2024 Volume 31 Issue 5 Pages 550-558
2024 Volume 31 Issue 5 Pages 550-558
Aim: In 2022, the Japan Atherosclerosis Society (JAS) has revised its clinical diagnostic criteria of familial hypercholesterolemia (FH) and adopted the use of definite, probable, possible, and unlikely FH according to the Dutch Lipid Clinic Network (DLCN) FH criteria. However, these strata have not been validated and their impact on coronary artery disease (CAD) is yet to be elucidated.
Methods: In this study, we retrospectively examined the patients with FH aged ≥ 15 years (N=857, male=431) who were admitted to Kanazawa University Hospital between 2010 and 2022. We assessed the prevalence of patients with a pathogenic variant as FH and odds ratio (OR) of CAD among each group determined by the JAS criteria 2022 for adults.
Results: In total, 414, 128, 142, and 173 patients were found to have definite, probable, possible, and unlikely FH, respectively, in this population. The prevalences of patients with a pathogenic variant as FH were 77.1%, 28.7%, 13.0%, and 1.2 %, respectively, among the definite, probable, possible, and unlikely FH patients (P-trend <0.001). Compared with the reference group of unlikely FH, patients with definite, probable, and possible FH were noted to have significantly higher adjusted odds of developing CAD (OR, 9.1; 95% confidence interval [CI], 3.2–12.6; P<0.001 and OR, 4.2; 95% CI, 1.7–6.4; P<0.001, and OR, 2.8; 95% CI, 1.2–4.4; P=0.002, respectively).
Conclusion: The new JAS diagnostic criteria for FH have been noted to work well in terms of diagnosing definitive, probable, or possible FH patients. Thus, it is seen to be of great help in terms of risk discrimination.
Familial hypercholesterolemia (FH) is among the most common inherited disorders caused by genetic mutation(s) in low-density lipoprotein (LDL) receptor (LDLR) and its associated genes, including apolipoprotein B (APOB), proprotein convertase subtilisin/kexin type 9 (PCSK9), and LDLR adaptor protein 1 (LDLRAP1)1-4). Worldwide, there exist several diagnostic criteria of FH, including the Dutch Lipid Clinic Network (DLCN), Simon Broome, and Japan Atherosclerosis Society (JAS)5-7). In 2022, the JAS has revised its clinical diagnostic criteria of FH to facilitate more proactive diagnosis of this disease. One of the major changes include the threshold of Achilles tendon thickening based on accumulated clinical and genetic data to increase the sensitivity of clinical diagnosis. The new criteria also adopted the strata of definite, probable, possible, and unlikely FH according to the DLCN FH criteria. In fact, the previous version of the JAS criteria 2017 used a “dichotomous” system where patients who met the clinical criteria were diagnosed as FH, whereas those who did not were deferred for FH diagnosis. Thus, the introduction of these strata should prevent physicians from deferring the FH diagnosis where the elements needed to their diagnosis are unclear. However, it is quite important to validate these changes of the new clinical criteria, especially the diagnostic specificity of each stratum. Moreover, the impact of this stratum on coronary artery disease (CAD) should also be determined. Accordingly, we aimed to assess the prevalence of patients with a pathogenic variant as FH among each stratum of diagnosis and the odds ratio (OR) of CAD in comparison with that of patients classified as having unlikely FH.
In this study, we analyzed the data collected from 872 patients (outpatients as well as hospitalized patients) who underwent the FH clinical assessments and collected DNA samples at Kanazawa University Hospital between 2010 and 2022. Some of the patients had already been genetically diagnosed using Sanger sequencing for coding regions of LDLR, PCSK9, and APOB (only the hot spot) until 2015 in our institute. Some patients were excluded due to missing data (n=15). In total, 857 patients were included in this present study.
Diagnosis of FHWe used the clinical criteria of FH 2022 by JAS for FH diagnosis (Fig.1). The three major elements of the clinical criteria are as follows: (1) hyper-LDL cholesterol (LDL cholesterol ≥ 180 mg/dL); (2) tendon or cutaneous xanthomas; and (3) family history of FH or premature CAD (first-degree relatives). When a patient meets two or more criteria, then he/she is diagnosed as having definite FH. Even when he/she does not meet two or more criteria, the patient may be diagnosed either as having probable, possible, or unlikely FH under the circumstances illustrated in Fig.1.

Red indicates definite FH. Orange indicates probable FH. Yellow indicates possible FH. Green indicates unlikely FH.
