Abstract
Aim: Superficial erosion accounts for approximately one-third of all cases of acute coronary syndrome (ACS). Previously, we found that a nearby bifurcation is independently associated with superficial erosion; however, the effect of long-term oscillatory flow on superficial erosion remains unexplored. Endothelial-to-mesenchymal transition (EndMT) is a dynamic process in which endothelial cells acquire mesenchymal properties and, in turn, give rise to smooth muscle cell (SMC)-like cells and extracellular matrix (ECM) accumulation, similar to the autopsy pathology of superficial erosion. This finding prompted us to suspect that EndMT plays a role in the effect of chronic oscillatory flow on superficial erosion.
Methods: We established oscillatory flow in mouse carotid arteries and analyzed neointimal hyperplasia, endothelial continuity, ECM content, and EndMT markers 4 weeks later. Furthermore, bioinformatic data analyses and in vitro studies were performed to elucidate the underlying mechanisms.
Results: Carotid arteries exposed to long-term oscillatory flow exhibited hyperplastic neointima, reduced endothelial continuity, and increased SMC-like cells and ECM, indicating superficial erosion-prone lesions. In addition, oscillatory flow significantly induced EndMT, whereas inhibition of EndMT ameliorated the formation of superficial erosion-prone lesions. Bioinformatic data analyses and in vitro studies showed a remarkable reduction in anti-EndMT KLF2 and KLF4 in a DNA methyltransferase (DNMT)-dependent manner, and the suppression of DNMTs attenuated oscillatory flow-induced EndMT and superficial erosion-prone lesions.
Conclusions: Chronic oscillatory flow causes superficial erosion-prone lesions by activating EndMT in a DNMT-dependent manner. Our findings highlight a promising therapeutic strategy for the prevention of superficial erosions.
Caiying Tang and Guoxia Shi contributed equally to this work.
Abbreviations: ACS = acute coronary syndrome, 5Aza = 5-Aza-2′-deoxycytidine, CFD = computational fluid dynamics, DEGs = differentially expressed genes, DNMT = DNA methyltransferase, ECM = extracellular matrix, EC = endothelial cell, EndMT = endothelial-to-mesenchymal transition, GEO = Gene Expression Omnibus, GSE = gene data sets, HA = hyaluronic acid, HAECs = human arterial endothelial cells, HUVECs = human umbilical vein endothelial cells, LCCA = left common carotid arteriy, LSS = laminar shear stress, NETs = neutrophil extracellular traps, OSI = oscillatory shear index, OSS = oscillatory shear stress, PSS = pulsatile shear stress, qRT-PCR = quantitative reverse transcription polymerase chain reaction, SMC = smooth muscle cell
Introduction
Superficial erosion, only secondary to plaque rupture, is responsible for approximately one-third of all acute coronary syndrome (ACS) cases1). Our previous study demonstrated that superficial erosion most frequently occurs in the proximal segment of the left anterior descending artery and in proximity to the coronary bifurcation2). Proximity to bifurcation emerged as the strongest predictor of superficial erosion, even stronger than current smoking status. These observations were further supported by a prospective translational OPTICO-ACS study3). Using three-dimensional coronary arterial reconstruction and computational fluid dynamics (CFD) in patients with plaque erosion, it was shown that a high oscillatory shear index (OSI) correlated with the location and extension of the thrombus, and that a high OSI was independently associated with superficial erosion4, 5). Several studies have demonstrated that acute oscillatory shear stress (OSS) can promote endothelial cell (EC) activation, neutrophil recruitment, and neutrophil extracellular traps (NETs) formation, which cooperate to trigger arterial intimal injury and thrombosis in murine models of superficial erosion6-8). Notably, the unique anatomical distribution (i.e. near bifurcation) has been associated with long-term OSS. However, whether or not long-term OSS affects the formation of superficial erosion-prone lesions remains unclear.
Erosion-prone lesions are characterized by intact fibrous caps with EC denudation and few inflammatory cells. These lesions are rich in smooth muscle cells (SMCs) and extracellular matrix (ECM), particularly collagen III and hyaluronic acid (HA)9, 10). ECs have been reported to express mesenchymal markers in regions exposed to OSS in experimental atherosclerosis murine models, which is known as endothelial-to-mesenchymal transition (EndMT)11). During EndMT, endothelial-derived mesenchymal descendants give rise to fibroblasts or mesenchymal cells, which subsequently contribute to the accumulation of SMC-like cells and the ECM12). In addition, a significant proportion of ECs undergoing EndMT may lead to discontinuity in the endothelium, indicating the potential role of EndMT in long-term OSS-induced superficial erosion-prone lesion formation.
The mechanisms linking hemodynamic characteristics to atherosclerosis are being extensively studied at multiple levels of regulation, most recently epigenetic13). One of the major epigenetic mechanisms implicated is DNA methylation, which primarily involves covalent transfer of a methyl group to C-5 of the cytosine ring of DNA by DNA methyltransferase (DNMT), thus regulating the chromatin structure and function14, 15). A previous study performed in partial carotid ligated mice suggested that DNMT1 responds to disturbed flow and results in altered methylation patterns in vascular ECs, thus promoting local inflammation and typical vulnerable atherosclerotic plaque formation16). However, whether or not DNA methylation participates in the development of superficial erosion-prone lesions, a unique plaque phenotype distinct from that of vulnerable plaques, has not been investigated.
We hypothesized that long-term oscillatory flow induces the formation of superficial erosion-prone lesions via EndMT activation. This hypothesis was tested using an in vivo animal model in which four-week-long disturbed flow was established in the carotid arteries. In addition, an orbital shaking system was constructed, and bioinformatic data were analyzed to uncover the underlying mechanism and identify novel therapeutic targets for the prevention of superficial erosions.
Materials and Methods
Detailed descriptions of the methods are available in the Supplemental Materials.
Animals and Arterial Injury
Eight-week-old male Apoe−/− mice with C57BL/6 background were purchased from the Beijing Vital River Laboratory Animal Technology and fed a normal chow diet to avoid lipid-rich vulnerable plaque formation. The animals were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). In brief, the left common carotid artery (LCCA) was electrically current injured using a bipolar microcoagulator with a current pulse of 5 W, and the intima healed and completely reendothelialized 4 weeks later (Supplemental Fig.1). Constrictive or non-constrictive cuffs were then placed on the LCCA to establish OSS or laminar shear stress (LSS)6, 7). After another 4 weeks, the mice were euthanized by exsanguination under isoflurane anesthesia (3%), and the LCCA was harvested (Fig.1A). Body weight and blood lipid profiles were measured, and no significant differences were found between the two groups (Supplemental Fig.2).


All procedures were approved by the Animal Care and Use Committee of the Second Affiliated Hospital of Harbin Medical University (approval no. SYDWGZR2020-05) and conformed to the recommendations of the European Ethical Committee (EEC) (2010/63/EU).
