2024 Volume 31 Issue 12 Pages 1733-1747
2024 Volume 31 Issue 12 Pages 1733-1747
Aim: Evidence regarding the association between various tumor necrosis factor-α (TNF-α) inhibitors and cardiovascular adverse events (AEs) is both limited and contradictory.
Methods: A retrospective pharmacovigilance study was conducted using the FDA Adverse Event Reporting System (FAERS) database. Cardiovascular AEs associated with TNF-α inhibitors (adalimumab, infliximab, etanercept, golimumab, and certolizumab) were evaluated using a disproportionality analysis. To reduce potential confounders, adjusted ROR and subgroup analyses were performed.
Results: After excluding duplicates, 9,817 cardiovascular reports were associated with the five TNF-α inhibitors. Only adalimumab had positive signals for myocardial infarction (ROR=1.58, 95%CI=1.51-1.64) and arterial thrombosis (ROR=1.54, 95%CI=1.49-1.58). The remaining four TNF-α inhibitors did not show a risk association with any type of cardiovascular event. Further analyses of specific indication subgroups and after adjusting for any confounding factors demonstrated that adalimumab was still significantly associated with cardiovascular events, especially in patients with psoriasis (adjusted ROR=2.16, 95%CI=1.95-2.39).
Conclusions: This study revealed that adalimumab was the only TNF-α inhibitor associated with an elevated risk of thrombotic cardiovascular AEs, whereas the other four TNF-α inhibitors did not show any risk effect. However, given the limitations of such pharmacovigilance studies, it is necessary to validate these findings in prospective studies in the future.
Immune-mediated inflammatory diseases (IMIDs), such as rheumatoid arthritis (RA) and inflammatory bowel disease (IBD), are associated with increased cardiovascular morbidity and mortality compared with the general population1, 2). Several meta-analyses3, 4), incorporating various observational studies, have demonstrated an association between IMIDs and cardiovascular risk. As the population of individuals with IMIDs continues to increase, early recognition, management of risk factors, and rational medication therapy are thus considered to have substantial implications.
There is an increasing emphasis that decreasing the inflammatory burden may be beneficial for reducing cardiovascular adverse events (AEs) in IMIDs. As the most commonly used inflammatory factor modulator, tumor necrosis factor-alpha (TNF-α) inhibitors have emerged as the standard treatment for severe IMIDs, either as monotherapy or in combination with other medications5). A recent meta-analysis6) revealed a potential association between anti-TNF-α therapy and a decreased risk of cardiovascular events in a systemic inflammatory population. Furthermore, several publications7, 8) have suggested the beneficial effects of TNF-α inhibitors on cardiovascular outcomes in patients with RA. However, there is a scarcity of comprehensive studies available to assess the associations between TNF-α inhibitors and cardiovascular AEs in the treatment of IMIDs, and this topic thus remains a subject of debate. Some studies have proposed that physiological levels of TNF-α may have beneficial effects on heart remodeling and tissue repair9, 10), implying that TNF-α inhibitors could potentially increase the risk of cardiotoxicity11). Therefore, controversy persists regarding whether TNF-α inhibitors during the treatment of IMIDs can lead to cardiovascular AEs.
Currently, there are five FDA-approved biological TNF-α inhibitors: adalimumab, infliximab, etanercept, golimumab, and certolizumab. A recent global market report12) indicates that the TNF-α inhibitor market is expected to grow from $38.56 billion in 2021 to $40.73 billion in 2022, highlighting its significant potential. TNF-α inhibitors have demonstrated substantial therapeutic benefits in patients with various autoimmune diseases13, 14), such as RA, ankylosing spondylitis (AS), Crohn’s disease (CD), ulcerative colitis (UC), psoriasis (PS), and chronic endogenous uveitis. Although each of the five referenced TNF-α inhibitors effectively blocks soluble TNF from interacting with its receptors, thereby achieving therapeutic outcomes, they differ significantly in terms of their structure and function15, 16). Therefore, it is critical to explore the associations between various TNF-α inhibitors and cardiovascular AEs.
To comprehensively understand the potential effects of various TNF-α inhibitors on cardiovascular AEs, a case/non-case study was conducted using one of the most comprehensive pharmacovigilance databases, the US Food and Drug Administration Adverse Event Reporting System (FAERS). FAERS is a post-marketing safety surveillance database used to record over 20 million adverse drug events worldwide, providing a valuable data source for real-world studies. Moreover, the large sample size of FAERS provides the statistical capacity to detect rare AEs that may be challenging to identify through traditional epidemiological investigations17). Thus, this pharmacovigilance study aimed to explore the potential association between TNF-α inhibitors and cardiovascular AEs, and disproportionality analyses were performed to detect association signals.