We defined the baseline points for the initial assessments that were performed for various clinical conditions and parameters before initiating lipid-lowering therapies. Hypertension was defined as a condition wherein systolic blood pressure of ≥ 140 mmHg and/or diastolic blood pressure of ≥ 90 mmHg were observed, or situations where any antihypertensive medications were used. We defined diabetes according to the Japan Diabetes Society guidelines8). We defined smoking status of patients as current smoking at their clinical assessments. We defined CAD as having a history of myocardial infarction, unstable angina, coronary artery revascularization, or any severe stenotic lesions (≥ 75%) in coronary arteries by coronary angiogram and/or coronary computed tomography. The total serum cholesterol, triglyceride, and high-density lipoprotein (HDL) cholesterol levels were determined enzymatically using automated instrumentation. LDL cholesterol levels were calculated using the Friedewald formula when the triglyceride level was <400 mg/dL; otherwise, it was determined enzymatically under the standard procedure.
Genetic AnalysisThe coding regions of the so-called FH genes, including LDLR, APOB, PCSK9, and LDLRAP1, were sequenced for all of the patients in this study using a next-generation sequencer as described previously9). We assessed their copy number variations (CNVs) at the LDLR using the eXome Hidden Markov Model10). The pathogenicity of the genetic variations was determined using the standard American College of Medical Genetics and Genomics criteria (“pathogenic” or “likely pathogenic” was considered as pathogenic)11).
Ethical ConsiderationsThis present study was approved by the Ethics Committee of Kanazawa University (2019-114). All procedures were done in accordance to the ethical standards of the Human Research Committee (institutional and national), the guidelines stipulated in the 1975 Declaration of Helsinki (revised in 2008), and all the other laws as well as guidelines in Japan. All participants provided informed consent for genetic analysis.
Statistical AnalysisContinuous variables with normal distribution were presented as mean±standard deviation, while continuous variables without normal distribution were expressed as medians and interquartile ranges. All comparisons between categorical variables were performed using the Fisher’s exact or chi-square test. Data were presented as numbers or percentages. For independent variables, the Student’s t-test was used to compare the means of continuous variables, whereas the nonparametric Wilcoxon Mann–Whitney U test was used to compare the median values. The Jonckheere–Terpstra trend test was used to determine trends of continuous variables, while the Cochran–Armitage trend test was used to determine trends of proportions. A multivariable logistic regression model was used to identify the factors associated with CAD. ORs were also calculated using the unlikely FH group as the reference; meanwhile, P-values of <0.05 were used to denote statistical significance.
Table 1 shows the participants’ clinical characteristics. The patients’ mean age was 49 years and 50% of the participants were men. At baseline, the median LDL cholesterol level was 182 mg/dL. Overall, 320 (37.3%) patients were determined to have a pathogenic variant of FH (Supplemental Table 1). Significant trends were noted in the variables, such as age, hypertension, diabetes, smoking, total cholesterol level, triglycerides, HDL and LDL cholesterol levels, and CAD, except for sex. Under the circumstances, the prevalence rates of patients with a pathogenic variant as FH were 77.1%, 28.7%, 13.0%, and 1.2% among the definite, probable, possible, and unlikely FH patients, respectively (Fig.2, P-trend<0.001). We identified four patients with a CNV in LDLR, all of whom were classified as definite FH. In addition, we found that a proportion of patients with a particular variant in PCSK9 gene (p.Glu32Lys) was quite different according to the groups (Supplemental Fig.1).
