Abstract
Aim: Serum levels of cholesterol absorption and synthesis markers are known to be associated with cardiovascular risk. Familial hypercholesterolemia (FH) is a well-known inherited disorder presenting elevated low-density lipoprotein cholesterol (LDL-C) and total cholesterol (TC) levels and premature coronary disease. In this study, we aim to examine the differences in terms of serum markers of cholesterol metabolism between FH and non-FH individuals and to examine their associations with serum lipid levels.
Methods: In this study, we utilized data on serum markers of cholesterol metabolism, namely, lathosterol (Latho, synthesis marker), campesterol (Campe, absorption marker), and sitosterol (Sito, absorption marker) measured by gas chromatography of the CACHE consortium, which comprised of 13 research groups in Japan. Clinical data were compiled using REDCap system. Among the 2944 individuals in the CACHE population, we selected individuals without lipid-lowering medications and hemodialysis patients for this CACHE study FH analysis. Multivariable adjustment was performed to assess the associations.
Results: In this study, we analyzed data from 51 FH patients and 1924 non-FH individuals. After adjustment for possible confounders, the FH group was shown to have significantly higher Campe and Sito concentrations and insignificantly higher Latho concentrations than the non-FH group. These marker concentrations showed nonlinear associations with TC in the FH group. Campe/Latho and Sito/Latho ratios were significantly higher in the FH group than in the non-FH group.
Conclusion: FH group had significantly elevated serum Campe and Sito concentrations and insignificantly elevated Latho concentrations; thus, intestinal cholesterol absorption relative to hepatic cholesterol synthesis was suggested to be elevated in patients with FH. Serum Latho, Campe, and Sito concentrations showed nonlinear associations with TC in the FH group.
See editorial vol. 30: 1113-1114
Introduction
Familial hypercholesterolemia (FH) is a common inherited disorder characterized by abnormally elevated serum levels of low-density lipoprotein cholesterol (LDL-C), early-onset atherosclerotic cardiovascular disease (ASCVD), particularly coronary artery disease (CAD), and tendinous or cutaneous xanthomas1). Patients with FH are often accompanied by very high levels of serum LDL-C during the fetal stage and after birth. The risk of CAD can increase up to 13-fold (95% CI: 10- to 17-fold) among individuals with FH not receiving statins2), and the prevalence of peripheral arterial disease can increase from 5- to 10-fold in FH patients compared with non-FH controls3). By contrast, the risk of stroke remains equivocal4).
Apart from serum lipids and lipoprotein levels, alterations in cholesterol metabolism may affect risk of ASCVD. Dietary cholesterol is absorbed from the intestine via the key intestinal cholesterol transporter Niemann-Pick C1-like 1 (NPC1L1)5). Ezetimibe is a selective inhibitor of the NPC1L1-mediated cholesterol absorption; it is known to lower serum cholesterol levels5). Cholesterol absorption can be assessed by measuring serum levels of plant sterols such as campesterol (Campe) and sitosterol (Sito), which are not synthesized in humans but absorbed via the NPC1L1 in the intestine6). In addition to dietary cholesterol intake, cholesterol is synthesized mainly by the liver, and 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase is the rate-limiting enzyme in cholesterol biosynthesis. Statins selectively inhibit this enzyme7) and reduce cholesterol in plasma. Cholesterol synthesis can be evaluated by measuring serum levels of the precursors of cholesterol such as lathosterol (Latho)6). Previous studies showed that high cholesterol absorbers had higher risks for all-cause death and cardiovascular death in a cohort of home-dwelling elderly individuals8).
So far, information remains limited as regards the possible changes in cholesterol absorption and synthesis in patients with FH. Since FH is caused by the impaired uptake of plasma LDL into cells due to mutations in the LDLR, APOB, PCSK9, and related genes1), intracellular cholesterol pool could be reduced, and resultant activation of sterol-responsive element binding protein 2 (SREBP2) would upregulate the genes involved in hepatic cholesterol synthesis9) and intestinal cholesterol absorption10, 11). Therefore, we hypothesized that both synthesis and absorption of cholesterol would be elevated in FH.
Aim
In the present study, we addressed the following two research questions: first, “Are there any differences in serum levels of the biomarkers for cholesterol metabolism between non-FH individuals and FH patients without lipid-lowering medication?” and second, “Are serum lipids associated with the biomarker levels for cholesterol metabolism in FH patients without lipid-lowering medication?”
Methods
Ethical Consideration
This study was performed in conformity with the latest version of Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects by the Ministry of Health, Labor and Welfare and Ministry of Education, Japan (the original version was in 2016 and was modified in 2017). The study protocol was reviewed and approved by the Ethics Committee, Osaka City University Graduate School of Medicine, Osaka, Japan (Approval No. 3871) and the National Cerebral and Cardiovascular Center, Suita, Japan (Approval No. M30-075) and registered at UMIN-CTR (UMIN000030635). Also, the protocol of this study was approved by the review board of each participating institution prior to the study.
