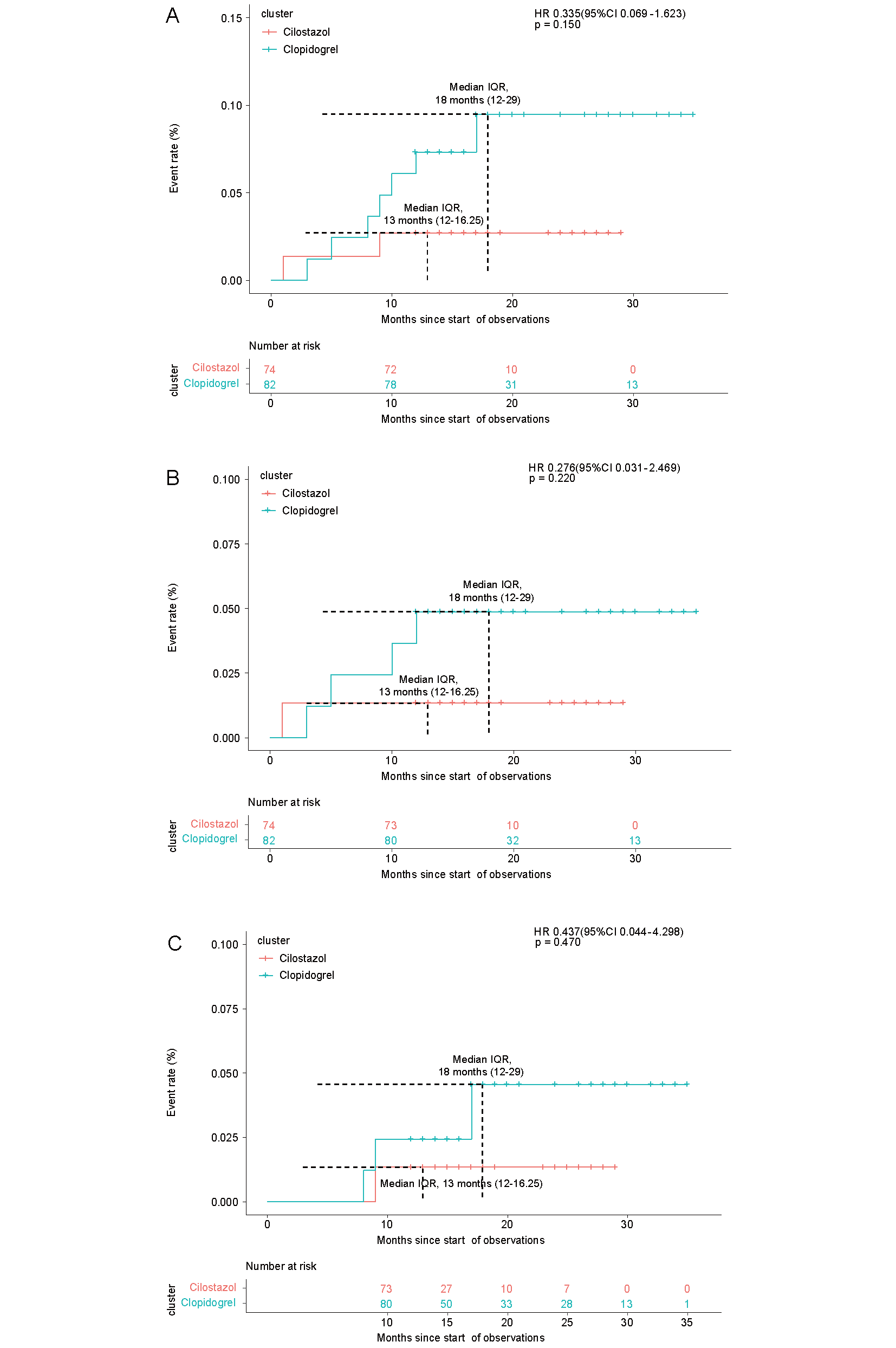
Kaplan–Meier curves for the time to the first event of ischemic events (A), ischemic stroke (B), and transient ischemic attack (C) at follow-up
During the follow-up period, we did not find a significant increase in the ADRs of palpitations in the cilostazol group (cilostazol group vs. clopidogrel group; 4.05% vs. 2.44%; HR, 1.66; 95% CI, 0.28–9.95; P=0.58; Table 4, Fig.4A). There was no difference in gastrointestinal discomfort between the two groups (cilostazol group vs. clopidogrel group; 8.11% vs. 12.20%; HR, 0.87; 95% CI, 0.31–2.46; P=0.80; Table 4, Fig.4B), and no patients reported headaches.