| Variables |
All (N= 857) |
Definite FH (N= 314) |
Probable FH (N= 178) |
Possible FH (N= 192) |
Unlikely FH (N= 173) |
P-value for trend |
|---|---|---|---|---|---|---|
| Age (years) | 49±17 | 46±16 | 48±19 | 51±15 | 53±17 | <0.001 |
| Male (%) | 431 (50.3 %) | 154 (49.0 %) | 90 (50.6 %) | 86 (44.8 %) | 101 (58.4 %) | 0.78 |
| Hypertension (%) | 211 (24.6 %) | 66 (21.0 %) | 34 (19.1 %) | 55 (28.6 %) | 56 (32.4 %) | 0.026 |
| Diabetes (%) | 69 (8.1 %) | 16 (5.1 %) | 12 (6.7 %) | 19 (9.9 %) | 22 (12.7 %) | <0.001 |
| Smoking (%) | 201 (23.5 %) | 63 (20.1 %) | 40 (22.5 %) | 48 (25.0 %) | 50 (28.9 %) | <0.001 |
| Total cholesterol (mg/dL) | 269 [221–325] | 328 [281–390] | 273 [240–315] | 242 [201–291] | 232 [192–285] | <0.001 |
| Triglyceride (mg/dL) | 115 [78– 177] | 103 [67–163] | 121 [88– 172] | 116 [78– 156] | 146 [112 –204] | <0.001 |
| HDL cholesterol (mg/dL) | 51 [42–62] | 48 [40– 59] | 49 [41– 63] | 52 [42– 64] | 53 [42–68] | <0.001 |
| LDL cholesterol (mg/dL) | 182 [135–240] | 253 [203– 311] | 182 [154– 234] | 157 [115– 195] | 147 [108–180] | <0.001 |
| CAD (%) | 228 (26.6 %) | 119 (37.9 %) | 52 (29.2 %) | 34 (17.8 %) | 23 (13.3 %) | <0.001 |
FH, familial hypercholesterolemia; CAD, coronary artery disease
| Gene | Nucleotide Change | Mutation Type | Effect on Protein | Number of Patients | ACMG | |
|---|---|---|---|---|---|---|
| LDLR | c.68-1G>C | Splice-cite | NA | 2 | PVS1/PM2/PM4/PP1/PP5 | Pathogenic |
| LDLR | c.191-2A>G | Splice-cite | NA | 2 | PVS1/PM2/PM4/PP1 | Pathogenic |
| LDLR | c.283T>G | Missense | p.Cys95Gly | 4 | PM1/PM2/PP3/PP4 | Likely pathogenic |
| LDLR | c.284G>T | Missense | p.Cys95Phe | 3 | PM2/PM5/PP1/PP5 | Likely pathogenic |
| LDLR | c.313+1G>A | Splice-cite | NA | 2 | PVS1/PM2/PM4 | Pathogenic |
| LDLR | c.344G>A | Missense | p.Arg115His | 3 | PS1/PM2/PP3 | Likely pathogenic |
| LDLR | c.378del | Frameshift | p.Phe126LeufsTer80 | 2 | PVS1/PM2/PM4/PP1 | Pathogenic |
| LDLR | c.389dupC | Frameshift | p.Asp131ArgfsTer49 | 1 | PVS1/PM1/PM2/PM4/PP5 | Pathogenic |
| LDLR | c.413C>G | Nonsense | p.Ser138Ter | 3 | PVS1/PM2/PM4/PP1/PP5 | Pathogenic |
| LDLR | c.418G>A | Missense | p.Glu140Lys | 2 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.478T>C | Missense | p.Cys160Arg | 2 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.489G>T | Missense | p.Gln163His | 2 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.530C>T | Missense | p.Ser177Leu | 2 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.532G>T | Missense | p.Asp178Tyr | 1 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.611G>C | Missense | p.Cys204Ser | 3 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.642G>C | Missense | p.Trp214Cys | 2 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.682G>A | Missense | p.Glu228Gln | 5 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.686_689del | Frameshift | p.Glu229AlafsTer35 | 3 | PVS1/PM2/PM4/PP1 | Pathogenic |
| LDLR | c.694+1G>A | Splice-cite | NA | 2 | PVS1/PM2/PM4/PP1 | Pathogenic |
| LDLR | c.718G>A | Missense | p.Glu240Lys | 3 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.718G>T | Nonsense | p.Glu240Ter | 2 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.726G>C | Missense | p.Gln242His | 2 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.796G>A | Missense | p.Asp266Asn | 4 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.797A>G | Missense | p.Asp266Gly | 4 | PM1/PM2/PM5/PP1/PP3 | Likely Pathogenic |
| LDLR | c.829G>T | Nonsense | p.Glu277Ter | 2 | PVS1/PM2/PM4/PP1 | Pathogenic |