Endothelial Permeability
Endothelial permeability was investigated by monitoring extravasation of Evans blue dye. Blue staining of the Evans blue dye-albumin conjugate was evident in LCCA sections subjected to OSS or LSS. Mice were injected immediately or 4 weeks after flow perturbation, via tail intravenous injection of 0.2 ml of 1 mg/ml Evans blue (Solarbio). Twenty minutes later, mice were euthanized, perfused by left ventricular apical cannulation with phosphate-buffered saline at 4℃ supplemented with heparin 10U/ml, and fixed with a perfusion of 10% formalin. The LCCA was opened longitudinally and examined by bright-field imaging.
In Vitro Shear Stress Model
An orbital shaking platform was used to apply shear stress to human umbilical vein endothelial cells (HUVECs) following previously established methods (Supplemental Fig.3)17, 18). In brief, HUVECs were seeded in 6-well plates and cultured to 70%–80% confluency. Fresh medium was added, and the plates were placed on an orbital shaker (r=10 mm, GSP-20, CBIO, China) at 210 rpm for 24 h to generate a defined shear stress pattern across the well, with a shear stress of approximately 4.8 dyn/cm2 in the center (OSS) and 11.1 dyn/cm2 in the periphery (LSS).
Bisulfite DNA Sequencing
HUVECs were then exposed to OSS or LSS for 24 h. Genomic DNA was isolated using an Ezup Column Animal Genomic DNA Purification Kit (B518251; ZIKER Biotechnology, Shenzhen, China). Purified DNA was modified with bisulfite using the EZ DNA Methylation-Gold Kit (D5005, Zymo Research, Irvine, CA, USA). The CpG islands in the promoter regions of KLF2 and KLF4 were identified by “Methprimer” (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi). The fragments between -473 bp and -132 bp of KLF2 (ID: 10365) and -1183 bp to -839 bp of KLF4 (ID: 9314) promoter regions were amplified using BigDye Terminator v1.1 (A500737; Applied Biosystems, Foster City, CA, USA). Information regarding the primer sequences is provided in Supplemental Table 1. Methylated sites are displayed with filled circles, and unmethylated sites with hollow circles.
Supplemental Table 1.Primers used for Bisulfite DNA Sequencing
| Gene |
Forward |
Reverse |
| KLF2
|
GTTTTTTTGAGAGTTTTTGAGAGGT
|
ACCCRCCCAAACCTTATAAA
|
| KLF4
|
AGGAATYGTGYGAGGTTAGG
|
AAACAACTAACRAACTAAAACCRAA
|
The RNA-seq data from human vascular ECs were downloaded from the Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/), three gene data sets (GSE) were selected: GSE92506, GSE103672 and GSE160611 19-21). HUVECs in GSE92506 were exposed to OSS (0.5±4 dyn/cm2) or pulsatile shear stress (PSS, 12±4 dyn/cm2), which is similar to the shear stress defined in the present study22). The magnitude of OSS and PSS in GSE103672 (HUVECs) was 0.5±5 dyn/cm2 and 12±5 dyn/cm2, while that in GSE160611 (human arterial endothelial cells [HAECs]) was the same as that in GSE92506. The mRNA expression profiles of HUVECs exposed to OSS and PSS were then compared. The package “limma” (v 3.52.3) of the R software program (v. 4.2.0) was used to identify differentially expressed genes (DEGs) between OSS and PSS. An absolute log2(fold change) >1 and p<0.05 were set as the threshold for DEGs.
Statistical Analyses
Animals were randomly allocated to treatment or control groups. The investigator was blinded to the group allocation during the experiment. Data are expressed as the mean±standard error of the mean. Unpaired Student’s t test and Mann-Whitney U test were used to evaluate differences between two groups, and a one-way analysis of variance with Tukey’s post hoc test was used for multiple groups (GraphPad Prism 9; GraphPad Software, America). Differences were considered statistically significant at p<0.05.
Results
Chronic Oscillatory Flow Induces the Development of Superficial Erosion-Prone Lesion
We previously reported that superficial erosion preferentially occurs at arterial branching points with abnormal oscillatory flow2). However, the long-term effects of oscillatory flow on superficial erosion-prone lesion formation have not yet been studied. Using previously described techniques7), electrical injury was applied to the LCCA of eight-week-old Apoe−/− mice, followed by constrictive or non-constrictive cuff placement around the proximal regions four weeks later to generate downstream OSS and LSS (Fig.1A, Supplemental Fig.1).
Four weeks after placement, the downstream segments of the LCCA were analyzed. The OSS group showed a disturbed EC barrier function (Fig.1B) and increased neointimal hyperplasia (Fig.1C-D) in the carotid arteries. The continuity of the endothelium, which was determined by immunohistochemical staining of ECs, showed that the OSS group exhibited a significantly greater loss of cell junctions (Fig.1E-F). Collagen III, an important component of ECM remodeling in superficial erosion, appeared to increase in carotid arteries exposed to OSS (Fig.1G-H). These findings confirmed the hypothesis that chronic oscillatory flow induces the formation of superficial erosion-prone lesions.
OSS Promotes in vivo and in vitro EndMT
Studies have shown that shear stress induces abnormal EndMT, which drives ECM deposition, thereby resulting in the development of renal fibrosis23), varicose veins24), and aortic valve calcification25). This pathological progression is consistent with the vascular characteristics observed in the mouse model. To test whether or not EndMT appeared in the OSS group, immunohistochemical staining of α-SMA, a mesenchymal marker, was performed, and significantly elevated intimal staining was observed (Fig.1I-J). However, this abnormal pathological staining may have resulted not only from EndMT but also from the migration of SMCs from the media.
To further investigate whether vascular ECs underwent EndMT, double immunofluorescence staining was performed. CD31+αSMA+ double-positive cells, suggestive of cells in the intermediate stage of EndMT, were observed in the endothelium under OSS (Fig.1K-L), indicating that oscillatory flow can induce EndMT in the carotid arteries.