Clinical Data Collection
Thirteen research groups in Japan that possessed data on the serum markers of cholesterol metabolism made up the CACHE consortium. CACHE stands for cholesterol absorption and cholesterol synthesis in high-risk patients. Clinical data including serum biomarkers of cholesterol metabolism were collected and compiled using the web-based system Research Electronic Data Capture (REDCap)12, 13) (https://projectredcap.org/about/) at Osaka City University (http://www.hosp.med.osaka-cu.ac.jp/self/hyokac/redcap/index.shtml).
Selection of the CACHE Population and Participants for this Analysis
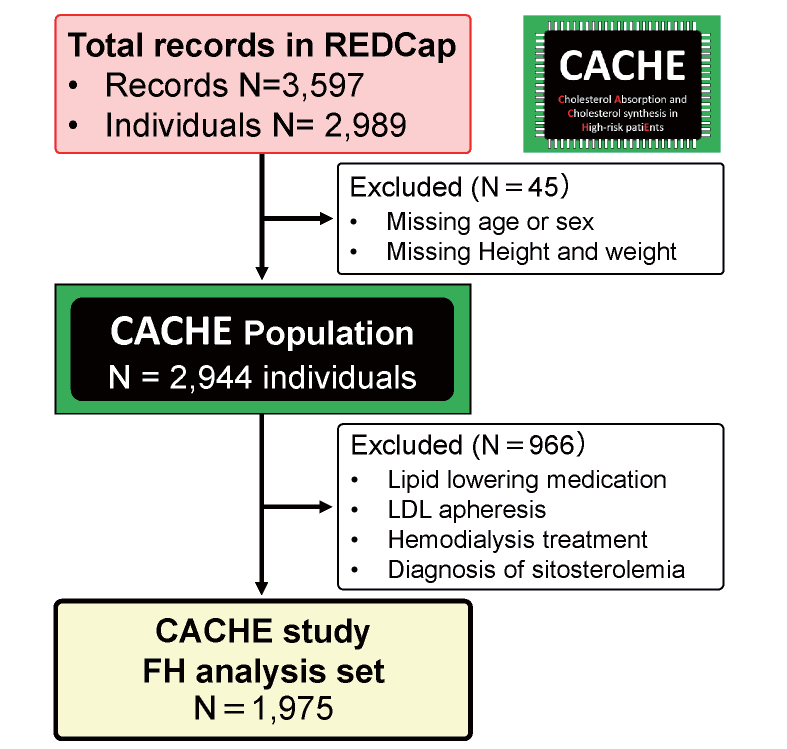
The inclusion criteria for the CACHE study were as follows: (1) patients at high risk of cardiovascular disease (coronary arterial disease, cerebrovascular disease, peripheral arterial disease, diabetes mellitus, chronic kidney disease including those treated with dialysis, and FH), or individuals who were examined for the screening of these conditions, and (2) individuals whose cholesterol metabolism markers were already measured (serum lathosterol, campesterol, and sitosterol levels). From the total of 3,597 records accumulated in the REDCap system, we selected the CACHE population for analysis (N=2,944) by excluding (1) the second records of the same individuals and (2) participants with missing values of age, sex, or both height and weight. For this CACHE study FH analysis, individuals with lipid-lowering medication, LDL apheresis, or hemodialysis treatment and patients with known diagnosis of sitosterolemia were further excluded, and the remaining participants were divided into two groups, i.e., those with and without FH.
Diagnosis of FH
FH was diagnosed based on the criteria from Japan Atherosclerosis Society published in 2017 14). Briefly, at least two of the following criteria should be satisfied: (1) LDL-C ≥ 180 mg/dL, (2) tendon/skin xanthomas, and (3) history of FH or premature CAD in second-degree blood relatives.
Assays for Latho, Campe, and Sito Concentrations
Serum concentrations of Latho, Campe, and Sito were measured by gas chromatography at SRL Inc., Tokyo, Japan. The procedure for gas chromatography has been described elsewhere in detail15). Latho is considered the biomarker for hepatic cholesterol synthesis, whereas Campe and Sito are considered the biomarkers for intestinal cholesterol absorption. Campe-to-Latho ratio (Campe/Latho ratio) was calculated for the assessment of the relative status of cholesterol absorption and cholesterol synthesis16).
Other Variables
The CACHE study has collected clinical data from medical records or data sets regarding the following items for research purposes: (1) clinical background including age, sex, smoking status, high-risk conditions (prior CAD, prior stroke, prior peripheral artery disease, diabetes mellitus (DM), chronic kidney disease (CKD) including dialysis, and FH), and comorbidity such as hypertension and hyperuricemia; (2) blood tests including total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), LDL-C, fasting plasma glucose, hemoglobin A1c (HbA1c), serum creatinine, estimated glomerular filtration rate (eGFR), uric acid, serum albumin, aspartate transaminase, alanine transaminase, C-reactive protein, and red blood cells, hemoglobin, mean corpuscular volume, white blood cells, and platelet counts; (3) physical examination and vital signs including height, body weight, body mass index (BMI), systolic blood pressure, diastolic blood pressure, and pulse rate; (4) medication use including drugs for dyslipidemia [statin, fibrate, ezetimibe, resin, probucol, omega-3-polyunsaturated fatty acid (PUFA), nicotinic acid, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, and microsomal triglyceride transfer protein inhibitor], hypertension, DM, and hyperuricemia; and (5) specific treatments including hemodialysis and LDL apheresis.