| LDLR | c.874delC | Frameshift | p.Leu292TrpfsTer78 | 1 | PVS1/PM2/PM4/PP1 | Pathogenic |
| LDLR | c.939C>A | Missense | p.Cys313Ter | 2 | PVS1/PM2/PM4/PP5 | Pathogenic |
| LDLR | c.940+2T>C | Splice-cite | NA | 1 | PVS1/PM2/PM4/PP1 | Pathogenic |
| LDLR | c.967G>A | Missense | p.Gly323Ser | 2 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.1012T>A | Missense | p.Cys338Ser | 1 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.1056C>A | Nonsense | p.Cys352Ter | 4 | PVS1/PM2/PM4/PP5 | Pathogenic |
| LDLR | c.1062dupT | Frameshift | p.Ile355TyrfsTer3 | 2 | PVS1/PM2/PM4/PP5 | Pathogenic |
| LDLR | c.1067A>T | Missense | p.Asp356Val | 2 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.1069G>T | Nonsense | p.Glu357Ter | 1 | PVS1/PM2/PM4 | Pathogenic |
| LDLR | c.1114_1115insC | Frameshift | p.Glu372AlafsTer9 | 2 | PVS1/PM2/PM4/PP5 | Pathogenic |
| LDLR | c.1123_1124insGA | Nonsense | p.Tyr375Ter | 2 | PVS1/PM2/PM4/PP5 | Pathogenic |
| LDLR | c.1183_1184insC | Frameshift | p.Val395AlafsTer46 | 2 | PVS1/PM2/PM4/PP5 | Pathogenic |
| LDLR | c.1187-2A>G | Splice-cite | NA | 1 | PVS1/PM2/PM4/PP5 | Pathogenic |
| LDLR | c.1245_1249dupCCGGA | Frameshift | p.Ser417ThrfsTer12 | 2 | PVS1/PM2/PM4/PP4 | Pathogenic |
| LDLR | c.1252G>A | Missense | p.Glu418Lys | 3 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.1285G>A | Missense | p.Val429Leu | 4 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.1297G>C | Missense | p.Asp433His | 3 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.1328G>C | Missense | p.Trp443Ser | 2 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.1339T>C | Missense | p.Ser447Pro | 2 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.1340C>G | Missense | p.Ser447Cys | 3 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.1432G>A | Missense | p.Gly478Arg | 4 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.1466A>G | Missense | p.Tyr489Cys | 4 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.1469G>A | Nonsense | p.Trp490Ter | 1 | PVS1/PM2/PM4/PP4 | Pathogenic |
| LDLR | c.1502C>T | Missense | p.Ala501Val | 3 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.1702C>G | Missense | p.Leu568Val | 2 | PM1/PM2/PP3/PP5 | Likely Pathogenic |
| LDLR | c.1705+1G>C | Splice-cite | NA | 2 | PVS1/PM2/PM4/PP4 | Pathogenic |
| LDLR | c.1706A>G | Missense | p.Asp569Gly | 1 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.1783C>T | Missense | p.Arg595Trp | 4 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.1845+2T>C | Splice-cite | NA | 14 | PVS1/PM2/PM4/PP4 | Pathogenic |
| LDLR | c.1859G>C | Missense | p.Trp620Ser | 4 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.1897C>T | Missense | p.Arg633Cys | 2 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.1925T>C | Missense | p.Leu642Ser | 2 | PM1/PM2/PP3/PP4/PP5 | Likely Pathogenic |
| LDLR | c.2054C>T | Missense | p.Pro685Leu | 20 | PM1/PM2/PP3/PP4/PP5 | Likely Pathogenic |
| LDLR | c.2096C>T | Missense | p.Pro699Leu | 2 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.2389G>A | Missense | p.Val797Met | 13 | PM1/PM2/PP3/PP4 | Likely Pathogenic |
| LDLR | c.2431A>T | Nonsense | p.Lys811Ter | 112 | PVS1/PM2/PM4/PP4 | Pathogenic |
| LDLR | c.1845-?_2141+?del | Large deletion | Truncated protein | 2 | PVS1/PM2/PM4/PP4 | Pathogenic |
| LDLR | c.2141-?_2311+?del | Large deletion | Truncated protein | 2 | PVS1/PM2/PM4/PP4 | Pathogenic |
| PCSK9 | c.94G>A | Missense | p.Glu32Lys | 21 | PS1/PS3/PP3/PP4/PP5 | Pathogenic |

Red indicates patients with an FH pathogenic variant. Gray indicates patients without an FH pathogenic variant.