Kaplan–Meier curves for the time to the first event of palpitation (A) and gastrointestinal discomfort (B) at follow-up
Bleeding rate was lower in the cilostazol group than in the clopidogrel group (cilostazol group vs. clopidogrel group; 5.41% vs. 20.73%; HR, 0.27; 95% CI, 0.09–0.81; P=0.02; Table 4, Fig.5A). Type 1 bleeding events (including bruising, hematoma, nosebleeds, and gingival bleeding) were more common in the clopidogrel group (cilostazol group vs. clopidogrel group; 2.7% vs.17.07%; HR, 0.16; 95% CI, 0.04–0.72; P=0.02; Table 4, Fig.5B), whereas the risk of type 2 bleeding events (including tarry stool) was similar between the two groups.

Kaplan–Meier curves for the time to the first event of bleeding events (A), type 1 bleeding events (B), and type 2 bleeding events (C) at follow-up
There were no differences in the distribution of mRS scores among hospitalized patients (P=0.053), with median mRS scores of 2 (2, 3) and 2 (1, 3) in the cilostazol and clopidogrel groups, respectively (Table 1). With respect to neurological outcome, we analyzed the change in mRS score at 12 months, and 71 (45.5%) patients had an improvement in mRS score after 1 year (mRS Δs ≥ 1). The difference of neurological function prognosis was not significant in the two groups (cilostazol group vs. clopidogrel group; 37 (50.0%) vs. 34 (41.5%), P=0.29) (Table 4).
Cilostazol-based DAPT is an empirical strategy widely used in aspirin-intolerant patients after coronary drug-eluting stent implantation6). However, the efficacy and safety of cilostazol-based DAPT has not been well studied in intracranial or extracranial arterial stenting. Here we reported the efficacy and safety of cilostazol-based DAPT versus clopidogrel-based DAPT in the specific population.
We found that cilostazol-based DAPT was as effective in preventing ischemic events as clopidogrel-based DAPT and even showed a slight advantage at follow-up, but did not reach statistical significance. Several previous studies had confirmed that cilostazol played a key role in the long-term prevention of secondary stroke8, 19, 24, 25). Given that except its antiplatelet effect, cilostazol also has vascular protection, antiproliferation, alleviation of ischemia–reperfusion injury, and antiatherosclerosis effects16, 21-23), which may improve the prognosis of patients with stroke, especially after intracranial or extracranial arterial stenting. In addition, the antiplatelet effect of cilostazol is not inferior to aspirin or clopidogrel, and the risk of bleeding is lower. However, cilostazol-based DAPT therapy has been poorly studied in patients with intracranial and extracranial artery stenting. Our study provided a preliminary exploration of that hypotheses and confirmed the efficacy of cilostazol.
The advantage of cilostazol-based DAPT was not significant compared to clopidogrel-based DAPT, especially in hospital time. Interestingly, we noticed a reduction in the incidence of ischemic events over time in the cilostazol group. These results were consistent with several previous studies6, 26). The PONIT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) trial reported that the benefit of clopidogrel plus aspirin was concentrated in the first month of trial27). In contrast, Dai et al.6) found that the cumulative incidence of major adverse cardiovascular and cerebrovascular events in the cilostazol group decreases only after 150 days of follow-up. Further studies showed more cases of stent thrombosis and nonfatal stroke in the cilostazol group within 48 days of follow-up. Exactly as it had been known, the pleiotropic effect of cilostazol was not apparent in the short term, which might appear to explain why cilostazol was better during the long-term follow-up, and the superiority of prevention of ischemic events in the cilostazol group was not obvious in the early stage.