This retrospective study was based on pharmacovigilance data obtained from the FAERS database and covers the period from the third quarter of 2017 to the third quarter of 2022. The FAERS database is updated quarterly and publicly available, containing information on AEs reported by healthcare professionals, patients, and drug manufacturers worldwide since 1968. It adheres to the international safety reporting guidelines issued by the International Conference on Harmonization (ICH E2b), in which all AEs are coded using terms from the Medical Dictionary of Regulatory Activities18). The FAERS data files contained seven types of datasets: demographic and administrative information (DEMO), drug information (DRUG), adverse events (REAC), patient outcomes (OUTC), source of reports (RPSR), therapy start and end dates of drug therapy (THER), and indications for use (INDI). The above files can be linked by “primaryid” and “caseid” variables. As this was an observational study using an anonymous database that did not involve either treatment intervention or the collection of blood samples, ethics committee approval was not required.
2.2 Data Pre-ProcessingA total of 8,599,840 reports were obtained from the FAERS database and imported into the MySQL 8.0. According to the FDA recommendations, the variable matching method19) was applied to remove duplicate reports due to the complexity of the sources of reports, which is usually used by the Medicines and Healthcare Products Regulatory Agency (MHRA) and Danish Health and Medicines Authority (DHMA). The main process is selecting the latest FDA-_DT and the higher PRIMARYID when CASEID is the same, thus resulting in a reduction of 1,118,093 duplicated reports (Fig.1).

A flow diagram of the study
Both generic and brand names were used to identify TNF-α inhibitor-associated cardiovascular records: adalimumab (HUMIRA, HYRIMOZ, AMGEVITA, IMRALDI, or HULIO), etanercept (ENBREL or ETICOVO), infliximab (AVSOLA, INFLECTRA, REMICADE, RENFLEXIS, or FLIXABI), certolizumab pegol (CIMZIA), and golimumab (SIMPONI). The role_code for AEs was assigned by reporters, including primary suspected (PS), secondary suspect drug (SS), concomitant (C), and interacting (I). To improve accuracy and obtain a better signal intensity, TNF-α inhibitors with a reported role code as ‘primary suspect’ (PS) were evaluated in the primary analysis.
In the FAERS database, AEs are classified and represented by preferred terms (PTs), which are distinct descriptors of a single medical concept, such as signs, symptoms, and disease diagnoses. Cardiovascular AEs from the ‘REAC’ dataset were identified by the Medical Dictionary for Regulatory Activities 25.0 (MedDRA 25.0). PTs included ‘acute coronary syndrome’, ‘acute myocardial infarction’, ‘angina pectoris’, ‘angina unstable’, ‘atrial fibrillation’, ‘cardiac failure’, ‘cardiac failure acute’, ‘cardiac failure chronic’, ‘cardiac failure congestive’, ‘cardiomyopathy’, ‘coronary artery disease’, ‘coronary artery occlusion’, ‘myocardial infarction’, ‘cerebral thrombosis’, ‘deep vein thrombosis’, ‘pulmonary embolism’, ‘pulmonary thrombosis’, ‘thrombosis’, ‘venous thrombosis’, and ‘portal vein thrombosis’. Furthermore, PTs can be grouped into broader categories known as Standardized MedDRA Queries (SMQs), which provide a concise classification for defining medical conditions or areas of interest20). The details of SMQs related to cardiovascular events in our study were ‘Cardiac failure’, ‘Cardiomyopathy’, ‘Myocardial infarction’, ‘Embolic and thrombotic events, arterial’ and ‘Embolic and thrombotic events, venous’.
After the above steps of deduplication and screening, 9,817 unique cardiovascular AE reports from patients who received TNF-α inhibitors in the FAERS database were ultimately obtained for further analysis.
2.4 Data Mining and Statistical AnalysisDemographic data (sex, age, reporting region, outcomes, and indications of TNF-α inhibitor users) were analyzed in cardiovascular AE reports associated with TNF-α inhibitors. Categorical variables are reported as frequencies and percentages, and continuous variables are summarized as medians with interquartile ranges.
To determine the association between a drug and an adverse event (AE), we utilized a 2×2 contingency table to perform a disproportionality analysis. A disproportionality analysis was conducted at the PT or SMQ level, which allowed us to assess whether the proportion of reporting an event of interest for a particular drug (e.g., TNF-α inhibitors) differed from the proportion of the same AEs in the drug control group (i.e., all other drugs in the full database)21). Both Frequentist (the reporting odds ratio [ROR])22) and Bayesian (the information component [IC])23) methods in the disproportionality analysis were used to explore the association between TNF-α inhibitors and cardiovascular AEs. The lower limit of the 95% confidence interval (CI) for ROR (ROR025) >1 and the lower limit of the 95% confidence interval for IC (IC025) >0 served as the signal detection criterion, indicating that this drug-AE of interest is more frequently reported compared to the control. Statistical shrinkage transformation was performed to obtain more robust results. The formulae for the transformed ROR and IC are presented in Supplementary Table S1.
| Algorithms | Equation | Criteria |
|---|---|---|
| ROR | ROR=ad/b/c | ROR025 >1 |
| 95%CI=eln(ROR)±1.96(1/a+1/b+1/c+1/d)^0.5 | ||
| IC | IC=log2a(a+b+c+d)(a+c)(a+b) | IC025 >0 |
| 95%CI=E(IC)±2V(IC)^0.5 |
Abbreviations: a, number of reports containing both the target drug and target adverse event; b, number of reports containing other adverse event of the target drug; c, number of reports containing the target adverse event of other drugs; d, number of reports containing other drugs and other adverse event. 95%CI, 95% confidence interval; N, the number of reports; E(IC), the IC expectations; V(IC), the variance of IC.
