2013 Volume 82 Issue 3 Pages 242-254
2013 Volume 82 Issue 3 Pages 242-254
In senescing carnation (Dianthus caryophyllus L.) flowers, ethylene production begins in the gynoecium, and the resulting ethylene acts on petals, inducing autocatalytic ethylene production. We investigated the role of abscisic acid (ABA) in ethylene production in the gynoecium of flowers. First, cDNAs of major genes involved in ABA biosynthesis and signaling were cloned from carnation flower tissues. Then, changes in ABA content and gene expression of ABA biosynthesis and signaling in the ovary were examined using three cultivars, ‘Light Pink Barbara (LPB)’ and ‘Excerea’, whose cut flowers produce ethylene during senescence and have an ordinary vase-life of about one week, and ‘Miracle Rouge’, whose cut flowers produce no detectable ethylene during senescence and have a vase-life of about three weeks. ABA content in the ovary was 530–710 pmol·g−1 fresh weight (FW) from Os 2 (early opening stage) to Os 6 (end of opening stage) in ‘LPB’, and at 200–380 pmol·g−1 FW in ‘Excerea’ at the same stages; but 930 pmol·g−1 FW at Ss 1 (early senescence stage). The ABA content remained at 70– 160 pmol·g−1 FW in ‘Miracle Rouge’. The changes in ABA content were in parallel with the transcript levels of DcNCED1 (carnation gene for 9-cis-epoxycarotenoid dioxygenase). DcPYR1 (ABA receptor gene) transcript was 0.004–0.007 relative expression level (r.e.l.) in ‘LPB’ ovary at Os 1–Os 3, and 0.028 r.e.l. at Ss 1 (beginning of senescence stage). In ‘Excerea’ ovary, DcPYR1 transcript was 0.025–0.037 r.e.l. during flower opening and higher at Ss 1. By contrast, DcPYR1 transcript remained at 0.002–0.006 r.e.l. in ‘Miracle Rouge’ ovary during flower opening and senescence. The transcripts of DcACS1, the key gene for ethylene biosynthesis, were detected at Ss 1 in ‘LPB’, and at Ss 2 in ‘Excerea’, but not in ‘Miracle Rouge’ throughout flower opening and senescence stages. These findings suggest that ABA plays a causal role in inducing the expression of the DcACS1 gene in the gynoecium, leading to ethylene biosynthesis, and that both the ABA content and DcPYR1 expression must be above putative threshold levels for ABA to exert its action.
Ethylene is a primary plant hormone involved in the senescence of cut carnation flowers (Abeles et al., 1992; Borochov and Woodson, 1989; Reid and Wu, 1992; Satoh, 2011). In flowers undergoing natural senescence, ethylene is first produced from the gynoecium and induces autocatalytic ethylene production in petals (Shibuya et al., 2000; ten Have and Woltering, 1997). Eventually, ethylene produced in the petals accelerates in-rolling of the petals, resulting in wilting of the flowers (Manning, 1985; Peiser, 1986; Woodson et al., 1992); therefore, the gynoecium plays a causal role in controlling petal senescence.
In carnation flowers, ethylene is synthesized through the following pathway, the same as that in other plants: L-methionine → S-adenosyl-L-methionine → 1-aminocyclopropane-1-carboxylate (ACC) → ethylene. ACC synthase and ACC oxidase catalyze the last two reactions. So far, three genes encoding ACC synthase (DcACS1, DcACS2, and DcACS3) and a gene encoding ACC oxidase (DcACO1) have been identified from carnation (Henskens et al., 1994; Jones and Woodson, 1999; Park et al., 1992). These genes are expressed in a tissue-specific manner in senescing carnation flowers; DcACO1 and DcACS1 are expressed in both the gynoecium and petals of carnation flowers, whereas DcACS2 and DcACS3 are expressed in the gynoecium but not as much as DcACS1 (Henskens et al., 1994; Jones and Woodson, 1999; ten Have and Woltering, 1997). DcACO1 is expressed in both the gynoecium and petals of carnation flowers undergoing senescence (Park et al., 1992). Nukui et al. (2004) investigated the expression of the genes in flowers of ‘White Candle’ carnation, whose flowers produce little ethylene and have a longer vase-life. ‘White Candle’ flowers had transcripts of DcACS3 and DcACO1, but not that of DcACS1 in the gynoecium, suggesting that DcACS1 expression plays a regulatory role in ethylene production in the gynoecium of this cultivar. Now it is thought that DcACS1 and DcACO1 are key genes for ethylene biosynthesis in the gynoecium and petals of senescing carnation flowers.