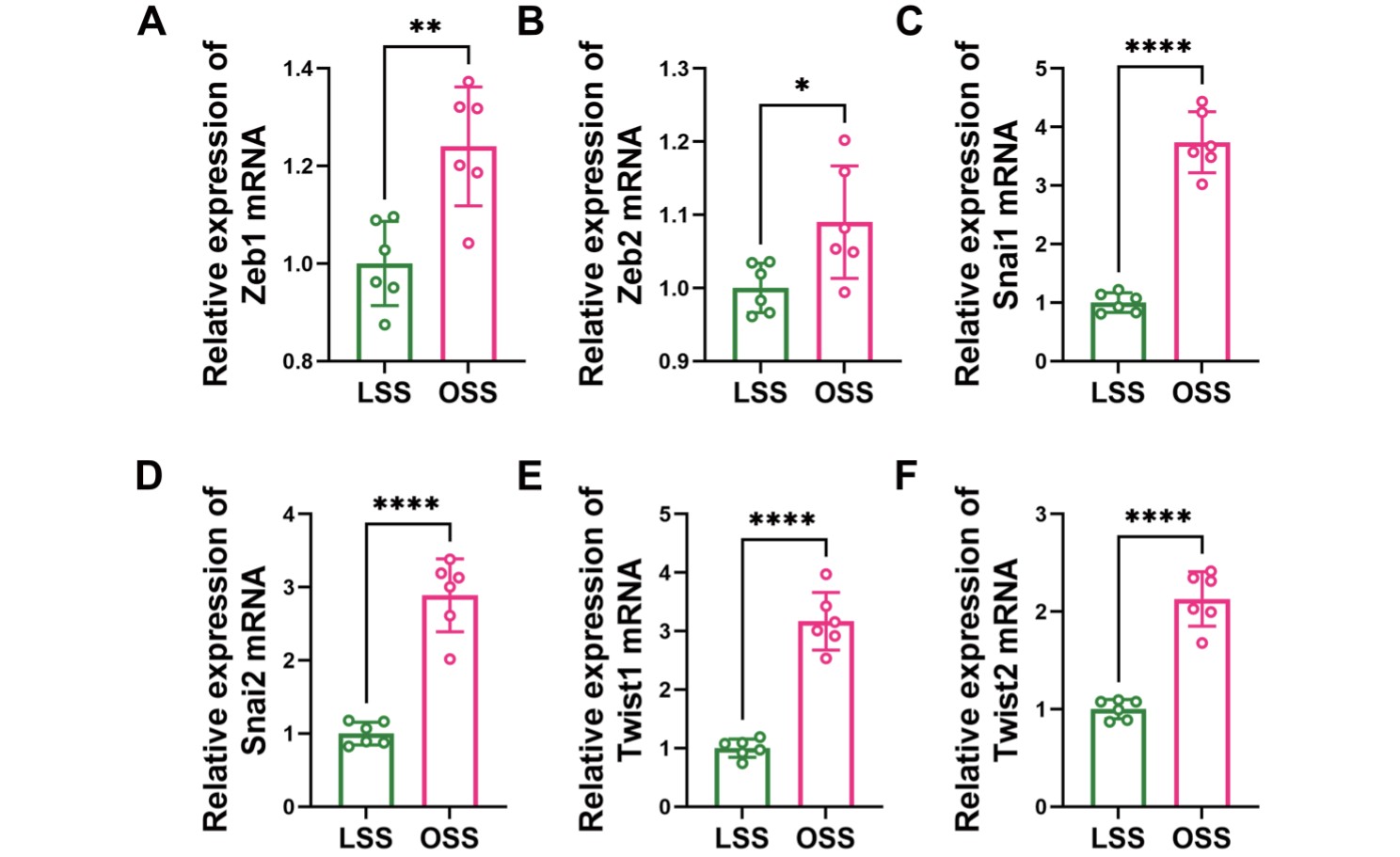
To further validate the association between the flow pattern and EndMT, HUVECs were subjected to different flows using an orbital shaking system (Supplemental Fig.3). The shear stress was approximately 11.1 dyn/cm2 at the periphery and 4.8 dyn/cm2 at the center of the well, with a rotation rate of 210 rpm18). We observed that HUVECs changed from a cobblestone appearance to an elongated spindle-shaped morphology similar to mesenchymal cells upon OSS treatment (Fig.2A-B). In addition, quantitative reverse transcription polymerase chain reaction (qRT-PCR) data showed reduced expression of endothelial markers (CD31, endocan, and VE-cadherin) and elevated expression of mesenchymal markers (N-cadherin, vimentin, and αSMA) and EndMT markers (Zeb1, Zeb2, Snai1, Snai2, Twist1 and Twist2) in the OSS group (Fig.2C-E, Supplemental Fig.4), suggesting that OSS promotes EndMT, through which ECs gain mesenchymal properties, such as ECM generation. Consistent with this, an increased expression of collagen I, collagen III and HA was observed in the OSS group (Fig.2F). Therefore, OSS promotes EndMT, both in vivo and in vitro.

Numerous mechanisms have been identified in EndMT, among which TGF-β/SMAD signaling is the most canonical26). Our in vitro experiments showed that OSS promoted the expression of TGF-β1 and TGF-β2 (Fig.3A) as well as the translocation of SMAD2/3 into the nucleus (Fig.2F). To test whether TGF-β/SMAD signaling plays a role in OSS-mediated EndMT, the ALK5 (TGF-β receptor kinase) inhibitor, SB431542, was added to OSS-treated ECs. We found that SB431542 markedly inhibited the promotional effect of OSS on EndMT, as evidenced by the elevated expression of endothelial markers and decreased expression of mesenchymal markers (Fig.3B-D).

To further confirm the in vivo role of TGF-β/SMAD signaling in OSS-induced EndMT, SB431542 was administered to block the TGF-β receptor in Apoe−/− mice. In brief, the LCCAs of Apoe−/− mice were electrically injured, and constrictive cuffs were placed to generate OSS. Mice were intraperitoneally injected with SB431542 (0.1 mg/10 g) or vehicle every other day for 4 weeks (Fig.3E). In agreement with in vitro observations, the expression of collagen III and αSMA (Fig.3F-I) and the proportion of CD31+αSMA+ double-positive cells (Fig.3J-K) were significantly reduced. Collectively, these results suggest that TGF-β/SMAD signaling mediates OSS-induced EndMT.
Inhibition of EndMT Prevents Chronic OSS-Induced Superficial Erosion-Prone Lesion
Given that EndMT reduces normal ECs and increases SMC-like cells and ECM formation, which is consistent with the pathological characteristics of superficial erosion, we suspect that EndMT may play a critical role in the formation of chronic OSS-induced superficial erosion-prone lesions. To test this hypothesis, we measured the intima/media ratio in the carotid arteries of mice treated with OSS and SB431542 and observed a significant reduction in neointimal hyperplasia (Fig.3L-M). Apoe−/− mice receiving SB431542 exhibited reduced ECM remodeling (Fig.3N-O) and increased intimal integrity in the carotid arteries (Fig.3P-Q). These data provided further evidence that EndMT can be triggered by oscillatory flow, which contributes to the formation of superficial erosion-prone lesions. We also conclude that TGF-β/SMAD signaling is indispensable for OSS-mediated EndMT, thus making it a potential target for the treatment of superficial erosion-prone lesions.
DNA Methylation Pattern Alters in Response to Different Shear Stresses
Recent studies have revealed that DNMTs are activated under pro-atherogenic conditions, such as disturbed flow, a high-fat diet, or high low-density lipoprotein cholesterol levels, to drive the progression of atherosclerosis13). However, the role of DNA methylation in superficial erosion development has not yet been explored. To investigate whether or not DNA methylation plays a role in our model, LCCA intima from the LSS or OSS groups was digested to extract DNA, which was then used for the dot blot assay. The data showed that the total genomic DNA from the OSS group exhibited multiple methylation signals, indicating alterations in the DNA methylation patterns in response to different flows (Fig.4A). To further understand the effects of OSS on DNA methylation, DNA methylation-related enzymes were examined, and increased expression of DNMT1 and DNMT3a was observed in the LCCA intima (Fig.4B). Similar results were observed in our in vitro model (Fig.4C and Supplemental Fig.5). Therefore, it can be inferred that OSS facilitates DNA hypermethylation in ECs.