DM was defined by previous diagnosis of DM, use of any antidiabetic medication, fasting plasma glucose of 126 mg/dL or higher, or HbA1c by the National Glycohemoglobin Standardization Program (NGSP) value of 6.5% or higher according to the diagnostic criteria by the American Diabetes Association and the Japan Diabetes Society17, 18). If the previously used HbA1c value by the Japan Diabetes Society (JDS) was entered, it was converted to the NGSP value using a conversion formula provided by JDS19).
Hypertension was defined by use of any antihypertensive medication, systolic blood pressure of 140 mmHg or higher, or diastolic blood pressure of 90 mmHg or higher according to the criteria of the Japanese Society of Hypertension20).
CKD was defined in this study by eGFR lower than 60 mL/min/1.73 m2 using the equation for the Japanese21). Because the CACHE study did not collect data on proteinuria, proteinuria was not considered for the definition of CKD in this study. Patients with kidney failure treated with hemodialysis were included in patients with CKD.
Regarding lipid parameters, we used the following rules: (1) TG and HDL-C values were used as entered. (2) Using TC, TG, and HDL-C, non-HDL-C was calculated by subtracting HDL-C from TC, and LDL-C was calculated using the Friedewald formula22). (3) If LDL-C measured by a homogenous assay was entered but TC was unavailable, the LDL-C by a homogenous assay was used for analysis, and non-HDL-C was calculated as LDL-C plus TG/5. (4) If LDL-C or non-HDL-C cannot be calculated because of the missing value of TG or HDL-C, it is handled as missing. In the CACHE study FH analysis, we presented data on TG, HDL-C, non-HDL-C, and LDL-C thus determined.
Statistical Analysis
In the clinical characteristics, continuous and categorical variables were summarized by medians (interquartile ranges, IQR) and numbers (percentages), respectively, and these were compared using the Kruskal–Wallis test or Fisher’s exact test, respectively.
Association between the serum markers for cholesterol metabolism and the presence of FH was examined using linear regression models with covariates’ adjustment. The adjustment was made for age, sex, smoking status, BMI eGFR, presence of any cardiovascular disease (CVD), DM, hypertension, CKD, or hyperuricemia, and use of antidiabetic, antihypertensive, and antihyperuricemic medication. The estimated means (95% confidence intervals) were then reported.
Association of serum lipids (TC, TG, HDL-C, or LDL-C) (exposure) with serum markers of cholesterol metabolism (outcomes) in the FH group was examined using nonlinear regression models, which consider the restricted cubic spline term for serum lipids with 3 knots (10th, 50th, and 90th percentile levels). Since the sample size was limited, the models were adjusted only for age and sex. To meet the normal assumption of the regression model, we logarithmically transformed the objective variables and then used them in the regression models. In the above regression models, all missing values were complemented through multiple imputation methods based on the predictive mean matching approach.
All statistical inferences were conducted with a two-sided 5% significance level using R software version 4.0.3 (https://cran.r-project.org/).
Results
Selection of Participants for this Analysis
Fig.1 shows the selection of participants for this CACHE study FH analysis. We collected 3,597 records for 2,989 individuals, and the repeated records were not used. By excluding 45 subjects with missing data on age, sex, or both height and weight, the CACHE population (N=2,944) was determined. For the purpose of this analysis, 966 subjects were further excluded to avoid the effects of lipid-lowering medication and hemodialysis treatment. Finally, 1,975 individuals were selected for this analysis.
Table 1 summarizes the clinical characteristics of the study participants by the presence or absence of FH. As compared with the non-FH group, those in the FH group were noted to be younger and had lower prevalence of DM, higher prevalence of dyslipidemia, lower HDL-C, higher LDL-C, and lower fasting glucose and HbA1c levels.
Table 1.