Ethylene production is induced by pollination in the carnation gynoecium (Jones and Woodson, 1997; Nichols, 1977); however, many carnation cultivars do not have anthers and cannot be pollinated by their own pollen. They nonetheless show an increase in ethylene production in the gynoecium, probably due to factors other than pollination. So far, however, few studies have been carried out on the mechanism of ethylene production in the gynoecium without pollination.
Exogenously-applied ABA accelerated the senescence of cut carnation flowers through the stimulation of ethylene biosynthesis (Mayak and Dilley, 1976a, b; Nowak and Veen, 1982; Ronen and Mayak, 1981). Nowak and Veen (1982) reported that ABA content increased transiently in the gynoecium of cut carnation flowers after harvest, reaching a maximum content before the surge in ethylene production in the flowers, whereas in the petals it increased steadily, undergoing a significant rise concomitant with the surge of ethylene production. Onoue et al. (2000) confirmed that during the natural senescence of cut carnation flowers, ABA accumulated in the gynoecium and petals before the onset of ethylene production in the flowers, and showed that application of exogenous ABA promoted ethylene production in the flowers. Moreover, Shibuya et al. (2000) showed that ABA applied from the cut stem end of carnation flowers stimulated ethylene production in flowers with the gynoecia left intact, but not in flowers whose gynoecia were removed, indicating that ABA action was expressed in the gynoecia but not in the petals. These results suggested that ABA is a crucial factor in the induction of ethylene biosynthesis in carnation flowers.
In plants, ABA is synthesized through the carotenoid (C40) pathway (Fig. 1). In this pathway, zeaxanthin is converted to all-trans-violaxanthin via antheraxanthin by two epoxidation reactions mediated by zeaxanthin epoxidase (ZEP). All-trans-violaxanthin is then converted to two 9-cis-epoxycarotenoids, 9′-cis-neoxanthin and 9-cis-violaxanthin. Two 9-cis-epoxycarotenoids are cleaved by 9-cis-epoxycarotenoid dioxygenase (NCED) to form a C15 precursor, xanthoxin. This reaction has been recognized as the rate-determining step in ABA biosynthesis in plants (Burbidge et al., 1999; Iuchi et al., 2001; Tan et al., 1997; Wang et al., 2011; Zhu et al., 2011). ABA content in plant tissues is also regulated by catabolism mediated by ABA 8′-hydroxylases (Cytochrome P450 CYP707A enzymes) (Kushiro et al., 2004). The enzyme mediates the hydroxylation of ABA at C-8′ producing 8′-hydroxy ABA, which is then spontaneously isomerized to form phaseic acid (Cutler and Krochko, 1999).

Pathway for ABA biosynthesis, catabolism, and action. ZEP, zeaxanthin epoxidase; NCED, 9-cis-epoxycarotenoid dioxygenase; CYP707A, ABA 8′-hydroxylase; PYR/PYL/RCAR, ABA receptor; PP2C, type 2C protein phosphatase; SnRK2, subfamily 2 of SNF1-related kinase; AREBF/ABF, ABA responsive element binding factor.
In the ABA signal transduction pathway, core components are ABA receptor (PYLs: PYR/PYL/RCAR) (Ma et al., 2009; Melcher et al., 2009), negative regulators (PP2Cs: type 2C protein phosphatases), and positive regulators (SnRK2s: subfamily 2 of SNF1-related kinases). In this pathway, PYLs (ABA receptors) bind ABA to form a complex (Ma et al., 2009; Melcher et al., 2009; Nishimura et al., 2009; Park et al., 2009), then the complex inhibits PP2C from dephosphorylating SnRK2 (Fujii et al., 2009; Melcher et al., 2009; Yoshida et al., 2006). SnRK2 is activated and phosphorylates downstream effectors, thus switching on ABA-responsive genes such as AREBFs (ABA responsive element binding factor) (Furihata et al., 2006; Kobayashi et al., 2005). Sun et al. (2011) recently identified many genes homologous to PYL, PP2C, and SnRK2 in tomato and characterized the possible transcriptional regulation of the genes in tomato fruit development.