To further determine whether or not DNA methylation participates in OSS-induced endothelial morphological and metabolic changes, we applied 5-Aza-2′-deoxycytidine (5Aza) to repress DNMTs. 5Aza is a nucleoside analog that traps DNMTs in a covalent complex with DNA, resulting in DNMTs inhibition, and the inhibition efficiency was validated (Supplemental Fig.6). In HUVECs cultured in OSS, qRT-PCR showed that 5Aza significantly inhibited OSS-induced EndMT (Fig.4D). Similar observations were also observed by Western blotting and immunofluorescence staining (Fig.4E-G). Collectively, these results demonstrated the involvement of DNA methylation in OSS-induced EndMT.

Furthermore, the LCCAs of Apoe−/− mice were electrically injured and constrictive cuffs were placed, as described above. The mice were subjected to 5Aza (0.2 mg/kg) or vehicle via intraperitoneal injection daily for 4 weeks (Fig.4H). As expected, EndMT markers were decreased in Apoe−/− mice injected with 5Aza (Fig.4I-L), further indicating that OSS promoted EndMT in a DNMT-dependent manner.
Inhibition of DNMTs Ameliorates the Formation of OSS-Induced Superficial Erosion-Prone Lesion
Having demonstrated the role of DNMTs in OSS-induced EndMT, we tested the contribution of DNMT in the formation of superficial erosion-prone lesions under long-term disturbed flow. As shown in Fig.4M-N, although the carotid arterial intima remained hyperplastic, there was a significant decrease in the fibrotic area in the 5Aza-treated Apoe−/− mice. Combined with the reduced expression of collagen III (Fig.4I-J), these data indicate a strong association between ECM remodeling and DNA methylation. Furthermore, endothelial continuity in the LCCA appeared to be better preserved in mice injected with 5Aza than mice injected with vehicle, suggesting reduced endothelial denudation (Fig.4O-P). Taken together, these results provide strong evidence that inhibition of DNMTs prevents chronic oscillatory flow-induced EndMT and thus ameliorates the development of superficial erosion-prone lesions. Therefore, it could be considered as a novel therapeutic target.
OSS Inhibits KLF2 and KLF4 via DNA Hypermethylation
Using the GEO database (https://www.ncbi.nlm.nih.gov/geo/), we investigated the mechanisms by which DNMTs facilitate EndMT. Three GSEs from different shear stress-treated human vascular cells were acquired. The mRNA expression profiles of the OSS-treated HUVECs and the control group were compared (Fig.5A-B, Supplemental Fig.7A). Given the same cell type and similar interference, we analyzed the common DEGs in GSE92506 and GSE103672 and identified 89 co-downregulated and 10 co-upregulated genes. Considering that DNA methylation of the promoters within CpG islands correlates with reduced chromatin accessibility and leads to transcriptional repression, we focused on the 89 co-downregulated genes. A further analysis using the “MethPrimer” (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi) revealed several genes containing CpG islands in their promoter, among which KLF2 and KLF4 attracted our attention because of their previously reported role in the inhibition of TGF-β signaling and EndMT (Fig.5A-C)27-29).


To investigate whether or not KLF2 and KLF4 were downregulated under chronic OSS, RNA extracted from the LCCA intima was analyzed. The results showed that the expression of KLF2 and KLF4 was significantly decreased in the OSS group (Fig.5D). We then used the Pearson correlation analysis method and revealed that the expression of KLF2 was negatively correlated with that of DNMT1, while the expression of KLF4 was negatively correlated with that of DNMT1 and DNMT3a (Fig.5E). These results suggest that OSS may reduce the KLF2 and KLF4 expression in a DNMT-dependent manner. To test this hypothesis, we subjected HUVECs to LSS or OSS for 24 h, and Bisulfite DNA Sequencing showed that methylation of the KLF2 and KLF4 promoters was higher in group OSS than group LSS (Fig.5F). In addition, 5Aza treatment rescued the OSS-induced KLF2 and KLF4 downregulation, suggesting that OSS reduced KLF2 and KLF4 through DNA hypermethylation (Fig.5G). Taken together, these findings indicate that the DNA methylation-mediated downregulation of KLF2 and KLF4 is responsible for OSS-induced EndMT and contributes to the formation of superficial erosion-prone lesions.
Discussion
In this study, we demonstrated that chronic oscillatory flow induces superficial erosion-prone lesions and elucidated the underlying mechanism. The three main findings of this study were as follows: 1) long-term oscillatory flow promoted neointimal hyperplasia, EC denudation, accumulation of SMC-like cells, and ECM remodeling, suggesting the formation of a superficial erosion-prone lesion; 2) EndMT was triggered by chronic OSS via activation of TGF-β/SMAD signaling, which contributed to the development of superficial erosion-prone lesions; and 3) OSS-evoked DNMTs were identified as important epigenetic modifying molecules that promoted EndMT and subsequent superficial erosion-prone lesion formation.
We previously showed that superficial erosion preferentially develops at the arterial bifurcation, featuring an OSS2). CFD studies have further revealed that a high OSI is independently associated with the formation of superficial erosion and thrombus4, 5). Libby et al. used a mouse model with carotid arteries exposed to laminar or oscillatory flow for 1 or 6 h demonstrated that acute flow perturbation promoted EC denudation, recruitment of neutrophils for NETs formation, and subsequent arterial thrombosis development in superficial erosion6, 7). However, whether and how long-term OSS affects superficial erosion formation has not yet been explored. In the present study, we showed that carotid arteries subjected to OSS for four weeks exhibited denuded endothelium and hyperplastic neointima rich in SMC-like cells and remodeled ECM, particularly collagen III, resembling the substrate of eroded human plaques9, 10). Our study demonstrated the formation of a superficial erosion-prone lesion under chronic oscillatory flow, further explaining the focal nature of superficial erosion.