To reduce any possible bias and improve the reliability of the signals, we conducted a sensitivity analysis via multivariate logistic regression to evaluate the ROR adjusted for age and sex. Cardiovascular risk varies according to age and sex demographics. A subgroup analysis was applied to limit the analysis to a population with the same indications. This indication-focused strategy is frequently employed in disproportionality analyses to control for the effects of underlying diseases. Particular populations were identified by the corresponding indications to avoid any variations in the impact of various inflammatory conditions on cardiovascular outcomes. Moreover, we restricted the population reporting AEs to a group of healthcare professionals to conduct further sensitivity analysis. Based on the prominent results of various sensitivity analyses, we performed a statistical analysis of co-administration, incorporating concomitant medications that may influence cardiovascular outcomes in multivariate logistic regression. The time to onset was defined as the duration between the administration of TNF-α inhibitors and the occurrence of cardiovascular AEs. The median number of days and corresponding interquartile range encompassing the first and third quartiles are presented in Supplementary Fig.1.

Time to onset of cardiovascular adverse events
All data analyses were independently performed by two authors. Data extraction was conducted using the MySQL 8.0 software program, and statistical analyses were performed using the R version 4.10 software program.
Between July 2017 and September 2022, 7,481,747 reports were included after removing duplicates. The numbers of cardiovascular AE reports for adalimumab, infliximab, golimumab, etanercept, and certolizumab were 6,076 (61.89%), 1,200 (12.22%), 341 (3.47%), 1,735 (17.67%), and 465 (4.74%), respectively (Fig.1).
The characteristics of the AE reports for different TNF-α inhibitors are presented in Table 1. With the exception of infliximab, more female patients (57.70%) reported cardiovascular AEs induced by TNF-α inhibitors. Patients aged 45– 64 years accounted for a greater proportion (44.30%). The median age (interquartile range) of all patients was 63 (54–71) years, and the highest median age was observed in the etanercept group. The United States was the main reporting country for these AEs. RA was the predominant indication for TNF-α inhibitors, comprising the highest proportion (38.87%) of reported cardiovascular AEs associated with TNF-α inhibitors. Furthermore, hospitalization (N=5,043, 53.63%) and death (N=1,021, 10.86%) were common outcomes, followed by disability (N=126, 1.34%) and life threating (N=81, 0.86%). Over 70% of cardiovascular AEs were reported beyond one month following TNF-α inhibitor treatment (Supplementary Fig.1), with a median onset time of 79.5 days (13.5–229).
| Characteristic | Cases, N (%) | |||||
|---|---|---|---|---|---|---|
| All TNF-α inhibitors | Adalimumab | Infliximab | Golimumab | Etanercept | Certolizumab | |
| Total Cases | 9,817 | 6,076 | 1,200 | 341 | 1,735 | 465 |
| Gender | ||||||
| Data available | 9,208 | 5,970 | 771 | 318 | 1,696 | 453 |
| Female | 5,313 (57.70%) | 3,360 (56.28%) | 369 (47.86%) | 190 (59.75%) | 1,082 (63.80%) | 312 (68.87%) |
| Male | 3,895 (42.30%) | 2,610 (43.72%) | 402 (52.14%) | 128 (40.25%) | 614 (36.20%) | 141 (31.13%) |
| Age | ||||||
| Data available | 6,453 | 3,886 | 680 | 257 | 1,345 | 285 |
| <18 | 47 (0.73%) | 22 (0.57%) | 20 (2.94%) | 0 (0.00%) | 3 (0.22%) | 2 (0.70%) |
| 18–44 | 677 (10.49%) | 366 (9.42%) | 172 (25.29%) | 27 (10.51%) | 79 (5.87%) | 33 (11.58%) |
| 45–64 | 2,859 (44.30%) | 1,735 (44.65%) | 284 (41.76%) | 116 (45.14%) | 596 (44.31%) | 128 (44.91%) |
| 65–74 | 1,871 (28.99%) | 1,168 (30.06%) | 145 (21.32%) | 60 (23.35%) | 415 (30.86%) | 83 (29.12%) |
| >74 | 999 (15.48%) | 595 (15.31%) | 59 (8.68%) | 54 (21.01%) | 252 (18.74%) | 39 (13.68%) |
| Median (IQR) | 63 (54~71) | 63 (55~71) | 57 (42~67) | 62 (53~73) | 64 (57~72) | 62 (54~70) |
| Indications | ||||||
| Data available | 9,700 | 6,029 | 1,176 | 338 | 1,693 | 464 |
| Rheumatoid arthritis | 3,770 (38.87%) | 2,203 (36.54%) | 152 (12.93%) | 168 (49.70%) | 970 (57.29%) | 277 (59.70%) |
| Psoriasis | 997 (10.28%) | 857 (14.21%) | 24 (2.04%) | 0 (0.00%) | 102 (6.02%) | 14 (3.02%) |
| Crohn disease | 1,398 (14.41%) | 970 (16.09%) | 425 (36.14%) | 3 (0.89%) | 0 (0.00%) | 0 (0.00%) |
| Colitis ulcerative | 713 (7.35%) | 396 (6.57%) | 273 (23.21%) | 14 (4.14%) | 0 (0.00%) | 30 (6.47%) |