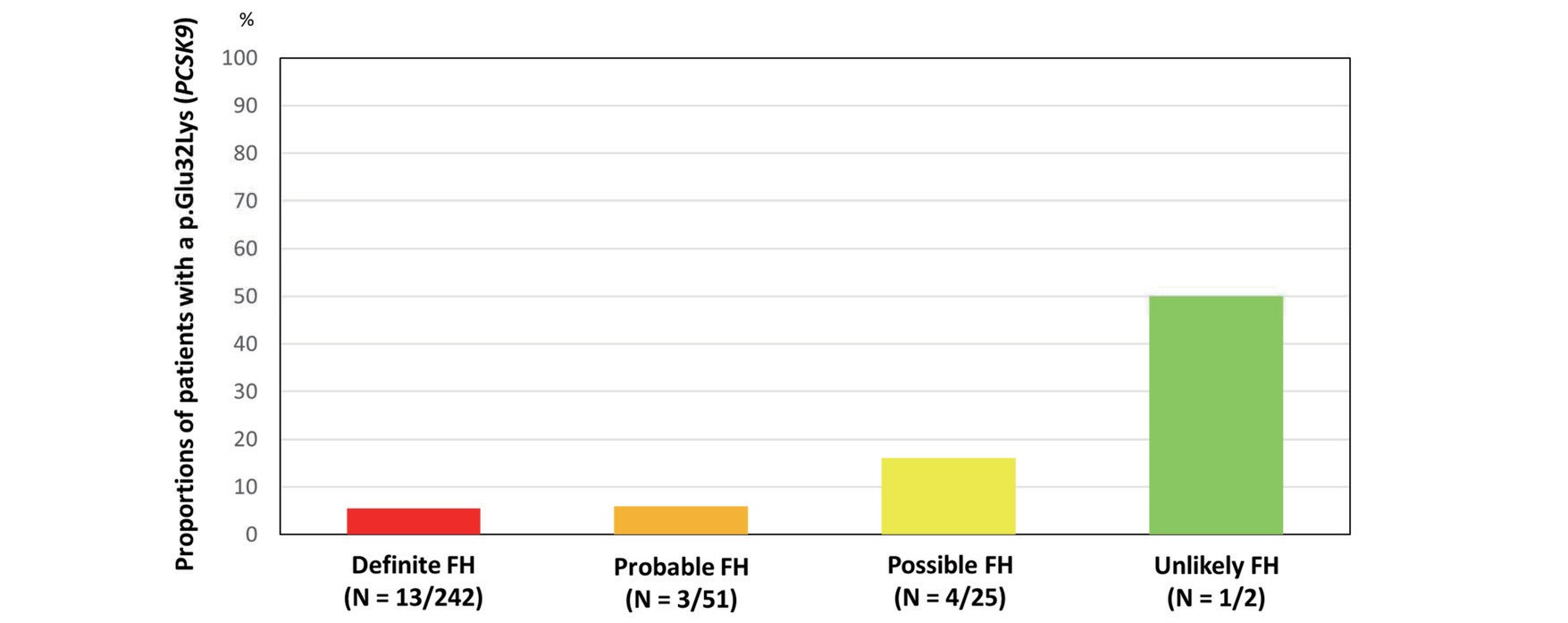
Proportions of patients with a p.Glu32Lys in PCSK9
As per our findings, age (OR=1.06, 95% confidence interval [CI]=1.03–1.09, P<0.001, Table 2), male sex (OR=1.82, 95% CI=1.04–2.60, P<0.001), hypertension (OR=3.32, 95% CI=1.54–5.10, P<0.001), diabetes (OR=2.02, 95% CI=1.10–2.94, P<0.001), smoking (OR=3.98, 95% CI=1.80–6.16, P<0.001), LDL cholesterol (OR=1.01, 95% CI=1.00–1.01, P=0.033, per 10 mg/dL), and pathogenic variants (OR=2.10, 95% CI=1.10–3.10, P<0.001) were determined to be associated with CAD.
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Age (per year) | 1.06 | 1.03–1.09 | <0.001 |
| Male (yes vs. no) | 1.82 | 1.04–2.60 | <0.001 |
| Hypertension (yes vs. no) | 3.32 | 1.54–5.10 | <0.001 |
| Diabetes (yes vs. no) | 2.02 | 1.10–2.94 | <0.001 |
| Smoking (yes vs. no) | 3.98 | 1.80–6.16 | <0.001 |
| LDL cholesterol (per 10 mg/dl) | 1.01 | 1.00–1.01 | 0.033 |
| pathogenic variants (vs. without variants) | 2.10 | 1.10–3.10 | <0.001 |
FH, familial hypercholesterolemia; CAD, coronary artery disease; OR, odds ratio; CI, confidence interval
Compared with the reference group of unlikely FH, patients with definite, probable, and possible FH had significantly higher adjusted odds of developing CAD (Fig.3, OR, 9.1; 95% CI, 3.2–12.6; P<0.001 and OR, 4.2; 95% CI, 1.7–6.4; P<0.001, and OR, 2.8; 95% CI, 1.2–4.4; P=0.002, respectively).