Considering the similar morphological and biochemical characteristics of superficial erosion-prone lesions and EndMT, we hypothesized that EndMT is involved in the formation of chronic OSS-induced arterial lesions. We showed that EndMT developed in carotid arteries and HUVECs subjected to OSS. Furthermore, we also demonstrated that inhibition of TGF-β/SMAD signaling via SB431542 prevented EndMT, suggesting that OSS promotes EndMT in a TGF-β/SMAD signaling-dependent manner. Furthermore, neointimal hyperplasia, the accumulation of SMC-like cells and ECM, and endothelium denudation induced by chronic oscillatory flow were ameliorated in SB431542-treated Apoe−/− mice, which was consistent with the reduction in EndMT. Thus, our study delineates the critical role of EndMT in the development of superficial erosion-prone lesions under long-term oscillatory flow. Notably, a previous study on high-fat diet-treated mice suggested that EndMT is associated with increased necrotic core area and decreased fibrous cap area in vascular cross-sections, which are characteristics of vulnerable plaques11). However, no study has investigated its role in eroded lesions, which are characterized by a lower frequency of lipid plaques, smaller lipid arcs, and thicker fibrous cap1). In the present study, considering the fact that patients with plaque erosion less frequently suffered from hyperlipidemia2), we fed Apoe−/− mice a normal chow rather than a cholesterol-rich diet to better understand the underlying mechanism. In addition, pathological observations in this study were explored in the context of chronic oscillatory flow, which mimics the actual situation in which vascular lesions are long-term rather than short-term when exposed to disturbed blood flow. Previous studies have shown that although EndMT can occur under different stimuli such as inflammation, oxidative stress, high blood sugar, and hypoxia, the resulting mesenchymal phenotype varies26, 30, 31). EndMT can give rise to SMC-like cells, fibroblast-like cells, and even osteoprogenitor cells, thus promoting the progression of pulmonary arterial hypertension, cardiac fibrosis, wound healing, and vascular calcification26). Our study described the effect of long-term oscillatory flow on EndMT, which resulted in increased SMC-like cells and ECM-producing mesenchymal properties and subsequent formation of superficial erosion-prone lesions. Furthermore, a recent review noted that EndMT can be inhibited by a few commonly used cardiovascular medicines, including aspirin, simvastatin, losartan, and spironolactone, suggesting that EndMT inhibition could help optimize clinical treatment for patients at a high risk of superficial erosion26).
Previous studies have reported that epigenetic mechanisms may contribute to atherosclerosis development13). However, few studies have focused on superficial erosion, which is a special subtype of atherosclerosis. To advance precision medicine, this study explored the mechanism of superficial erosion-prone lesion formation and demonstrated that DNA methylation participates in OSS-induced EndMT and formation of superficial erosion-prone lesions. We showed that the 5-mC signal increased in carotid arteries with constrictive cuffs in Apoe−/− mice, and the endothelial expression of DNMT1 and DNMT3a was upregulated under OSS both in vivo and in vitro. Inhibition of DNMTs by 5Aza led to reduced EndMT and significantly ameliorated OSS-mediated endothelial detachment and ECM remodeling. In addition, 5Aza has been approved (as Vidaza) by the United States Food and Drug Administration for the treatment of myelodysplastic syndromes, including leukemia, suggesting that inhibition of aberrant DNA methylation could be a promising therapy for superficial erosion-prone lesions.
KLF2 and KLF4 are flow-sensitive transcription factors that play critical roles in repressing TGF-β signaling and EndMT27-29). The expression of KLF2 and KLF4 in HAECs increased under LSS and decreased under OSS32). KLF2 has been reported to be repressed in carotid arteries exposed to disturbed flow in the mouse carotid artery collar model, while overexpression of KLF2 results in reduced expression of TGF-β target genes27). In addition, silencing KLF2 in HUVECs has been reported to increase EndMT, thus contributing to atherosclerosis28). KLF4 is also increased in ECs subjected to laminar flow, wherein it inhibits the TGF-β signaling pathway and subsequent EndMT29). Consistent with the previous reports, our study showed that OSS downregulated the expression of KLF2 and KLF4. It has also been reported that KLF2 and KLF4 coordinate the regulation of the endothelial function and the establishment of a quiescent, anti-inflammatory, and anti-thrombotic phenotype33-35). Given the importance of KLF2 and KLF4 in endothelial homeostasis and EndMT inhibition, their suppression by OSS through DNA methylation most likely accounts for OSS-induced EndMT and superficial erosion-prone lesions.
The present study suggests that KLF2 and KLF4 inhibit EndMT by suppressing the TGF-β/SMAD signaling. However, KLF4 was reported to inhibit epithelial-to-mesenchymal transition (a process studied more widely than EndMT) via the activation of E-cadherin and suppression of Snail, suggesting that KLF2 and KLF4 may affect EndMT through other mechanisms36, 37). In addition, a variety of mechanosensors in ECs have been identified, including integrins, junctional complexes, ion channels, G proteins, G protein-coupled receptors, caveolae, the glycocalyx, and primary cilia38, 39). Mechanosensors can recognize different flows and convert mechanical forces into biochemical stimuli, which in turn activates downstream signaling pathways and regulates EC functions. For example, Zhang et al. demonstrated that the mechanosensitive cation channel Piezo1 activates annexin A2 in response to OSS, which then mediates the lipid raft translocation of integrin α5 and EC activation, thereby contributing to the development of atherosclerosis40). Future studies on the mechanosensors through which chronic OSS induces superficial erosion-prone lesions may shed light on potential therapeutic targets.
Study Limitations
Several limitations associated with the present study warrant mention. First, although double immunofluorescence staining of CD31 and αSMA can be used to detect ECs in the intermediate stage of EndMT, ECs that have undergone complete mesenchymal transition cannot be identified due to complete loss of endothelial markers. We propose that the use of EC lineage tracking systems may address this limitation26). Second, the ECs used in this study were HUVECs. However, arterial ECs are more suitable for modeling in vivo responses. Our analysis of DEGs in GSE160611 (RNA-seq dataset of HAECs) revealed 234 upregulated and 823 downregulated genes, among which FGFR1 and NFATC1 attracted our attention because they are known to inhibit EndMT11, 41). Furthermore, “Methprimer” showed that NFATC1 is rich in CpG islands in the promotor, and inhibition of DNMTs was able to rescue the expression of NFATC1 in HUVECs exposed to OSS, suggesting a possible role of DNA methylation in OSS-induced NFATC1 down-regulation (Supplemental Fig.7). Further detailed mechanistic studies are required in the future.
Conclusions
Our study demonstrated that chronic OSS promotes the formation of superficial erosion-prone lesions by activating EndMT. In addition, we provide novel insights into the epigenetic mechanisms by which OSS induces the development of superficial erosion-prone lesions in a DNMT-dependent manner. This is the first study to elucidate the roles of EndMT and DNA methylation in the formation of superficial erosion-prone lesions in the context of long-term oscillatory flow, thereby shedding light on new therapeutic targets for the prevention of superficial erosion.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82072091 to J.D., No. 82102068 to P.S.), the Natural Science Foundation of Heilongjiang Province (YQ2020H017 to J.D), and the Hei Long Jiang Postdoctoral Foundation (grant No. LBH-Q21117 to J.D).
Author Contributions
C.T., G.S., P.S., and J.D. designed the research; C.T., G.S., R.J, X.P., C.W., Z.D., S.L., and P.W. performed the research; P.S., B.Y., and J.D. provided essential material input; S.S., C.P., and S.L. provided intellectual input; and C.T., G.S., P.S., and J.D. wrote the manuscript, which was approved by all authors.
Declarations of Interest
None.
Data Availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Graphical Abstract
OSS promotes hypermethylation of KLF2 and KLF4 via DNMT1/3a, resulting in decreased gene expression. This process leads to the activation of TGF-β/SMAD signaling, which promotes EndMT and subsequent superficial erosion-prone lesion formation.