Clinical characteristics of the CACHE study FH analysis
| Variables |
non-FH |
FH |
Overall |
Missing (N, %) |
P value
|
| Number of subjects |
1924 |
51 |
1975 |
|
|
| Age (years) |
54 [42–62] |
43 [31–57] |
54 [42–62] |
0 (0.0%) |
<0.001 |
| Male (N, %) |
1055 (54.8%) |
22 (43.1%) |
1077, (54.4%) |
0 (0.0%) |
0.098 |
| BMI (kg/m2)
|
22.7 [20.7–24.8] |
21.7 [19.9–25.3] |
22.7 [20.7–24.8] |
0 (0.0%) |
0.258 |
| Current smoker (N, %) |
191 (13.2%) |
3 (5.9%) |
194 (13.0%) |
479 (24.3%) |
0.125 |
| CAD (N, %) |
55 (2.9%) |
1 (2.0%) |
56 (2.8%) |
0 (0.0%) |
0.703 |
| Stroke (N, %) |
17 (0.9%) |
0 (0.0%) |
17 (0.9%) |
0 (0.0%) |
0.500 |
| PAD (N, %) |
29 (1.5%) |
0 (0.0%) |
29 (1.5%) |
0, 0.0% |
0.377 |
| CVD (N, %) |
85 (4.4%) |
1 (2.0%) |
86 (4.4%) |
0 (0.0%) |
0.396 |
| Diabetes mellitus (N, %) |
483 (38.5%) |
3 (6.2%) |
486 (37.3%) |
673 (34.1%) |
<0.001 |
| Hypertension (N, %) |
553 (28.8%) |
11 (32.4%) |
564 (28.9%) |
21 (1.1%) |
0.651 |
| CKD (N, %) |
176 (14.0%) |
5 (9.8%) |
181 (13.8%) |
663 (33.6%) |
0.399 |
| Dyslipidemia (N, %) |
950 (49.5%) |
51 (100%) |
1001 (50.8%) |
6 (0.3%) |
<0.001 |
| TC (mg/dL) |
210 [185–233] |
308 [286–342] |
211 [185–235] |
137 (6.9%) |
<0.001 |
| TG (mg/dL) |
94 [68–137] |
101 [70–139] |
95 [68–137] |
1 (0.1%) |
0.652 |
| HDL-C (mg/dL) |
60.0 [49.0–73.0] |
55.0 [45.5–62.5] |
59.6 [48.9–73.0] |
1 (0.1%) |
0.016 |
| LDL-C (mg/dL) |
126 [103–149] |
230 [201–257] |
127 [104–151] |
6 (0.3%) |
<0.001 |
| HbA1c (%) |
5.7 [5.4–6.5] |
5.4 [5.3–5.6] |
5.6 [5.3–6.4] |
728 (36.9%) |
0.001 |
| Glucose (mg/dL) |
99 [92–109] |
90 [86–99] |
99 [91–109] |
8 (0.4%) |
<0.001 |
Continuous variables are summarized as median [interquartile range], and categorical variables are given as number (percentage).
Abbreviations: FH, familial hypercholesterolemia; N, number; BMI body mass index; CAD, coronary artery disease; PAD, peripheral artery disease; CVD, cardiovascular disease including CAD, stroke, and PAD; CKD, chronic kidney disease; TC, total cholesterol, TG, triglycerides; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; HbA1c, hemoglobin A1c.
The levels of serum Latho, Campe, and Sito were compared between the non-FH and FH groups (Fig.2) in two ways, namely, in concentration and in ratio to TC. The FH group showed significantly higher serum concentrations of Campe and Sito, whereas Latho concentration was not different between the two groups. The FH group showed significantly higher ratios of Campe/Latho. When serum levels of cholesterol metabolism marker levels were expressed as ratios to TC, the FH group showed significantly lower Latho/TC ratios than the non-FH controls, whereas both Campe/TC and Sito/TC ratios were not significantly different between the two groups.
Campe/Latho Ratio and Sito/Latho Ratio
Campe/Latho ratio and Sito/Latho ratio were calculated to examine the relative status of cholesterol absorption and cholesterol synthesis, and they were compared between the two groups (Fig.3). These ratios were significantly higher in the FH group than in the non-FH group.
Association of Serum Lipids with Concentrations of Cholesterol Metabolism Markers in the FH Group
Restricted cubic spline curves were used to show the nonlinear relationship between serum lipids and serum concentrations of Latho, Campe, and Sito adjusted for age and sex in the FH group. As shown in Fig.4, TC levels were found to be significantly associated with Latho, Campe, and Sito levels. The association appeared to be nonlinear, and the Latho concentration was similar in the TC range of 330 mg/dL and higher. In contrast, the associations of TC with Campe and Sito concentrations appeared less impressive than the TC range of 300 mg/dL or lower. Similar associations of LDL-C were noted with Latho, Campe, and Sito concentrations. In contrast, HDL-C did not show significant associations with Latho, Campe, or Sito concentrations. TG was associated positively with Latho but not significantly with Campe or Sito concentrations.
Association of Serum Lipids with Concentrations of Cholesterol Metabolism Markers in the Non-FH Group
Similar association studies between serum lipid variables and the three biomarker concentrations were conducted in the non-FH group (Fig.5). In the non-FH group, the associations of TC with the three biomarkers were almost linear in the range of TC up to 300 mg/dL. An almost linear association was also found between LDL-C and Latho in the range of LDL-C up to 250 mg/dL. HDL-C exhibited almost linear associations with all three biomarker concentrations in the non-FH group, which was in contrast to no significant association in the FH group. In the non-FH group, TG showed a positive association with Latho but not with Campe or Sito.
Discussion
This CACHE study FH analysis compared the serum levels of Latho, Campe, Sito, Campe/Latho ratio, and Sito/Latho ratio between the FH and non-FH groups, and their associations with serum lipid levels were examined in the FH group. Serum concentration of Latho was shown to be not significantly different between the two groups, whereas Campe and Sito concentrations were significantly higher in the FH group. When the marker levels were expressed as ratios to TC, Latho/TC level was significantly lower in the FH group, whereas Campe/TC and Sito/TC ratios were not significantly different between the groups. Both Campe/Latho ratio and Sito/Latho ratio were significantly higher in the FH group. The three marker concentrations were positively but nonlinearly associated with TC and LDL-C, but not significantly with HDL-C. TG was significantly associated only with Campe.