With respect to repeat revascularization, only one case happened during our follow-up period, which occurred in the clopidogrel-based group. This result was consistent with previous studies, in which cilostazol could significantly reduce the rate of in-stent restenosis and thrombosis, independent of the type of stent received23, 28, 29). The reduction of in-stent restenosis may be related with the mechanism to suppress smooth muscle proliferation and neointimal growth as previously reported30). Moreover, considering that atherosclerosis is a high risk factor for stroke recurrence, the antiatherogenic effects of cilostazol may be beneficial against in-stent restenosis.
We observed changes in scores on mRS (delta mRS), a scale widely used to measure disability in daily activities in patients with ischemic stroke, as a reference for neurological recovery. As a result, we found a slight advantage of improving neurological recovery in the cilostazol-based group, though there was no statistical significance. In addition, our findings demonstrated a high proportion of patients with diabetes, which had been shown to be associated with stroke and poor post-stroke outcomes31-33). At the same time, previous studies suggested that cilostazol is beneficial to attenuate the progression of coronary atherosclerosis in patients with diabetes33-35). Therefore, we believe that cilostazol may be a good preventive regimen for patients with ischemic stroke with diabetes mellitus.
In addition, the low risk of systemic and cerebral hemorrhage would account for its benefit over other antiplatelet agents, in that the longer patients are treated, the higher the cumulative risk of bleeding36). In the combination therapy, the performance of cilostazol is promising to improve the long-term prognosis.
There was significant advantage in bleeding events in the cilostazol group compared with the clopidogrel group. This finding was compatible with previous pooled data showing that the use of cilostazol does not increase the incidence of bleeding compared with other pharmacotherapies8, 19, 23). In fact, our results suggested that the risk of hemorrhagic complications may be relatively reduced. As previously reported, DAPT is limited due to its higher risk of bleeding5, 16, 27). In contrast, cilostazol-based DAPT showing a lower risk of bleeding may be a safety alternative regimen.
As pointed out in the PICASSO trial (Prevention of Cardiovascular Events in Asian Patients with Ischemic Stroke at High Risk of Cerebral Hemorrhage), cilostazol is also effective in preventing cardiovascular events in patients with high risk of cerebral hemorrhage37). In contrast, the incidence of antiplatelet-related ADRs, including palpitation, and gastrointestinal discomfort were similar between the two groups. The incidence of palpitations was slightly higher in the cilostazol-based group, but not statistically significant. Furthermore, unlike a previous study, palpitations in our patients were acceptable38).
This study had several limitations. All the above conclusions should be interpreted with caution because of the retrospective and single-center design of this study. Although we observed the advantage of cilostazol in preventing ischemic stroke recurrence, this result was not significant. This might be due to the small sample size or short term of follow-up. Meanwhile, there were differences in age and BMI in the two groups, which might disrupt our results. Furthermore, there were some differences in the follow-up duration between the two groups. This might be related to the higher frequency of cilostazol used in our hospital in recent years. Therefore, although we collected data from 2019 to 2021, more patients were enrolled earlier in the clopidogrel group, and more patients were enrolled later in the cilostazol group. The differences described above might lead to strong biases, and these limitations will be addressed in the further prospective and larger patient population studies.
In conclusion, based on those findings, it was reasonable to predict that cilostazol-based DAPT could be recommend as an effective and safe therapy regimen among patients undergoing intracranial or extracranial artery stent implantation, especially complicated with diabetes. Additionally, cilostazol might be considered for long-term monotherapy maintenance therapy. In the future, large-scale prospective studies are needed to further validate our conclusions.
We thank Guochao Yang for his suggestions when writing this article. This research was supported by Hengrui Hospital Pharmacy foundation of Jiangsu Pharmaceutical Association (no. H202006), Jiangsu Research Hospital Association for Precision Medication (no. JY202014).
None.