Supplementary Methods
qRT-PCR Analyses
Animals were randomly divided into group OSS and LSS, and underwent carotid surgery as described above. Then the LCCA was harvested, briefly rinsed in PBS, and the intima was collected as previously reported1, 2). Total RNA was isolated from HUVECs or LCCA intima using TRIzol according to the manufacturer’s instructions. First-strand cDNA was generated from 1 µg of RNA using ReverTra Ace qPCR RT Kit (TOYOBO, China). qRT-PCR experiments were performed using ACEQ Universal SYBR qPCR Master Mix (Vazyme, Chnia). Amounts of mRNA of interest were normalized to that of GAPDH. Each cDNA sample was run in triplicate. Analysis was performed using the 2−ΔΔCT cycle threshold method. The information of primer sequences is provided in Supplemental Table 2 and Supplemental Table 3.
Supplemental Table 2.Primers used for qRT-PCR in HUVECs
| Gene |
Forward |
Reverse |
| αSMA
|
ACTGCCTTGGTGTGTGACAA
|
CACCATCACCCCCTGATGTC
|
| CD31
|
GGAGCCTTCCGTTCTAGAGT
|
ACGTGCAGTACACGGAAGTT
|
| DNMT1
|
AGGCGGCTCAAAGATTTGGAA
|
GCAGAAATTCGTGCAAGAGATTC
|
| DNMT3a
|
CCGATGCTGGGGACAAGAAT
|
CCCGTCATCCACCAAGACAC
|
| DNMT3b
|
AGGGAAGACTCGATCCTCGTC
|
GTGTGTAGCTTAGCAGACTGG
|
| Endocan
|
CTTGCTACCGCACAGTCTCA
|
TGCAATCCATCCCGAAGGTG
|
| FGFR1
|
ACCAAACCGTATGCCCGTAG
|
CCCACTGGAAGGGCATTTGA
|
| GAPDH
|
CTGGGCTACACTGAGCACC
|
AAGTGGTCGTTGAGGGCAATG
|
| KLF2
|
CTGAGTGAACCCATCCTGCC
|
ATGAAGTCCAGCACGCTGTT
|
| KLF4
|
GGGTCTTGAGGAAGTGCTGAG
|
AAATCCCCGGGACTGACCTT
|
| N-Cadherin
|
GGGATCAAAGCCTGGAACATA
|
GACACGATTCTGTACCTCAACA
|
| NFATC1
|
CAGCTTTCCAGTCCCTTCCAA
|
GATGCATAGCCATAGTGTTCTTCCT
|
| SNAI1
|
ACTGCAACAAGGAATACCTCAG
|
GCACTGGTACTTCTTGACATCTG
|
| SNAI2
|
CGAACTGGACACACATACAGTG
|
CTGAGGATCTCTGGTTGTGGT
|
| TET1
|
GGAAAGAAGAGGGCTGCGAT
|
ACGGTCTCAGTGTTACTCCCTAA
|
| TET2
|
TCATCCTGGTGTGGGAAGGA
|
CAGGCGCAAGTTCTCTCTTCA
|
| TET3
|
TGCACTAGCTGTACCAACCG
|
CTGAGCTCTGAGCCTGTCTTG
|
| TGF-β1
|
GGCCAGATCCTGTCCAAGC
|
GTGGGTTTCCACCATTAGCAC
|
| TGF-β2
|
CCCCGGAGGTGATTTCCATC
|
GGGCGGCATGTCTATTTTGTAAA
|
| TGF-β3
|
ACTTGCACCACCTTGGACTTC
|
GGTCATCACCGTTGGCTCA
|
| TWIST1
|
GTCCGCAGTCTTACGAGGAG
|
GCTTGAGGGTCTGAATCTTGCT
|
| TWIST2
|
AGCGGCGCTACAGCAAGAAGT
|
CGTCTGGATCTTGCTCAGCTTGT
|
| VE-Cadherin
|
GAGAAGCAGGCCAGGTATGAG
|
AAATGTGTACTTGGTCTGGGTG
|
| Vimentin
|
GGACCAGCTAACCAACGACA
|
TCCTCCTGCAATTTCTCCCG
|
| ZEB1
|
TTACACCTTTGCATACAGAACCC
|
TTTACGATTACACCCAGACTGC
|
| ZEB2
|
CAAGAGGCGCAAACAAGCC
|
GGTTGGCAATACCGTCATCC
|
Supplemental Table 3.Primers used for qRT-PCR in mice
| Gene |
Forward |
Reverse |
| DNMT1
|
CGGACAGTGACACCCTTTCAG
|
TCCGTTTAGTGGGGCCCTT
|
| DNMT3a
|
ACCGCAAAGCCATCTACGAA
|
AGCCTTGCCACTGTCACTTT
|
| DNMT3b
|
ATGAAGAAGAGGGTGCCAGC
|
CTCTGTGCAGATTGCCTCCA
|
| GAPDH
|
AGGTCGGTGTGAACGGATTTG
|
GGGGTCGTTGATGGCAACA
|
| KLF2
|
GCCTTCGGTCTTTTCGAGGAC
|
TGGTGTAGCTGCAAGTATGTG
|
| KLF4
|
GGCGAGTCTGACATGGCTG
|
GCTGGACGCAGTGTCTTCTC
|
| TET1
|
CGGGTTTACAATGGCTCTTCG
|
GGTTTGGGTGTGACTACTGGG
|
| TET2
|
AAGCAGCCGTCAGCCAAAT
|
TTCCGTGTTGGGAAAGCATCT
|
| TET3
|
CGCTGCTCGTCTGGAAGATG
|
GGCCCCGTAAGATGACACA
|
LCCA intima was harvested as previously described3, 4). Genomic DNA from LCCA intima was extracted using the TIANamp Genomic DNA Kit (DP304, TIANGEN, China). The isolated DNA was first denatured by heating at 95℃ for 5 min, followed by chilling on ice for 5 min. Then 2µl of DNA samples (50 ng/µl) were spotted onto the Hybond N+ membrane (0.45 µM) and fixed by UV cross-linking. After blocked in 5% skim milk and incubated with rabbit anti- 5-mC (1:1000, Abcam 214727) overnight at 4℃, the membrane was incubated with anti-rabbit horseradish peroxidase-linked secondary antibodies (1:10000, ZSGB-BIO). The blots were then detected by ECL and exposed to X-ray film. Equal DNA loading was verified by staining the membranes with 0.1% methylene blue dye buffer for 3 min.
Western Blot Analysis
Samples were macerated on ice and lysates and prepared with RIPA buffer supplemented with proteases inhibitors. Lysates were separated by SDS-PAGE, transferred to a PVDF membrane and incubated with primary antibodies against VE-Cadherin (1:1000, Abcam 33168), Vimentin (1:1000, Abcam 92547), DNMT1 (1:1000, Abcam 188453), DNMT3a (1:2000, Abcam 188470) and GAPDH (1:1000, Proteintech 60004) overnight at 4℃. After incubation with horseradish peroxidase-linked secondary antibodies (1:5000, ZSGB-BIO), immunoreactive proteins were detected by ECL and exposure to X-ray film.