| Ankylosing spondylitis | 524 (5.40%) | 301 (4.99%) | 68 (5.78%) | 45 (13.31%) | 70 (4.13%) | 40 (8.62%) |
| Other indications | 2,298 (23.69%) | 1,302 (21.60%) | 234 (19.90%) | 108 (31.95%) | 551 (32.55%) | 103 (22.20%) |
| Reporting region | ||||||
| Data available | 9,180 | 5,472 | 1,197 | 341 | 1,705 | 465 |
| United States, US | 5,091 (55.46%) | 3,740 (68.35%) | 183 (15.29%) | 78 (22.87%) | 862 (50.56%) | 228 (49.03%) |
| Other countries | 4,089 (44.54%) | 1,732 (31.65%) | 1,014 (84.71%) | 263 (77.13%) | 843 (49.44%) | 237 (50.97%) |
| Outcomes | ||||||
| Data available | 9,404 | 5,816 | 1,195 | 327 | 1,619 | 447 |
| Hospitalized | 5,043 (53.63%) | 3,356 (57.70%) | 593 (49.62%) | 165 (50.46%) | 703 (43.42%) | 226 (50.56%) |
| Disabled | 126 (1.34%) | 80 (1.38%) | 21 (1.76%) | 4 (1.22%) | 16 (0.99%) | 5 (1.12%) |
| Life threating | 81 (0.86%) | 38 (0.65%) | 15 (1.26%) | 3 (0.92%) | 20 (1.24%) | 5 (1.12%) |
| Died | 1021 (10.86%) | 670 (11.52%) | 101 (8.45%) | 36 (11.01%) | 163 (10.07%) | 51 (11.41%) |
| Other outcomes | 3133 (33.32%) | 1,672 (28.75%) | 465 (38.91%) | 119 (36.39%) | 717 (44.29%) | 160 (35.79%) |
As cardiovascular toxicity is relatively rare, we first explored the association between each specific cardiovascular AE at the PT level and TNF-α inhibitors. The association spectrum of the five TNF-α inhibitors is shown in Fig.2, where the lower limit of the 95% confidence interval of IC (IC025) represents the strength of association and IC025 >0 serves as the risk signal detection criterion. In both the complete dataset and the dataset limited to reports from healthcare professionals, apart from adalimumab, the other four TNF inhibitors demonstrated no significant cardiovascular AE risk and even exhibited a certain degree of cardiovascular protection. Among the protective signals, certolizumab showed the lowest IC025 value in acute heart failure (IC025=-6.88). Notably, the spectral distribution of adalimumab was quite different from that of other TNF-α inhibitors. Strong risk signals were detected in the adalimumab group and related to thrombosis (IC025=0.15), pulmonary thrombosis (IC025=1.72), myocardial infarction (IC025=0.37), coronary artery occlusion (IC025=2.99), and cerebral thrombosis (IC025=1.44) in the full dataset.

*represents the analysis restricted to reports from healthcare professionals.
In addition to performing a disproportionate analysis at the PT level, SMQs were selected to group PTs into broader categories and further assess the association between TNF-α inhibitors and cardiovascular events (Fig.3). Among the five classes of SMQs, only adalimumab had positive signals for myocardial infarction (ROR025=1.51, IC025=0.59) and arterial thrombosis (ROR025=1.49, IC025=0.57), which was consistent with the results of the associations between TNF-α inhibitors and each specific cardiovascular AE at the PT level. Most cardiovascular AEs were reported as arterial thrombosis (N=4,108) and myocardial infarction (N =2,508) with the use of adalimumab. Compared with adalimumab, the other four TNF-α inhibitors consistently showed negative signals in all SMQs, thus suggesting no significant cardiovascular risk during anti-TNF therapy.

A disproportionality analysis through information component (a) and reporting odds ratios (b) at the SMQ level
After adjusting for age and sex, or limiting the patients to the same indications, the association between cardiovascular AEs and adalimumab exposure still existed in different models (Table 2). In the stratification analysis, adalimumab was associated with a higher risk of cardiovascular events in psoriasis patients (adjusted ROR=2.16, 95%CI=1.95-2.39; IC=0.59, 95%CI= 0.52-0.65). Similar to the results of the main analysis, no significant positive association was found for the other four TNF-α inhibitors in any rheumatic or inflammatory diseases. A subgroup analysis based on sex for different autoimmune diseases in patients treated with adalimumab. As illustrated in Supplementary Fig.2, no significant statistical differences were observed among the various sex subgroups. Additionally, we conducted a statistical analysis to control for the influence of concomitant medications on cardiovascular AEs (Supplementary Table S2). By performing a multivariate regression analysis and adjusting for combined cardiovascular medications (aspirin, atorvastatin, metoprolol, and lisinopril), we found that adalimumab remained significantly associated with cardiovascular AEs across various inflammatory diseases (P<0.01) (Supplementary Table S3).