In the present study, we aimed to obtain further support for the role of abscisic acid (ABA) in ethylene production in the gynoecium of carnation by examining ABA content and gene expression of ABA biosynthesis and signaling. As the first step towards those studies, we cloned and characterized the cDNAs for ZEP, NCED, and CYP707A in the ABA biosynthesis and catabolism pathway, and those of PYR, PP2C, SnRK2, and AREBF in the ABA perception, signal transduction, and action pathway in carnation (Dianthus caryophyllus ‘Light Pink Barbara’) flowers. Then, we examined changes in the ABA content and expression of these genes in different floral tissues during opening and senescence of the flowers, using three cultivars, ‘Light Pink Barbara’ and ‘Excerea’, with ordinary ethylene production and senescence characteristics, and ‘Miracle Rouge’ with undetectable ethylene production and long-vase life of flowers. The ‘Miracle Rouge’ cultivar was recently generated by cross-breeding at NARO Institute for Floriculture Science (Onozaki et al., 2006).
Cut flowers of the spray carnation (Dianthus caryophyllus L. ‘Light Pink Barbara’) were harvested when the first flower out of five to six flower buds on a flower stem was nearly open at the nursery of a commercial grower in Miyagi prefecture. The flowers were transported dry to the laboratory at the Kyoto Prefectural Institute of Agricultural Biotechnology in Kyoto prefecture the day after harvest. On arrival, the flowers were placed in plastic buckets with their cut end in water under continuous light from white fluorescent lamps (14 μmol·m−2·s−1) at 23°C. Depending on the season the experiments were carried out, flowers of the same cultivar were obtained similarly from commercial nurseries in Hyogo and Nagano prefectures.
The flowers of standard carnation cultivars, ‘Excerea’ and ‘Miracle Rouge’, which have only one flower on each stem, were harvested at the nursery of NARO Institute of Floricultural Science, Tsukuba, Japan, and used for experiments at the Institute, similarly to the method described above.
The flower-opening process was separated into 6 stages (Os 1 to Os 6) as described by Harada et al. (2010), and the flower senescence process into 4 stages (Ss 1 to Ss 4) as described previously (Morita et al., 2011). In senescence, the numbers of days after the full opening of flowers, which corresponded to Os 6, is shown in addition to senescence stages (Ss 1 to Ss 4) according to days after full opening of the flowers (Fig. 3).

Profiles of flower opening and senescence in (A) ‘Light Pink Barbara’, (B) ‘Excerea’, and (C) ‘Miracle Rouge’ carnation. The floweropening process was separated into 6 stages (Os 1 to Os 6) according to Harada et al. (2010), and the flower senescence process into 4 stages (Ss 1 to Ss 4) according to Morita et al. (2011). Os 6 and Ss 1 can not be separated morphologically, but the two stages are shown separately for convenience. Senescence progress varied with the cultivar; therefore, days after the full opening of flowers (Os 6) are shown below the respective photographs. Bar shows 1 cm.
Ten outermost petals each were detached from 5 flowers at each stage, combined and mixed (making a sample of 50 petals) and stored at −80°C for extraction of RNA. The number of petals used for specific experiments were as designated. Different tissues, i.e., leaves, stem, calyx, ovary, and style were detached from fully open flowers of ‘Light Pink Barbara’ and stored as above. Here, style refers to the stigma plus style, considering its structure in carnation.
RNA extractionTotal RNA was isolated from floral tissues of ‘Light Pink Barbara’ carnation by the phenol-chloroform method. Samples of about 1 g frozen petals were pulverized in liquid N2 with a mortar and pestle, and extracted with 5 ml extraction buffer [1% SDS, 50 mM Tris-HCl (pH7.5), 50 mM EDTA] plus an equal volume of water-saturated phenol. Total RNA was recovered in the supernatant after centrifugation, purified by treatment with phenol-chloroform solution (1:1, v/v) followed by chloroform, and finally precipitated with isopropanol at −20°C. Later, total RNA was extracted with an RNeasy Plant Mini kit (Qiagen, Hilden, Germany) from floral tissues of ‘Excerea’ and ‘Miracle Rouge’.