Immunofluorescence
For in vitro experiments, HUVECs subjected to shear stress for 24 h were fixed in 4% PFA for 10 min. The cells were permeabilized with 0.1% Triton X-100 and blocked with 5% BSA for 30 min, followed by incubation with the primary antibodies against CD31 (1:200, Proteintech 28083), VE-Cadherin (1:100), vWF (1:300, Abcam 154193), SM22α (1:200, Abcam 14106), HA (1:100, GeneTex 17370), Collagen III (1:200, Abcam 7778), SMAD2/3 (1:400, Cell Signaling Technology 8685) or Collagen I (1:500, Abcam 138492) at 4℃ overnight. Fluorophore-coupled anti-species antibody (1:500, Abcam) were used for 1h to visualize the corresponding proteins. The cell nuclei were stained with DAPI and the cytoskeleton with Phalloidin.
For arterial samples, frozen cross sections were permeabilized, blocked, and incubated with primary antibodies against CD31 (1:200), αSMA (1:100, Abcam 240654) at 4℃ overnight. Vessels showing total occlusion or aneurysmal features were excluded from the analysis. Then they were incubated with secondary antibodies (1:500, Abcam). Images were obtained under a confocal microscope.
Immunohistochemistry
The paraffin cross sections were incubation with the primary antibody against CD31 (1:200), αSMA (1:100) and Collagen III (1:200) at 4℃ overnight. Then they were incubated with biotin-conjugated secondary antibodies (ZSGB-BIO), and visualized with 3-3’di- aminobenzidin DAB.
Supplemental References
1)Chen J, Zhuang R, Cheng HS, Jamaiyar A, Assa C, McCoy M, Rawal S, Pérez-Cremades D, Feinberg MW. Isolation and culture of murine aortic cells and RNA isolation of aortic intima and media: Rapid and optimized approaches for atherosclerosis research. Atherosclerosis, 2022; 347: 39-46
2)Nevado RM, Hamczyk MR, Andrés V. Isolation of Mouse Aortic RNA for Transcriptomics. In: Atherosclerosis, 2022: 611-627
3)Behringer EJ, Pires PW, Hakim MA. Isolation and Functional Analysis of Arteriolar Endothelium of Mouse Brain Parenchyma. Journal of Visualized Experiments, 2022
4)Hakim CH, Jackson WF, Segal SS. Connexin Isoform Expression in Smooth Muscle Cells and Endothelial Cells of Hamster Cheek Pouch Arterioles and Retractor Feed Arteries. Microcirculation, 2010; 15: 503-514
References
- 1) Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park SJ, Jang YS, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi SY, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R, Jang IK. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol, 2013; 62: 1748-1758
- 2) Dai J, Xing L, Jia H, Zhu Y, Zhang S, Hu S, Lin L, Ma L, Liu H, Xu M, Ren X, Yu H, Li L, Zou Y, Zhang S, Mintz GS, Hou J, Yu B. In vivo predictors of plaque erosion in patients with ST-segment elevation myocardial infarction: a clinical, angiographical, and intravascular optical coherence tomography study. Eur Heart J, 2018; 39: 2077-2085
- 3) Leistner DM, Krankel N, Meteva D, Abdelwahed YS, Seppelt C, Stahli BE, Rai H, Skurk C, Lauten A, Mochmann HC, Frohlich G, Rauch-Krohnert U, Flores E, Riedel M, Sieronski L, Kia S, Strassler E, Haghikia A, Dirks F, Steiner JK, Mueller DN, Volk HD, Klotsche J, Joner M, Libby P, Landmesser U. Differential immunological signature at the culprit site distinguishes acute coronary syndrome with intact from acute coronary syndrome with ruptured fibrous cap: results from the prospective translational OPTICO-ACS study. Eur Heart J, 2020; 41: 3549-3560
- 4) Yamamoto E, Thondapu V, Poon E, Sugiyama T, Fracassi F, Dijkstra J, Lee H, Ooi A, Barlis P, Jang IK. Endothelial Shear Stress and Plaque Erosion: A Computational Fluid Dynamics and Optical Coherence Tomography Study. JACC Cardiovasc Imaging, 2019; 12: 374-375
- 5) Thondapu V, Mamon C, Poon EKW, Kurihara O, Kim HO, Russo M, Araki M, Shinohara H, Yamamoto E, Dijkstra J, Tacey M, Lee H, Ooi A, Barlis P, Jang IK. High spatial endothelial shear stress gradient independently predicts site of acute coronary plaque rupture and erosion. Cardiovasc Res, 2021; 117: 1974-1985
- 6) Franck G, Mawson TL, Folco EJ, Molinaro R, Ruvkun V, Engelbertsen D, Liu X, Tesmenitsky Y, Shvartz E, Sukhova GK, Michel JB, Nicoletti A, Lichtman A, Wagner D, Croce KJ, Libby P. Roles of PAD4 and NETosis in Experimental Atherosclerosis and Arterial Injury: Implications for Superficial Erosion. Circ Res, 2018; 123: 33-42
- 7) Franck G, Mawson T, Sausen G, Salinas M, Masson GS, Cole A, Beltrami-Moreira M, Chatzizisis Y, Quillard T, Tesmenitsky Y, Shvartz E, Sukhova GK, Swirski FK, Nahrendorf M, Aikawa E, Croce KJ, Libby P. Flow Perturbation Mediates Neutrophil Recruitment and Potentiates Endothelial Injury via TLR2 in Mice: Implications for Superficial Erosion. Circ Res, 2017; 121: 31-42
- 8) Folco EJ, Mawson TL, Vromman A, Bernardes-Souza B, Franck G, Persson O, Nakamura M, Newton G, Luscinskas FW, Libby P. Neutrophil Extracellular Traps Induce Endothelial Cell Activation and Tissue Factor Production Through Interleukin-1alpha and Cathepsin G. Arterioscler Thromb Vasc Biol, 2018; 38: 1901-1912
- 9) Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J, Virmani R. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation, 1996; 93: 1354-1363
- 10) Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol, 2000; 20: 1262-1275
- 11) Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest, 2015; 125: 4514-4528
- 12) Kovacic JC, Dimmeler S, Harvey RP, Finkel T, Aikawa E, Krenning G, Baker AH. Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review. J Am Coll Cardiol, 2019; 73: 190-209
- 13) Lee DY, Chiu JJ. Atherosclerosis and flow: roles of epigenetic modulation in vascular endothelium. J Biomed Sci, 2019; 26: 56