The results of this analysis raise a fundamental question: which should we use, serum concentrations or their ratios to TC, as a more appropriate way to express cholesterol metabolism with serum biomarkers? The conclusion can change depending on the assessment method. The gold standard method for assessing intestinal cholesterol absorption and hepatic cholesterol synthesis is in vivo studies using radiolabeled tracers by which fractional cholesterol absorption, fecal excretion of sterols, and cholesterol synthesis can be calculated. Gylling and Miettinen23) conducted such studies as well as the measurements of serum markers of cholesterol absorption in 17 subjects and reported that the measured fractional absorption was significantly and positively correlated with both serum concentrations and ratios to serum cholesterol, inversely with fecal excretion of neutral sterols, and inversely with cholesterol synthesis. Regarding such correlations in FH, Gylling and Miettinen have performed similar studies in 22 patients with heterozygous FH24). The measured fractional absorption of cholesterol was directly correlated with plasma marker levels of cholesterol absorption markers, which were expressed as ratios to plasma TC. However, because they did not provide the correlation of measured fractional absorption of cholesterol with plasma concentrations of cholesterol absorption markers in their study, the degree of correlation between concentrations of the markers and directly measured parameters of cholesterol metabolism in patients with FH remains unknown.
What are the rationales for dividing the cholesterol metabolism marker concentrations by plasma TC? In case of estimating enzyme activity, it is reasonable to utilize the ratio of the product to the precursor concentrations in serum because the enzyme is directly involved in their concentrations. The rate-limiting enzyme of cholesterol biosynthesis is HMG-CoAR. Since both Latho and cholesterol are downstream of this enzyme, the ratio of Latho/TC does not directly indicate the enzyme activity of HMG-CoAR. Campe is not metabolized into cholesterol. Then, what is the reason for using the ratios to TC? It is presumably because both cholesterol and these markers are carried in lipoproteins in the circulation. The serum lipoprotein level is a common factor affecting both cholesterol and these markers. However, it may be too simple to normalize these marker levels by dividing TC. First, as shown in this study, the associations between TC and these markers are not necessarily linear. Second, the distributions of these markers among different lipoproteins appear to be uneven. Cholesterol synthesis markers are more enriched in very low-density lipoprotein and intermediate-density lipoprotein, whereas absorption markers are more enriched in HDL fraction25). Third, in some cases, the measured absorption parallels serum concentration better than its ratio to cholesterol. In patients with FH with and without operation of ileal bypass, no difference was noted in the measured fractional cholesterol absorption and serum concentrations of Sito or Campe between the two groups, while Sito/TC and Campe/TC ratios were higher in the operated group26). This may be an example where dividing the marker concentrations by TC gave misleading results. And fifth, division by TC may induce problems in the interpretation of their associations with serum lipids. These considerations suggest that use of the ratios of cholesterol metabolism biomarkers to TC can do more harm than good. We believe that serum concentrations are more appropriate than the ratios to TC. Because we showed both serum concentrations and their ratios to TC only for the purpose of discussion, the results in Fig.2 should be interpreted very carefully.
It is reasonable to hypothesize that both hepatic synthesis and intestinal absorption are elevated in patients with FH because they have impaired uptake of plasma LDL, which would cause decreased intracellular cholesterol pool, activation of SREBP2, and upregulation of transcription of genes involved in cholesterol synthesis including HMGCR gene9). NPC1L1 is also upregulated by SREBP2 10, 11), resulting in increased cholesterol absorption. Regarding possible change in cholesterol absorption in FH, Connor WE and Lin DS27) conducted an in vivo kinetic study using radiolabeled tracers in 6 patients with type 2 hypercholesterolemia with tendon xanthoma and 15 normocholesterolemic control subjects. When labeled cholesterol was taken via labeled egg yolk, the fractional absorption was 40.4% in the hypercholesterolemic group and 42.9% in the normocholesterolemic group. When labeled cholesterol was taken via crystalline cholesterol, the fractional absorption was 54.8% in the hypercholesterolemic group and 44.7% in the normocholesterolemic group. Although the authors concluded that cholesterol absorption was similar between groups, the sample size was small, and no statistical adjustment or test was performed for the between-group comparison. Another study by Koivisto and Miettinen28) measured cholestanol as a marker of intestinal cholesterol absorption in patients with FH and compared it with normal controls. The plasma concentration of cholestanol and its ratio to plasma cholesterol were both slightly higher in FH than in the normocholesterolemic control subjects, suggesting elevated cholesterol absorption in FH. Gylling and Miettinen24) measured plasma concentrations of Campe and Sito as cholesterol absorption biomarkers in 22 patients with heterozygous FH, wherein they reported the ratios of the absorption marker levels to plasma cholesterol were higher in the FH patients. In this CACHE study FH analysis, the concentrations of Campe and Sito were higher in the FH group, but their ratios to TC were not significantly different from the non-FH group. Collectively, these studies are consistent in that cholesterol absorption is normal or elevated in FH. In our study, we showed significantly higher Campe/Latho and Sito/Latho ratios in the FH group than in the non-FH group. As Campe/Latho and Sito/Latho ratios are the same as the ratio of Campe/TC to Latho/TC and the ratio of Sito/TC to Latho/TC, respectively, it is possible to avoid discussing the benefit and harm of dividing the concentration by TC by using these ratios of absorption/synthesis markers. Therefore, we can conclude that cholesterol absorption relative to cholesterol synthesis is deemed to be elevated in patients with FH.