PCR cloning of cDNAs of genes for ABA biosynthesis, catabolism, signaling, and actionTo obtain full-length composite cDNAs for ABA biosynthesis (ZEP and NCED), catabolism (CYP707A), signaling (PYR, PP2C, and SnRK2), and action (AREBF), we used a nearly identical strategy in which three partial-length cDNAs were obtained by ordinary RT-PCR, 5′-RACE (rapid amplification of cDNA end), and 3′-RACE techniques (Frohman et al., 1988). The cDNAs were combined to make composite cDNAs. Then, to confirm complete nucleotide sequences, full-length cDNAs were amplified with primers derived from both ends of the composite cDNAs and total RNAs obtained from carnation petals. The forward- and reverse-primer pairs for PCR to obtain partial length cDNAs and sizes (in base pairs) of amplificates are summarized in Table 1.
| Genes, purpose and target | Foward (primer name; sequence, 5′ to 3′) | Reverse (primer name; sequence, 5′ to 3′) | Amplificate (bp) |
|---|---|---|---|
| PCR cloning (First round PCR) | |||
| DcNCED1a/b and DcNCED2a/b | NCED-F; TTYGAYGGNGAYGGNATGGTNCA | NCED-R; TCCCANGCRTTCCANARRTGRAA | 743 |
| DcZEP1 | ZEP-F; AARATGCARTGGTAYGCNTTYTA | ZEP-R; GCRTCRTCRTCYTCRAACCAYTT | 668 |
| DcCYP707 | CYP707A-F; TYTTYTTYCAYCARGGWGAYTAYCAT | CYP707A-R; CRAAWGGCATRAANGTRTTSGGYTT | 876 |
| DcPYR1 | PYR-F; TTYGAYCARCCNCARAARTAYAARCC | PYR-R; ACRAARTARCANGTYTCRTCYTTNG | 320 |
| DcPP2C | PP2C-F; AATTGYGGYGAYTCHMGDGCNGTKC | PP2C-R; ACRTCCCAHAVNCCRTCRCTHGC | 284 |
| DcSnRK2.1 and DcSnRK2.2 | SNRK2-F; GTNATGGARTATGCWKCTGGHGGWGARC | SNRK2-R; GRTCYTCRAANGGRTAHGCTCCMAC | 382/380 |
| DcAREBF1 | AREBF-F; ATHTGGAAYGCNGARGARAAYCARAC | AREBF-R; TTCATCATYTCDATNACYTGRTTYTTYTGC | 983 |
| RT-PCR amplification | |||
| DcNCED1a/b | DcNCED1-rF1; CACTCTCAAGTCTCAACACACACATTC | DcNCED1-rR1; CAGAAACAAGCTGAGCTTCGATCC | 223/216 |
| DcNCED2a/b | DcNCED2-rF1; GCTGAAGTTCAGTGTTGATGGGTAG | DcNCED2-rR1; GGTGTCTCGGGGACGTATTC | 194/194 |
| DcZEP1 | DcZEP1-rF1; GACAGAAAGGTAGCGTTCCGAG | DcZEP1-rR1; CCATTGTATGAATGAAGTTGCAACTATAGC | 229 |
| DcCYP707A1 | DcCYP707A1-rF1; GACTCGAATAATTTCACCCCGTAATCG | DcCYP707A1-rR1; GAGCCTGCTGTACAATTGCTGTG | 190 |
| DcPYR1 | DcPYR1-rF1; CCAAAAGCTTTGTGGTCTCGTGC | DcPYR1-rR1; GATACCGACGAATACTAATTACACGACTC | 197 |
| DcPP2C | DcPP2C1-rF1; GACCTCATGTTGCAATCTTATCTCCC | DcPP2C1-rR1; CTGCAAGGTAAAAAGCGTTTCAAAAGACG | 159 |
| DcSnRK2.1 | DcSNRK2.1-rF1; CTTCTGTTCGACCTCCGTTTCTC | DcSNRK2.1-rR1; CATAGTACGTAGTAGTACTTACTGAGTAG | 297 |
| DcSnRK2.2 | DcSNRK2.2-rF1; CTGCTGATGGTAATTACATAAATGTTCGG | DcSNRK2.2-rR1; GGCAAAATGCCACGATTTCGAGG | 154 |
| DcAREBF1 | DcAREBF1-rF1; GTTGGATAGTTTACGATGAGTGGACTAC | DcAREBF1-rR1; GTCCTCGGAAGACTGACGTTTACAAG | 207 |
| DcACS1 | DcACS1-rF1; CGAGGGCAAATGGAGCTTCGA | DcACS1-rR1; CATCTGAATGTGGTCTTTCACACCG | 233 |
| DcACO1 | DcACO1-rF1; CGGGTCCCATTCCAACTGCTT | DcACO1-rR1; GTAGACCATACAATCCATAGGACATGG | 209 |
| DcUbq3-7 | Ubq-rF; GCTCCATCTGTCTGTGGTTGTTG | Ubq-rR; GAGAATTCACACCGAAATGTAGCAGC | 183 |
| Real-time RT-PCR analysis* | |||
| DcNCED1a/b | DcNCED1-rF1; CACTCTCAAGTCTCAACACACACATTC | DcNCED1-rR1; CAGAAACAAGCTGAGCTTCGATCC | 223/216 |