- 14) Wang M, Ngo V, Wang W. Deciphering the genetic code of DNA methylation. Brief Bioinform, 2021; 22
- 15) Parry A, Rulands S, Reik W. Active turnover of DNA methylation during cell fate decisions. Nat Rev Genet, 2021; 22: 59-66
- 16) Dunn J, Qiu H, Kim S, Jjingo D, Hoffman R, Kim CW, Jang I, Son DJ, Kim D, Pan C, Fan Y, Jordan IK, Jo H. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest, 2014; 124: 3187-3199
- 17) Warboys CM, de Luca A, Amini N, Luong L, Duckles H, Hsiao S, White A, Biswas S, Khamis R, Chong CK, Cheung WM, Sherwin SJ, Bennett MR, Gil J, Mason JC, Haskard DO, Evans PC. Disturbed flow promotes endothelial senescence via a p53-dependent pathway. Arterioscler Thromb Vasc Biol, 2014; 34: 985-995
- 18) Dardik A, Chen L, Frattini J, Asada H, Aziz F, Kudo FA, Sumpio BE. Differential effects of orbital and laminar shear stress on endothelial cells. J Vasc Surg, 2005; 41: 869-880
- 19) Huang T-S, Wang K-C, Quon S, Nguyen P, Chang T-Y, Chen Z, Li Y-S, Subramaniam S, Shyy J, Chien S. LINC00341 exerts an anti-inflammatory effect on endothelial cells by repressing VCAM1. Physiological Genomics, 2017; 49: 339-345
- 20) Ajami NE, Gupta S, Maurya MR, Nguyen P, Li JY-S, Shyy JYJ, Chen Z, Chien S, Subramaniam S. Systems biology analysis of longitudinal functional response of endothelial cells to shear stress. Proceedings of the National Academy of Sciences, 2017; 114: 10990-10995
- 21) Maurya MR, Gupta S, Li JY-S, Ajami NE, Chen ZB, Shyy JYJ, Chien S, Subramaniam S. Longitudinal shear stress response in human endothelial cells to atheroprone and atheroprotective conditions. Proceedings of the National Academy of Sciences, 2021; 118
- 22) Warboys CM, Ghim M, Weinberg PD. Understanding mechanobiology in cultured endothelium: A review of the orbital shaker method. Atherosclerosis, 2019; 285: 170-177
- 23) Patschan D, Schwarze K, Henze E, Patschan S, Muller GA. The endothelial-to-mesenchymal transition and endothelial cilia in EPC-mediated postischemic kidney protection. Am J Physiol Renal Physiol, 2016; 310: F679-F687
- 24) Chandran Latha K, Sreekumar A, Beena V, S SB, Lakkappa RB, Kalyani R, Nair R, Kalpana SR, Kartha CC, Surendran S. Shear Stress Alterations Activate BMP4/pSMAD5 Signaling and Induce Endothelial Mesenchymal Transition in Varicose Veins. Cells, 2021; 10
- 25) Fernandez Esmerats J, Villa-Roel N, Kumar S, Gu L, Salim MT, Ohh M, Taylor WR, Nerem RM, Yoganathan AP, Jo H. Disturbed Flow Increases UBE2C (Ubiquitin E2 Ligase C) via Loss of miR-483-3p, Inducing Aortic Valve Calcification by the pVHL (von Hippel-Lindau Protein) and HIF-1alpha (Hypoxia-Inducible Factor-1alpha) Pathway in Endothelial Cells. Arterioscler Thromb Vasc Biol, 2019; 39: 467-481
- 26) Xu Y, Kovacic JC. Endothelial to Mesenchymal Transition in Health and Disease. Annu Rev Physiol, 2023; 85: 245-267
- 27) Boon RA, Fledderus JO, Volger OL, van Wanrooij EJ, Pardali E, Weesie F, Kuiper J, Pannekoek H, ten Dijke P, Horrevoets AJ. KLF2 suppresses TGF-beta signaling in endothelium through induction of Smad7 and inhibition of AP-1. Arterioscler Thromb Vasc Biol, 2007; 27: 532-539
- 28) Jiang Y, Shen Q. IRF2BP2 prevents ox-LDL-induced inflammation and EMT in endothelial cells via regulation of KLF2. Exp Ther Med, 2021; 21: 481
- 29) Liang G, Wang S, Shao J, Jin YJ, Xu L, Yan Y, Gunther S, Wang L, Offermanns S. Tenascin-X Mediates Flow-Induced Suppression of EndMT and Atherosclerosis. Circ Res, 2022; 130: 1647-1659
- 30) Ma J, Sanchez-Duffhues G, Goumans MJ, Ten Dijke P. TGF-beta-Induced Endothelial to Mesenchymal Transition in Disease and Tissue Engineering. Front Cell Dev Biol, 2020; 8: 260
- 31) Testai L, Brancaleone V, Flori L, Montanaro R, Calderone V. Modulation of EndMT by Hydrogen Sulfide in the Prevention of Cardiovascular Fibrosis. Antioxidants (Basel), 2021; 10
- 32) Andueza A, Kumar S, Kim J, Kang DW, Mumme HL, Perez JI, Villa-Roel N, Jo H. Endothelial Reprogramming by Disturbed Flow Revealed by Single-Cell RNA and Chromatin Accessibility Study. Cell Rep, 2020; 33: 108491
- 33) Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr., Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest, 2006; 116: 49-58
- 34) Dekker RJ, Boon RA, Rondaij MG, Kragt A, Volger OL, Elderkamp YW, Meijers JC, Voorberg J, Pannekoek H, Horrevoets AJ. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood, 2006; 107: 4354-4363
- 35) Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerzsten RE, Edelman ER, Jain MK. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem, 2007; 282: 13769-13779
- 36) Yori JL, Seachrist DD, Johnson E, Lozada KL, Abdul-Karim FW, Chodosh LA, Schiemann WP, Keri RA. Kruppel-like factor 4 inhibits tumorigenic progression and metastasis in a mouse model of breast cancer. Neoplasia, 2011; 13: 601-610
- 37) Yori JL, Johnson E, Zhou G, Jain MK, Keri RA. Kruppel-like factor 4 inhibits epithelial-to-mesenchymal transition through regulation of E-cadherin gene expression. J Biol Chem, 2010; 285: 16854-16863
- 38) He L, Zhang CL, Chen Q, Wang L, Huang Y. Endothelial shear stress signal transduction and atherogenesis: From mechanisms to therapeutics. Pharmacol Ther, 2022; 235: 108152
- 39) Wang X, Shen Y, Shang M, Liu X, Munn LL. Endothelial mechanobiology in atherosclerosis. Cardiovasc Res, 2023; 119: 1656-1675
- 40) Zhang C, Zhou T, Chen Z, Yan M, Li B, Lv H, Wang C, Xiang S, Shi L, Zhu Y, Ai D. Coupling of Integrin alpha5 to Annexin A2 by Flow Drives Endothelial Activation. Circ Res, 2020; 127: 1074-1090
- 41) Wu B, Wang Y, Lui W, Langworthy M, Tompkins KL, Hatzopoulos AK, Baldwin HS, Zhou B. Nfatc1 coordinates valve endocardial cell lineage development required for heart valve formation. Circ Res, 2011; 109: 183-192