| TNF-α inhibitor | Cases | ROR (95%CI) | IC (95%CI) | |
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| adalimumab | RA: 2840 | 1.47 (1.41, 1.53)* | 1.63 (1.53, 1.74)* | 0.55 (0.49, 0.60)* |
| PS: 1871 | 1.51 (1.41, 1.61)* | 2.16 (1.95, 2.39)* | 0.59 (0.52, 0.65)* | |
| CD: 1210 | 1.39 (1.28, 1.52)* | 1.63 (1.44, 1.85)* | 0.48 (0.38, 0.55)* | |
| CU: 583 | 1.49 (1.34, 1.65)* | 1.29 (1.11, 1.50)* | 0.57 (0.44, 0.67)* | |
| AS: 415 | 1.42 (1.24, 1.63)* | 1.11 (0.88, 1.39) | 0.50 (0.34, 0.62)* | |
| certolizumab | RA: 314 | 0.67 (0.59, 0.74) | 0.63 (0.54, 0.73) | -0.59 (-0.77, -0.45) |
| PS: 56 | 0.27 (0.21, 0.36) | 0.62 (0.34, 1.13) | -1.87 (-2.31, -1.55) | |
| CD: 42 | 0.55 (0.40, 0.74) | 0.46 (0.31, 0.70) | -0.87 (-1.38, -0.50) | |
| CU: 2 | 0.31 (0.08, 1.24) | ND | -1.70 (-4.29, -0.30) | |
| AS: 49 | 0.66 (0.49, 0.88) | 0.67 (0.44, 1.00) | -0.60 (-1.08, -0.26) | |
| etanercept | RA: 1130 | 0.73 (0.69, 0.78) | 0.53 (0.49, 0.57) | -0.45 (-0.55, -0.38) |
| PS: 311 | 0.65 (0.58, 0.74) | 0.78 (0.63, 0.97) | -0.61 (-0.80, -0.47) | |
| CD: 2 | 0.44 (0.11, 1.78) | ND | -1.17 (-3.77, 0.22) | |
| CU:1 | 0.74 (0.10, 5.28) | ND | -0.43 (-4.22, 1.25) | |
| AS: 91 | 0.87 (0.70, 1.08) | 0.42 (0.31, 0.58) | -0.20 (-0.55, 0.05) | |
| golimumab | RA: 191 | 0.90 (0.78, 1.04) | 0.71 (0.60, 0.85) | -0.15 (-0.39, 0.02) |
| PS: 48 | 0.80 (0.60, 1.07) | 0.61 (0.46, 0.79) | -0.32 (-0.80, 0.02) | |
| CD: 4 | 0.55 (0.21, 1.46) | 0.51 (0.13, 2.07) | -0.87 (-2.63, 0.21) | |
| CU: 17 | 0.46 (0.28, 0.74) | 0.25 (0.13, 0.49) | -1.13 (-1.95, -0.57) | |
| AS: 49 | 1.05 (0.79, 1.41) | 0.62 (0.43, 0.90) | 0.08 (-0.40, 0.42) | |
| infliximab | RA: 192 | 0.53 (0.46, 0.61) | 0.97 (0.78, 1.20) | -0.92 (-1.16, -0.74) |
| PS: 92 | 0.46 (0.38, 0.57) | 1.01 (0.58, 1,77) | -1.11 (-1.46, -0.86) | |
| CD: 512 | 0.64 (0.58, 0.71) | 1.02 (0.87, 1.19) | -0.64 (-0.79, -0.53) | |
| CU: 351 | 0.56 (0.50, 0.63) | 0.85 (0.71, 1.02) | -0.83 (-1.01, -0.70) | |
| AS: 91 | 0.65 (0.52, 0.81) | 0.82 (0.56, 1.18) | -0.62 (-0.97, -0.37) | |
ND: not determined because there were fewer than three reports of cardiovascular adverse events
The asterisk indicates statistical significance.
Model 1: crude ROR.
Model 2: adjusted for age and sex via a multivariable logistic regression.
Model 3: Information component.
RA, rheumatoid arthritis; PS, psoriasis; CD, Crohn’s disease; CU, ulcerative colitis; AS, ankylosing spondylitis.

Correlation between adalimumab and cardiovascular adverse events in different patient sex groups with various autoimmune diseases
| Concomitant drugs | N (%) | Indications (DrugBank) |
|---|---|---|
| Methotrexate | 761 (12.52%) | Treat psoriasis, cancer, rheumatoid arthritis |
| Aspirin | 589 (9.69%) | Treat pain, fever, inflammation, migraines and reduce the risk of major adverse cardiovascular events |
| Atorvastatin | 552 (9.08%) | Lower lipid levels and reduce the risk of cardiovascular disease |
| Folic acid | 475 (7.82%) | Treat megaloblastic anemia and is found in many supplements |
| Metoprolol | 475 (7.82%) | Treat hypertension and angina |
| Vitamin D | 470 (7.74%) | Vitamins |
| Prednisone | 452 (7.44%) | Treat inflammation or immune-mediated reactions and to treat endocrine or neoplastic diseases |
| Omeprazole | 366 (6.92%) | Treat gastroesophageal reflux disease |
| Lisinopril | 310 (5.10%) | Treat hypertension, heart failure, and acute myocardial infarction. |
| Metformin | 267 (4.39%) | Treat type 2 diabetes mellitus |
| Drug | Indications | Total cases | Adjusted OR (95%CI) | P value |
|---|---|---|---|---|
| Adalimumab | RA | 196,204 | 1.80 (1.69, 1.91) | <0.001 |
| PS | 81,915 | 2.04 (1.84, 2.25) | <0.001 | |
| CU | 33,074 | 1.34 (1.16, 1.55) | <0.001 | |
| CD | 57,250 | 1.57 (1.40, 1.75) | <0.001 | |
| AS | 18,529 | 1.49 (1.24, 1.80) | <0.001 |
Adjusted for age, sex and concomitant drugs via a multivariable logistic regression.