| DcNCED2a/b | DcNCED2-rF1; GCTGAAGTTCAGTGTTGATGGGTAG | DcNCED2-rR1; GGTGTCTCGGGGACGTATTC | 194 |
| DcPYR1 | DcPYR1-rF1; CCAAAAGCTTTGTGGTCTCGTGC | DcPYR1-rR1; GATACCGACGAATACTAATTACACGACTC | 197 |
| DcUbq3-7 | Ubq3F; GTTGTTGGTTTCAGGGCTGGTTTG | Ubq3R; CTACGGTAATTGAGAATTCACACCGAAATG | 178 |
Detailed procedures for PCR cloning are shown below with DcNCED1 and DcNCED2 cDNAs as examples. To clone DcNCED1 and DcNCED2 cDNAs, we initially amplified partial length cDNAs (743 bp) by RT-PCR using total RNA obtained from opening carnation petals and forward (NCED-F) and reverse (NCED-R) primers derived from sequences of NCED cDNAs reported in public databases. RT-PCR was performed according to the standard procedure with necessary optimization. The PCR products were cloned into pGEM-T Easy Vector (Promega, Madison, WI, USA) for sequencing, and two distinct nucleotide sequences were obtained. Using one of the two nucleotide sequences, sequence-specific primers were designed. Then, upstream cDNA of 713 bp (it was revealed later that this was a mixture of 713 bp and 695 bp) was obtained by 5′-RACE with the forward 5′RACE primer and the reverse DcNCED1-R1 primer, and downstream cDNA of 1019 bp (similarly as above, a mixture of 1019 bp and 1013 bp) (including polyA sequence) by 3′-RACE with the DcNCED1-F1 primer and the 3′RACE primer. The three partial cDNAs were reconstituted to make composite cDNA (DcNCED1) of ca. 2200 bp. On the other hand, using another nucleotide sequence, upstream cDNA of 1342 bp was obtained by 5′-RACE with the forward 5′RACE primer and the reverse DcNCED2-R1, and also the downstream cDNA of 813 bp (including polyA sequence) by 3′-RACE with the forward DcNCED2-F1 primer and the reverse 3′RACE primer. The three partial cDNAs were reconstituted to make composite cDNA (DcNCED2) of ca. 2100 bp. Finally, full-length cDNAs for DcNCED1 and DcNCED2 were amplified from total RNAs with primers derived from both ends of the composite cDNAs.
During full-length nucleotide sequence determination of amplified cDNAs, we recognized the presence of two nucleotide sequences for DcNCED1 with minor differences in nucleotide sequences, and designated then DcNCED1a and DcNCED1b. This was true for DcNCED2, giving two cDNAs, DcNCED2a and DcNCED2b. Nucleotide sequences were edited and analyzed by GENETYX-WIN and a homology search was performed using the BLASTX program, available from the DDBJ website.
RT-PCRTotal RNAs were isolated from the respective tissues of carnation flowers as described above. Reverse transcription was performed with ReverTra Ace (Toyobo, Osaka, Japan) using an oligo dT primer. The cDNAs were amplified with Taq DNA polymerase using appropriate primers (Table 1). The conditions were 96°C for 3 min followed by 38 cycles of 30 s at 96°C, 30 s at 53°C, and 1 min at 72°C. DcUbq3-7 cDNAs (Nomura et al., 2012) were also amplified as a standard under the conditions of 94°C for 3 min followed by 36 cycles of 30 s at 94°C, 30 s at 53°C, and 1 min at 72°C. The amplificates were separated by agarose gel electrophoresis, stained with ethidium bromide and photographed.