Regarding possible changes in cholesterol synthesis in FH, Gylling and Miettinen24) measured plasma concentrations of squalene, desmosterol, and Latho as cholesterol synthesis biomarkers in 22 patients with heterozygous FH. As compared to the normal control values, the concentrations of these markers were elevated in the FH patients, whereas their ratios to plasma cholesterol were lower in the FH group. Similar to that report, this CACHE study FH analysis showed that the concentration of Latho was insignificantly higher in the FH group due presumably to relatively small sample size of the FH group, and Latho/TC ratio was significantly lower in the FH group in multivariable-adjusted comparison. As far as we know, there is no study that compared cholesterol synthesis between FH and non-FH control groups by directly measuring in vivo cholesterol synthesis with radiolabeled tracers. Therefore, although direct evidence remains lacking, we interpret these results of concentrations of surrogate markers to suggest that cholesterol synthesis is normal or elevated in FH patients.
It is important to point out that the associations of Latho concentration with TC and LDL-C were nonlinear, and Latho concentration showed plateau in the range of higher TC (approximately 350 mg/dL or higher) and in the range of higher LDL-C (approximately 260 mg/dL or higher) in the FH group. Since the elevated LDL-C and TC levels in FH have resulted from lowered cellular uptake of plasma LDL due to impaired LDL receptor function, these associations may reflect cellular cholesterol pool. We speculate that lower hepatic cellular cholesterol pool was associated with higher cholesterol synthesis, but it reached a maximum level in FH patients with very high LDL-C and TC levels. It is possible that hepatic degradation of cholesterol into bile acid is downregulated to maintain intracellular cholesterol in the liver in patients with FH, which may affect hepatic cholesterol synthesis. Clearly, further studies are needed on this issue.
What is the implication of this study? First, in addition to the elevated LDL-C levels, the increased intestinal cholesterol absorption in patients with FH may partly contribute to the excess risk for ASCVD in these patients. Dietary 7-ketocholesterol, which is an oxysterol, could enter the circulation via enterocytes, induce plaque destabilization, and rupture in animal models29). Serum 7-ketocholesterol levels were shown to be higher in patients with CAD, particularly in those with unstable angina30). Second, the inhibition of intestinal cholesterol absorption may have additional benefit on top of LDL-C reduction in FH patients with increased cholesterol absorption. Ezetimibe is known to selectively inhibit cholesterol absorption via NPC1L1 5), and fenofibrate31) and pemafibrate32) were reported to upregulate mRNA expression of Npc1l1 gene in rodents. We need further studies on this possibility in patients with FH. Third, because high cholesterol absorbers were shown to have higher risk for cardiovascular mortality in non-FH populations8, 33), the measurement of the cholesterol metabolism biomarkers may be useful in the risk stratification and prediction of responsiveness to ezetimibe in patients with FH. So far, however, we need more direct epidemiologic evidence for this possibility in FH. Fourth, since the FH group showed elevated levels of serum Sito than the non-FH group, this may make it difficult to differentiate sitosterolemia from FH. In fact, 2 out of 49 patients (4.1%) in the FH group had serum Sito level of 10 µg/mL or higher (10.0 and 10.8 µg/mL), which is one of the diagnostic criteria for sitosterolemia34). In such cases, genetic analysis may be needed for differential diagnosis.
This study has several limitations. First, the sample size was relatively small. However, it was larger than early studies conducted in 1970s to 1990s and allowed statistical test in which statistical adjustment was done. Second, because this was a retrospective analysis, we were not able to correlate the serum marker levels of cholesterol metabolism with directly measured cholesterol absorption or synthesis. Third, although some previous studies showed higher cholesterol absorption was associated higher risk of all-cause and cardiovascular mortality8), this cross-sectional study examined nothing about such prognostic values of the cholesterol metabolism markers in patients with FH. Fourth, we did not examine the degradation of cholesterol, which is another important component determining cholesterol homeostasis. Fifth, since FH was clinically diagnosed in our study, the FH group might include some patients with a variant in the ABCG5 or ABCG8 gene. According to Tada et al.35), 7.6% of their 487 patients with clinically diagnosed FH did not have FH mutations but had ABCG5/G8 mutations. Such patients with ABCG5/G8 mutations are known to have increased cholesterol absorption marker levels and possibly different responsiveness to ezetimibe.
Conclusion
By measuring the serum concentrations of Latho, Campe, and Sito, we examined possible changes in cholesterol metabolism in patients with FH. The FH group had significantly elevated serum Campe and Sito concentrations and insignificantly elevated Latho concentrations as compared to the non-FH group. Campe/Latho ratio and Sito/Latho ratio were significantly higher in the FH group. These results suggest that cholesterol absorption and absorption relative to synthesis are elevated in patients with FH. To confirm these findings, we need further in vivo studies using radiolabeled tracers.