Abbreviations: RA, rheumatoid arthritis; PS, psoriasis; CD, Crohn disease; CU, colitis ulcerative; AS, ankylosing spondylitis.
TNF-α inhibitors are widely used to treat IMIDs. There is concern regarding TNF-α inhibitor-associated cardiovascular AEs, as the outcomes of such events are generally severe. Previous studies were limited by the number of subjects or cases of cardiovascular AEs, which could not guarantee assessment accuracy. To our knowledge, this is the first comprehensive pharmacovigilance study to evaluate the association between TNF-α inhibitors and cardiovascular AEs. In general, our study found that, with the exception of adalimumab, TNF-α inhibitors might not increase the risk of cardiovascular AEs during the treatment of IMIDs. We identified differential safety profiles among various TNF-α inhibitors and highlighted the need for awareness of the risk of certain drug-specific cardiac events, especially thrombotic cardiovascular events, related to adalimumab.
According to the descriptive analysis of TNF-α inhibitor-related cardiovascular reports, females accounted for a larger proportion. However, in the subgroup analysis, we found no statistically significant differences among the various sex subgroups. This inconsistency could be attributed to the excessive proportion of females in the background frequency of the FAERS database20). Elderly patients (>65 years) accounted for nearly half of the cardiovascular reports, potentially because aging itself is a risk factor for the development of cardiovascular disease24).
Owing to the significant role of chronic inflammation in cardiovascular disease development25, 26) and the association of serum TNF levels with cardiovascular outcomes27), concerns have arisen regarding the potential cardiovascular risks associated with TNF-α inhibitors. It is intuitive to assume that decreasing the inflammatory burden with TNF-α inhibitors can reduce cardiovascular risk. In a national cohort study28) conducted by the ARTIS Study Group, anti-TNF-α therapy was associated with a 50% lower risk of short-term acute coronary syndrome in RA patients who responded well to clinical drug treatment. Likewise, a meta-analysis29) of 28 studies (a total of 236,525 patients with RA) showed that TNF-α inhibitors could reduce cardiovascular risk. Anti-TNF-α therapy has been recommended by European experts for the treatment of severe psoriasis patients at a high risk of cardiovascular complications30). Our results align with these studies, thus suggesting a reduced risk of cardiovascular events associated with anti-TNF-α therapy in most cases. To validate our findings and explore the potential associations regarding the use of TNF-α inhibitors across different indications, we employed three distinct analytical models covering various rheumatic and inflammatory conditions. After adjusting for confounders and restricting the dataset to health professional reports, the results further support our findings.
However, based on the findings of the ATTACH31) and RENEWAL32), current guidelines recommend avoiding the use of TNF-α inhibitors in patients with moderate-to-severe heart failure. The reason for treatment failure in these trials is mostly attributed to the fact that the majority of included patients had ischemic cardiomyopathy at baseline. Therefore, this does not imply that the use of TNF-α inhibitors in patients with IMIDs carries a risk of heart failure. Studies conducted by the US National Data Bank for Rheumatic Diseases33) and the German biologics register34) have demonstrated the beneficial effects of TNF-α inhibitors on heart failure in patients with RA. TNF-α inhibitors are recommended for patients with severe cardiotoxicity who do not respond to steroid therapy. Our study similarly found that none of the five TNF-α inhibitors increased the risk of heart failure in patients with IMIDs.
In addition, our study revealed a differential association between TNF-α inhibitors and cardiovascular AEs, mainly adalimumab, which exhibited an exclusively positive risk association with thrombotic AEs. After adjusting for confounding factors, the results remained statistically significant. Korswagen et al.35) also observed serious arterial thromboembolic events in patients treated with adalimumab. In fact, the phenomenon of differential safety profiles among similar antibody drugs is common. Recently, a meta-analysis of trials revealed significant differences in safety profiles between golimumab and three other TNF-α inhibitors36). Real-world clinical data from Asian patients with psoriasis showed a differential safety profile between adalimumab and etanercept37). Moreover, infliximab exhibited a higher susceptibility to granulomatous infections than other TNF-α inhibitors38).