Real-time RT-PCRReal-time RT-PCR for the quantification of transcripts of DcNCED1a/b, DcNCED2a/b, and DcPYR1 was conducted using the LightCycler FastStart DNA Master SYBR Green I Kit and the LightCycler Instrument (Roche, Basel, Switzerland). The two isoforms of DcNCED1, DcNCED1a and DcNCED1b, and those of DcNCED2, DcNCED2a and DcNCED2b, were difficult to analyze separately by real-time RT-PCR. Therefore, we analyzed the mixture of transcripts of DcNCED1a and DcNCED1b as DcNCED1a/b transcripts, and that of DcNCED2a and DcNCED2b as DcNCED2a/b transcripts. cDNA was synthesized using ReverTra Ace according to the manufacturer’s instructions. Primer sets for cDNAs of respective genes and the sizes of amplificates are shown in Table 1. PCR conditions were initial heating for 10 min at 95°C followed by 40 cycles of 5 s at 95°C, 5 s at 53°C, 10 s at 72°C, in which the extension time varied depending on the length of amplificates (1 s per 25 mer). The absolute transcript level was calculated using the dilution series of a target sequence on LightCycler Software Ver. 3.5. Then, relative expression levels were calculated on the basis of DcUbq3-7 transcripts levels by dividing the transcript copy number of respective genes by that of DcUbq3-7 transcripts. Three RNA preparations per stage, obtained from three independent ovary samples at each stage as described above, were used for analyses, and data are shown as the mean ± SE of three independent preparations for each stage.
Assay of ABA in flower tissuesThe cut carnation flowers were placed with their cut stem ends in distilled water and left under the controlled environmental conditions described above. Flowers were observed for the development of petal opening and in-rolling symptoms once a day. Three flowers at the given opening or senescence stages were sampled, and the ovary and one petal in the outermost whorl were detached from their respective flowers, making 3 independent samples. The style, receptacle, and calyx tissues were sampled when necessary. They were weighed and stored at −80°C for determination of ABA content.
Extraction and assay of ABA were performed according to Dobrev and Kaminek (2002) with necessary modifications. Briefly, fresh or frozen tissues (50–100 mg) were pulverized in liquid N2, and ABA was extracted with methanol/water/formic acid (15 : 4 : 1, v/v/v). A known amount of [2H6](+)-2-cis, 4-trans-2H-ABA (OlChemIm Ltd., Olomouc, Czech Republic) was added to the extract as an internal standard. The extract was evaporated to an aqueous solution, and ABA was purified by passage through a Sep-Pak Plus C18 cartridge (400 mg sorbent; Waters Co., Milford, MA, USA) followed by elution with methanol/water/formic acid (15 : 4 : 1, v/v/v), and the eluate was dried by evaporation and the sample was taken up in 1M formic acid. Then the sample was applied to an Oasis MCX column (150 mg sorbent; Waters Co.), and eluted with l00% methanol. After evaporation of the methanol, the sample was dissolved again in 100% methanol. The sample was applied to a Bond Elut DEA cartridge (500 mg sorbent; Agilent Technologies Inc., Santa Clara, CA, USA), and eluted with 0.2% acetic acid in methanol. After evaporation of the solution, the sample was taken up in 100% methanol, and passed through Sephadex G-25 (GE Healthcare UK Ltd., Little Chalfont, Buckinghamshire, UK) to remove impurities. The eluate was concentrated under vacuum and finally taken up into 100 μl of 20% methanol. Portions of the solutions were analyzed for ABA by LC/MS/MS (UPLC/MS/MS Quattro premier XE, Waters Co.; column, ACQUITY UPLC BEH C18, 2.1 mm i.d. × 50 mm; column temperature 40°C; flow rate 0.2 mL·min−1) with a step-wise gradient elution with methanol containing 0.5% acetic acid (20 to 80% methanol from 0 to 5 min, 80 to 100% from 5 to 5.01 min, 100% from 5.01 to 6 min and 100 to 20% from 6 to 6.5 min). The LC/MS/MS instrument was operated at cone voltage 30 eV and collision energy 30 eV in negative ESI mode. The content of ABA in the given tissues is shown as the mean ± SE of 3 replications from 3 independent flowers.