Red indicates definite FH. Orange indicates probable FH. Yellow indicates possible FH. Green indicates unlikely FH.
In this present study, we validated the new clinical criteria of FH 2022 in Japan, especially the new strata of definite, probable, possible, and unlikely FH. We also investigated the impact of these strata on CAD among FH patients. As per our findings, the new clinical criteria of FH 2022 in Japan were noted to work effectively while diagnosing definite FH (where the proportions of a pathogenic variant as FH was quite high, whereas that of unlikely FH was close to 0). Significant trends were also noted in terms of OR of CAD in these strata (definite, probable, possible, and unlikely FH).
The diagnostic criteria of any diseases are commonly updated according to the identification of new findings. In FH, data based on genetic testing have been accumulated for decades, which could be used for more accurate diagnosis12-17); thus, the committee of JAS revised the clinical criteria of FH in 2022 to increase the sensitivity of their diagnosis, while trying to maintain its specificity. Indeed, the previous version of the clinical criteria of FH published in 2017 adopted a “dichotomous” diagnosis, that is, yes or no. However, this has resulted in many patients to be deferred in FH diagnosis when they did not meet the criteria even though they actually have FH18). Several reasons can be attributed to this situation. First, the threshold of the Achilles tendon thickness had been too large. Thus, this parameter has been revised from 9.0 mm in both sexes to 8.0 mm in men and 7.5 mm in women19). Second, genetic testing could not be used for the definitive diagnosis of FH7, 20). For this matter, genetic testing is now covered by the national health insurance in Japan since April 2022, and the new guideline has now accepted the use of genetic testing for FH. Finally, the “dichotomous” diagnostic criteria had hampered the proactive diagnosis of FH. To overcome this problem, the new clinical diagnostic criteria adopted the diagnostic strata (definitive, probable, possible, and unlikely FH), similar to that of the DLCN FH criteria. In this present study, we were able to clearly demonstrate the validity of these strata and confirm their clinical impact on CAD. We believe that these new clinical criteria will help identify more patients with FH without unnecessary deferral of FH diagnosis in Japan.
Another important aspect observed in this study is the difference of proportion of a particular missense variant in PCSK9 (p.Glu32Lys). In fact, the effect on CAD as well as on LDL cholesterol by this particular variant in PCSK9 (p.Glu32Lys) appears to be much smaller than that of a nonsense variant in LDLR (p.Lys811Ter) in a population-based study21). In addition, we have shown that LDL cholesterol level and the phenotype of patients with missense variants, including this particular variant in PCSK9 (p.Glu32Lys), were milder than that of patients with loss-of-function variants in LDLR in a hospital-based study22). Thus, it is reasonable to see that the prevalence of this particular variant in PCSK9 (p.Glu32Lys) associated with milder phenotype is higher in the lower category of FH (possible FH and unlikely FH).
This current study has several limitations. First, this is a single-center study; thus, results may not be applicable to patients referred to other hospitals. Second, some patients were excluded from this study due to missing data, which might have affected our results. Third, we used a short-read next-generation sequencer for genotyping, which may have missed the large CNVs of LDLR. Fourth, the current genetic testing for FH cannot perfectly capture the patients with FH. Thus, we believe that a multicenter study investigating this matter would be needed to fully validate this issue in the future.
The new JAS diagnostic criteria for FH have been noted to work well in terms of diagnosing definitive, probable, or possible FH patients. Additionally, these revised diagnostic criteria are deemed useful for risk discrimination.
We thank Ms. Kazuko Honda and Mr. Sachio Yamamoto for their outstanding technical assistance.
This work was supported by JSPS KAKENHI [grant numbers: 20H03927, 21H03179, and 22H03330]; a grant from the Ministry of Health, Labor, and Welfare of Japan (Sciences Research Grant for Research on Rare and Intractable Diseases) and Japanese Circulation Society (project for genome analysis in cardiovascular diseases); and the Japan Agency for Medical Research and Development [grant numbers: 20314864 and 22672854] to Dr. Hayato Tada.
None.