While all five TNF-α inhibitors mentioned above consistently achieve their clinical therapeutic effects by blocking the binding of soluble TNF to its receptors, the significant structural and functional differences among them may have contributed to the observed variations in cardiovascular AEs in our study15, 16). Compared with the other four TNF-α inhibitors, adalimumab is a fully human IgG1 monoclonal antibody that exhibits high affinity for TNF39). It has the unique ability to form distinct higher-order complexes, including 1:1, 1:2, 2:2, and 3:2 complexes between the IgG molecule and the TNF trimer40). The observed risk association between adalimumab and cardiovascular AEs may be attributed to its unique complex formation in vivo. Furthermore, recent studies have highlighted the additional and diverse biological effects of TNF-α inhibitors on transmembrane TNF and Fc receptors41-43). Compared to soluble TNF, transmembrane TNF predominantly binds to TNF receptor 2 (TNFR2), which exhibits opposing effects to TNFR1 in some respects44). TNFR2 has been found to have a cardioprotective effect, and its greater inhibition could lead to adverse cardiovascular outcomes45). Based on the strongest binding affinity of adalimumab to transmembrane TNF in peripheral blood mononuclear cells41), we hypothesized that the observed signals of cardiovascular AEs may also be associated with the inhibition of TNFR2. Moreover, the varying actions of TNF-α inhibitors on Fc receptor-expressing cells may also result in differences in efficacy or safety42). Ho et al. observed distinct drug-specific effects of TNF-α inhibitors on the transcriptional profile of Th cells46). Their findings also emphasized the unique effects of adalimumab, which occur through the Fc-facilitated physical interaction between Th cells and CD14+ monocytes. Wojtal KA and colleagues also found that adalimumab activated Fc receptors on peripheral blood mononuclear cells and thus led to the expression of pro-inflammatory cytokines, whereas infliximab or certolizumab did not have this effect47). Therefore, anti-TNF-α antibodies not only neutralize the target protein but also mediate other signaling pathways through unexpected receptors, such as TNFR2 and/or Fc receptors, which might contribute to the discordance in the side effects of TNF-α inhibitors.
Furthermore, since platelets are pivotal in the formation of arterial thrombosis, exploring the effects of TNF-α inhibitors on platelets may help understand their thromboembolic cardiovascular risk. In an in vitro experiment48), it was discovered that adalimumab can directly increase TRAP-induced platelet aggregation by ≥ 20%, potentially increasing the risk of thrombosis. In contrast to adalimumab, certolizumab and etanercept have been found to downregulate the expression of VCAM-1 and ICAM-1, which play crucial roles in platelet aggregation and thrombosis formation49, 50). Furthermore, in a recent in vivo study51), adalimumab exhibited contrasting effects on platelets compared to golimumab or etanercept, which can significantly increase platelet counts. These findings align with our results for adalimumab and partly explain the different characteristics of the spectrum of cardiovascular AEs exhibited by adalimumab. In addition to the possible mechanisms outlined above, TNF-α inhibitors have been linked to dyslipidemia52, 53). A 48-week comparative study revealed that patients treated with adalimumab exhibited higher levels of weight gain and adipokines than those receiving infliximab or etanercept54), which suggests that the differential impact of TNF-α inhibitors on lipid metabolism may also influence the occurrence of cardiovascular AEs.
Thus, based on the current literature and results of our study, TNF-α inhibitors appear to have paradoxical effects in different situations. Regarding their protective effects, owing to the pro-atherogenic actions of TNF-α, these inhibitors not only reduce systemic inflammation but also help improve the endothelial function and provide atherosclerotic protection55-57). However, in terms of potential risks, the signaling pathways mediated by TNFR2 or Fc receptors, as well as the direct effects on platelets, can all influence the occurrence of thrombotic events. Hence, a competing balancing mechanism may exist in the association between anti-TNF therapy and cardiovascular events (Fig.4). The eventual cardiovascular events may be a complex process that requires further study to confirm the specific association. However, it is important to note the compound-specific effects of individual TNF-α inhibitors, particularly adalimumab, on thrombotic cardiovascular outcomes. With the emergence of high-quality head-to-head clinical comparative studies, we will develop a more comprehensive understanding of the potential variances in efficacy or safety among similar biological agents. We believe that personalized therapy planning is necessary for patients with inflammatory diseases who receive anti-TNF-α therapy.