Acknowledgements
We are grateful to Ms. Megumu Horiuchi for processing the blood samples of the FH patients. Part of this study was presented at the 53rd Annual Meeting of the Japan Atherosclerosis Society (October 22-23, 2021, Kyoto, Hybrid version) and at the 18th International Symposium of Atherosclerosis (October 24-27, 2021, Kyoto, Hybrid version).
Funding
This study was supported by a grant to TS from Bayer Yakuhin Ltd. The funder played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest
Mariko Harada-Shiba has received stock holdings from Liid Pharma Inc., honoraria from Amgen Inc., and scholarship grants from Recordati Rare Diseases Japan. Masatsune Ogura reported personal fee from Kowa Company Ltd and Amgen. Tatsuro Ishida reported personal fee from Bayer Yakuhin Ltd and Kowa Inc. Yasushi Ishigaki reported personal fee from Bayer Yakuhin, Kowa Pharmaceutical Company, MSD, Novartis, Novo Nordisk, Ono Pharmaceutical, Sanofi K.K., and Takeda Pharmaceutica; research grant from Daiichi Sankyo, and Takeda Science Foundation; and Scholarship grant from MSD and Ono Pharmaceutical. Tetsuya Matoba reported personal fee from Bayer Yakuhin Ltd and MSD; and research grant from Amgen and Kowa. Takeshi Matsumura reported personal fee from Eli Lily Japan KK, and Boehringer Ingelheim Japan Inc; and research grant from Shimazu Corporation. Tomoko Nakagami reported personal fee from Sanwa Kagaku Kenkyusho Co Ltd, Novo Nordisk Pharma Ltd Japan, Eli Lily Japan KK, Sanofi K.K., Sumitomo Pharma, and Boehringer Ingelheim Japan Inc. Shizuya Yamashita reported personal fee from Kowa. Hiroshi Yoshida reported personal fee from Denka Company Ltd and Kowa Company Ltd. Tetsuo Shoji reported personal fee and research grant from Bayer Yakuhin Ltd. Other authors reported no financial conflict of interest relevant to this study.
References
- 1) Defesche JC, Gidding SS, Harada-Shiba M, Hegele RA, Santos RD and Wierzbicki AS: Familial hypercholesterolaemia. Nat Rev Dis Primers, 2017; 3: 17093
- 2) Benn M, Watts GF, Tybjaerg-Hansen A and Nordestgaard BG: Familial hypercholesterolemia in the danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab, 2012; 97: 3956-3964
- 3) Hutter CM, Austin MA and Humphries SE: Familial hypercholesterolemia, peripheral arterial disease, and stroke: a HuGE minireview. Am J Epidemiol, 2004; 160: 430-435
- 4) Akioyamen LE, Tu JV, Genest J, Ko DT, Coutin AJS, Shan SD and Chu A: Risk of Ischemic Stroke and Peripheral Arterial Disease in Heterozygous Familial Hypercholesterolemia: A Meta-Analysis. Angiology, 2019; 70: 726-736
- 5) Altmann SW, Davis HR, Jr., Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N and Graziano MP: Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science, 2004; 303: 1201-1204
- 6) Miettinen TA, Tilvis RS and Kesaniemi YA: Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol, 1990; 131: 20-31
- 7) Brown MS and Goldstein JL: Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res, 1980; 21: 505-517
- 8) Strandberg TE, Tilvis RS, Pitkala KH and Miettinen TA: Cholesterol and glucose metabolism and recurrent cardiovascular events among the elderly: a prospective study. J Am Coll Cardiol, 2006; 48: 708-714
- 9) Sakakura Y, Shimano H, Sone H, Takahashi A, Inoue N, Toyoshima H, Suzuki S and Yamada N: Sterol regulatory element-binding proteins induce an entire pathway of cholesterol synthesis. Biochem Biophys Res Commun, 2001; 286: 176-183
- 10) Pramfalk C, Jiang ZY, Cai Q, Hu H, Zhang SD, Han TQ, Eriksson M and Parini P: HNF1alpha and SREBP2 are important regulators of NPC1L1 in human liver. J Lipid Res, 2010; 51: 1354-1362
- 11) Gevry N, Schoonjans K, Guay F and Murphy BD: Cholesterol supply and SREBPs modulate transcription of the Niemann-Pick C-1 gene in steroidogenic tissues. J Lipid Res, 2008; 49: 1024-1033
- 12) Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N and Conde JG: Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform, 2009; 42: 377-381
- 13) Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN and Consortium RE: The REDCap consortium: Building an international community of software platform partners. J Biomed Inform, 2019; 95: 103208
- 14) Harada-Shiba M, Arai H, Ishigaki Y, Ishibashi S, Okamura T, Ogura M, Dobashi K, Nohara A, Bujo H, Miyauchi K, Yamashita S, Yokote K and Working Group by Japan Atherosclerosis Society for Making Guidance of Familial Hypercholesterolemia: Guidelines for Diagnosis and Treatment of Familial Hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 751-770