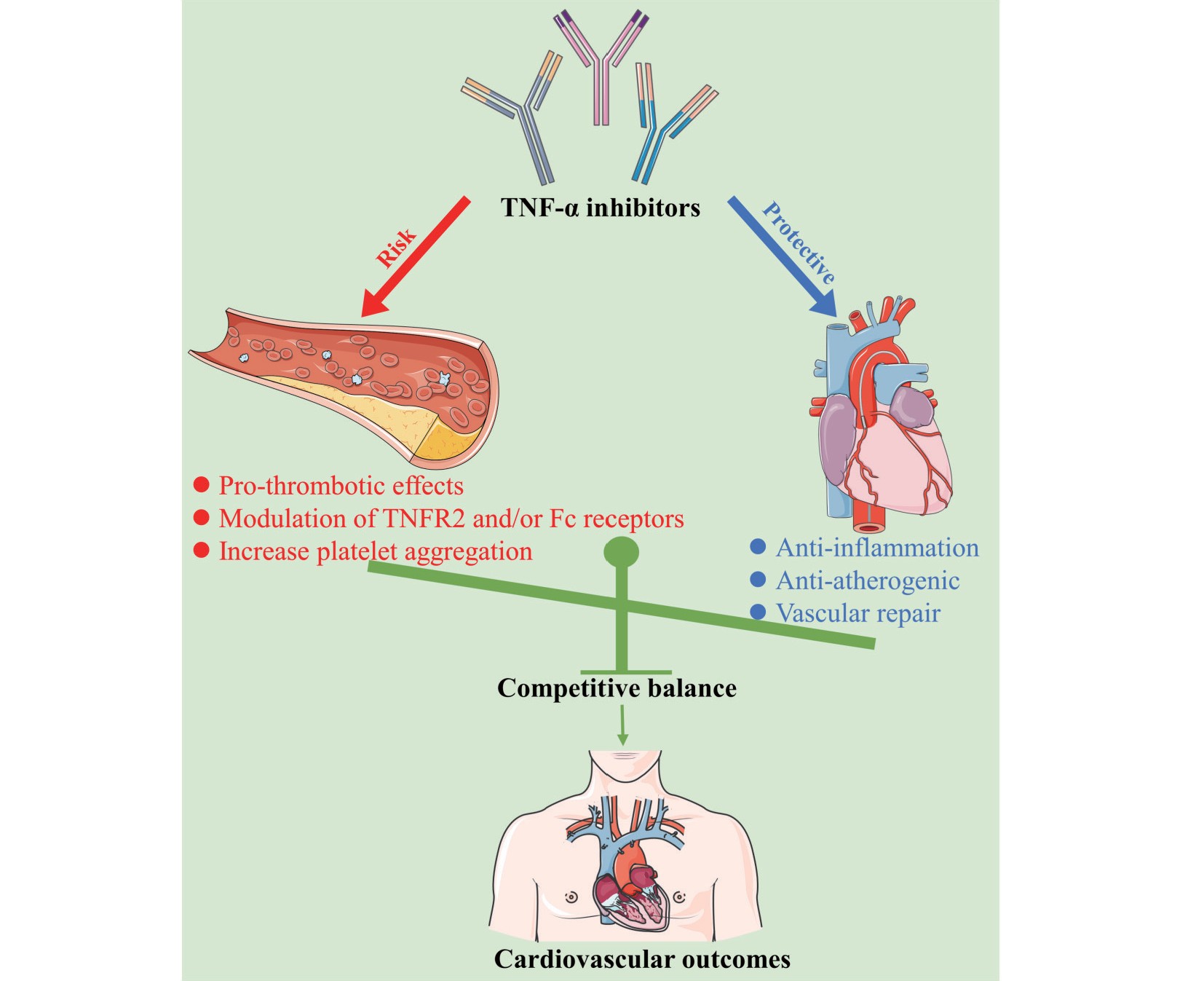
Concept of a competing balancing mechanism for the associations of TNF-α inhibitors with the cardiovascular outcomes
Our study relies on real-world pharmacovigilance data and it could therefore successfully complement the findings of previous studies: access to a population usually neglected in pre-marketing studies and obtaining large numbers of population reports from around the world, which is an obvious strength compared with previous studies. Nevertheless, this retrospective pharmacovigilance study is nevertheless associated with some limitations. First, it is difficult to make a causal inference, which is a common drawback in all pharmacovigilance studies. Second, the incidence of cardiovascular events could not be calculated because of the small number of patients receiving drug therapy. Third, the absence of detailed clinical information and specific diagnostic criteria may have introduced some potential bias in our analysis. Fourth, we did not include a comparison of different drug dosages; thus, it is uncertain whether the effects vary with the dose.
Based on real-world pharmacovigilance data, the current study systematically evaluated the association between TNF-α inhibitors and cardiovascular AEs, making significant contributions to clinical practice and drug safety research. Although limited by the inherent flaws in pharmacovigilance data, this analysis identified that TNF-α inhibitors, except for adalimumab, were not significantly associated with cardiovascular AEs. When adalimumab is used in IMIDs, the potential thrombotic risk should thus be considered. Further studies are needed to address the gaps in our study and to develop a more comprehensive understanding of the potential variances in the safety profiles of TNF-α inhibitors.
The authors thank FAERS for providing the data for this study.
The study was conceived and designed by JM, CH, and GY conceived and designed the study. The data were collected and analyzed by JM and JC. JM and ZF prepared the original draft of the manuscript. All authors have contributed to the manuscript and approved the submitted version.
This work was supported by the Hunan Provincial Natural Science Foundation of China (No. 2020JJ5852, No. 2023JJ60513) and the Key Research and Development Project of Hunan Province (No. 2020SK2010).
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript, apart from those disclosed.
FAERS database is publicly available without application and is derived from the following resources: https://fis.fda.gov/extensions/fpd-qde-faers/fpd-qde-faers.html.
Ethical approval was not required for this study. All the data used were publicly available in the FAERS database, which contains anonymized pharmacovigilance data.
Not applicable.
Not applicable.
Not applicable.