- 15) Yoshida H, Tada H, Ito K, Kishimoto Y, Yanai H, Okamura T, Ikewaki K, Inagaki K, Shoji T, Bujo H, Miida T, Yoshida M, Kuzuya M, Yamashita S: Reference Intervals of Serum Non-Cholesterol Sterols by Gender in Healthy Japanese Individuals. J Atheroscler Thromb, 2020; 27: 5: 409-417
- 16) Shoji T, Akiyama Y, Fujii H, Harada-Shiba M, Ishibashi Y, Ishida T, Ishigaki Y, Kabata D, Kihara Y, Kotani K, Kurisu S, Masuda D, Matoba T, Matsuki K, Matsumura T, Mori K, Nakagami T, Nakazato M, Taniuchi S, Ueno H, Yamashita S, Yoshida H and Yoshida H: Association of Kidney Function with Serum Levels of Cholesterol Absorption and Synthesis Markers: The CACHE Study CKD Analysis. J Atheroscler Thromb, 2022; 29: 1835-1848
- 17) American_Diabetes_Association: Diagnosis and classification of diabetes mellitus. Diabetes Care, 2014; 37 Suppl 1: S81-90
- 18) Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes M, Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, Ito C, Inagaki N, Iwamoto Y, Kasuga M, Hanafusa T, Haneda M and Ueki K: Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig, 2010; 1: 212-228
- 19) Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H and Committee on the Standardization of Diabetes Mellitus-Related Laboratory Testing of Japan Diabetes S: International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig, 2012; 3: 39-40
- 20) Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S and Japanese Society of Hypertension Committee for Guidelines for the Management of H: The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res, 2014; 37: 253-390
- 21) Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H and Hishida A: Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis, 2009; 53: 982-992
- 22) Friedewald WT, Levy RI and Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 1972; 18: 499-502
- 23) Tilvis RS and Miettinen TA: Serum plant sterols and their relation to cholesterol absorption. Am J Clin Nutr, 1986; 43: 92-97
- 24) Gylling H and Miettinen TA: Serum noncholesterol sterols related to cholesterol metabolism in familial hypercholesterolemia. Clin Chim Acta, 1988; 178: 41-49
- 25) Ketomaki A, Gylling H, Siimes MA, Vuorio A and Miettinen TA: Squalene and noncholesterol sterols in serum and lipoproteins of children with and without familial hypercholesterolemia. Pediatr Res, 2003; 53: 648-653
- 26) Koivisto PV and Miettinen TA: Effect of ileal exclusion on plant sterol metabolism in familial hypercholesterolemia. Digestion, 1987; 38: 133-141
- 27) Connor WE and Lin DS: The intestinal absorption of dietary cholesterol by hypercholesterolemic (type II) and normocholesterolemic humans. J Clin Invest, 1974; 53: 1062-1070
- 28) Koivisto PV and Miettinen TA: Plasma and biliary cholestanol related to steroid metabolism in familial hypercholesterolemia patients with and without ileal exclusion. Clin Chim Acta, 1988; 174: 197-205
- 29) Sato K, Nakano K, Katsuki S, Matoba T, Osada K, Sawamura T, Sunagawa K and Egashira K: Dietary cholesterol oxidation products accelerate plaque destabilization and rupture associated with monocyte infiltration/activation via the MCP-1-CCR2 pathway in mouse brachiocephalic arteries: therapeutic effects of ezetimibe. J Atheroscler Thromb, 2012; 19: 986-998
- 30) Hitsumoto T, Takahashi M, Iizuka T and Shirai K: Clinical significance of serum 7-ketocholesterol concentrations in the progression of coronary atherosclerosis. J Atheroscler Thromb, 2009; 16: 363-370
- 31) Valasek MA, Clarke SL and Repa JJ: Fenofibrate reduces intestinal cholesterol absorption via PPARalpha-dependent modulation of NPC1L1 expression in mouse. J Lipid Res, 2007; 48: 2725-2735
- 32) Sairyo M, Kobayashi T, Masuda D, Kanno K, Zhu Y, Okada T, Koseki M, Ohama T, Nishida M, Sakata Y and Yamashita S: A Novel Selective PPARalpha Modulator (SPPARMalpha), K-877 (Pemafibrate), Attenuates Postprandial Hypertriglyceridemia in Mice. J Atheroscler Thromb, 2018; 25: 142-152
- 33) Rogacev KS, Pinsdorf T, Weingartner O, Gerhart MK, Welzel E, van Bentum K, Popp J, Menzner A, Fliser D, Lutjohann D and Heine GH: Cholesterol synthesis, cholesterol absorption, and mortality in hemodialysis patients. Clin J Am Soc Nephrol, 2012; 7: 943-948
- 34) Tada H, Nomura A, Ogura M, Ikewaki K, Ishigaki Y, Inagaki K, Tsukamoto K, Dobashi K, Nakamura K, Hori M, Matsuki K, Yamashita S, Yokoyama S, Kawashiri MA and Harada-Shiba M: Diagnosis and Management of Sitosterolemia 2021. J Atheroscler Thromb, 2021; 28: 791-801
- 35) Tada H, Okada H, Nomura A, Yashiro S, Nohara A, Ishigaki Y, Takamura M and Kawashiri MA: Rare and Deleterious Mutations in ABCG5/ABCG8 Genes Contribute to Mimicking and Worsening of Familial Hypercholesterolemia Phenotype. Circ J, 2019; 83: 1917-1924