Statistical analysisStatistical analysis was carried out by Tukey’s multiple range test using an on-line statistical analysis program MEPHAS (http://www.gen-info.osaka-u.ac.jp/testdocs/tomocom/, April 10, 2013).
cDNAs of putative genes for ABA biosynthesis (four NCEDs and one ZEP), signaling (one PYR, one PP2C, and two SnRK2s), and action (one AREBF) were isolated from total RNAs obtained from opening petals of ‘Light Pink Barbara’ using a combination of RT-PCR, 5′-RACE and, 3′-RACE techniques. However, in the case of a cDNA encoding CYP707A (an enzyme for ABA catabolism), the initial partial length PCR-amplificate was obtained from the total RNA obtained from the gynoecium of opening ‘Light Pink Barbara’ flowers. After composite cDNAs were obtained, their entire nucleotide sequences were confirmed by cloning full-length cDNAs by PCR and sequencing. We designated the original genes for isolated cDNAs as follows and deposited then in DDBJ; DcNCED1a and DcNCED1b, and DcNCED2a and DcNCED2b [accession numbers (acc. no.) AB750605–AB750608], DcZEP1 (acc. no. AB750609), DcCYP707A1 (acc. no. AB750610), DcPYR1 (acc. no. AB750611), DcPP2C1 (acc. no. AB750612), DcSnRK2.1 and DcSnRK2.2 (acc. no. AB750613 and AB750614), and DcAREBF1 (acc. no. AB750615).
Figure 2 summarizes the structures of isolated cDNAs, showing 5′-flanking (5′-UTR), ORF (open reading frame) and 3′-flanking (3′-UTR) sequences with nucleotide numbers. The predicted proteins were compared with counterparts from other plant sources by alignment of amino-acid sequences and phylogenic analysis to confirm their identity (data not shown).
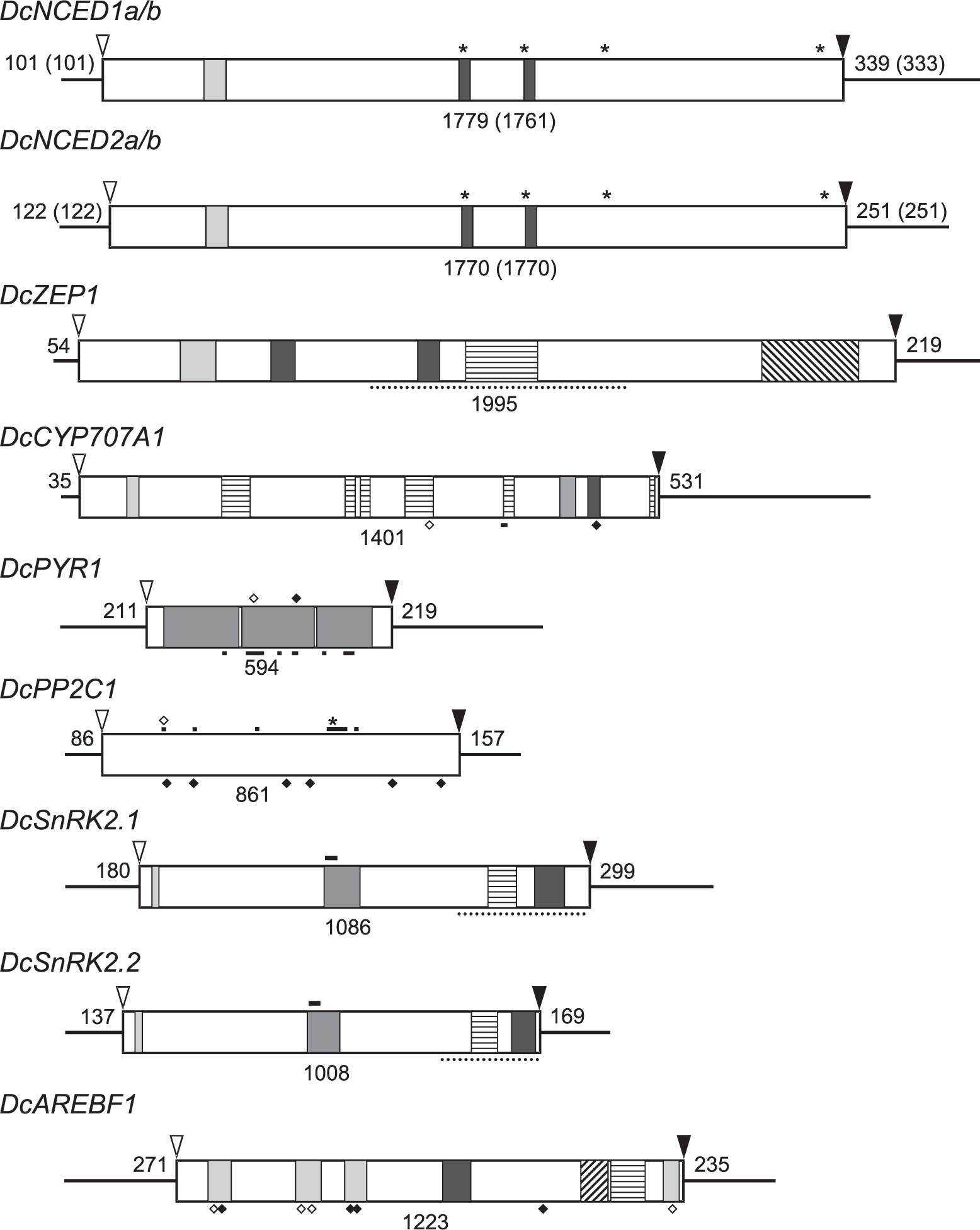
Structures of cDNAs of genes involved in ABA biosynthesis and action obtained from carnation flowers. Nucleotide numbers for the full-length region, 3′-UTR and 5′-UTR of each cDNA are shown in the diagrams. For DcNCED1a/b and DcNCED2a/b, the numbers in parentheses are those for 1b and 2b. Conserved amino acids, motif and regions in the deduced proteins are shown in the diagrams. For all diagrams:
 ATG start codon,
ATG start codon,
 stop codon (TGA for DcZEP1, DcPYR1, DcPP2C1 and DcAREBF1, and TAA for remaining others). DcNCED1a/b and DcNCED2a/b:
stop codon (TGA for DcZEP1, DcPYR1, DcPP2C1 and DcAREBF1, and TAA for remaining others). DcNCED1a/b and DcNCED2a/b:
 α-helix,
α-helix,
 featured regions of NCED, * iron-ligating histidines; DcZEP1:
featured regions of NCED, * iron-ligating histidines; DcZEP1:
 ADP binding region,
ADP binding region,
 lipocain conserved motif,
lipocain conserved motif,
 FAD binding region,
FAD binding region,
 FHA (Forkhead-associated) domain, ⋯ monooxygenase domain; DcCYP707A1:
FHA (Forkhead-associated) domain, ⋯ monooxygenase domain; DcCYP707A1:
 Pro rich region,
Pro rich region,
 aromatic region,
aromatic region,
 heme binding region, ♢ conserved Thr, ♦ proximal Cys, ─ K-region,
heme binding region, ♢ conserved Thr, ♦ proximal Cys, ─ K-region,
 SRS (substrate binding site); DcPYR1: conserved domain, ─ ABA interaction, ♢ Gate, ♦ Latch; DcPP2C1: ─ PYR/PYL/RCAR, ♦ Mn2+/Mg2+ binding sites, ♢ phosphate binding site, * ABA interaction site; DcSnRK2.1 and DcSnRK2.2,
SRS (substrate binding site); DcPYR1: conserved domain, ─ ABA interaction, ♢ Gate, ♦ Latch; DcPP2C1: ─ PYR/PYL/RCAR, ♦ Mn2+/Mg2+ binding sites, ♢ phosphate binding site, * ABA interaction site; DcSnRK2.1 and DcSnRK2.2,
 ATP interaction region,
ATP interaction region,
 activation loop,
activation loop,
 D/E rich domain acidic patch,
D/E rich domain acidic patch,
 Motif I, ─ phosphatase site, ⋯ regulatory domain; DcAREBF1:
Motif I, ─ phosphatase site, ⋯ regulatory domain; DcAREBF1:
 conserved region,
conserved region,
 glutamine-rich region,
glutamine-rich region,
 basic region,
basic region,
 Leu zipper region, ♢ R-X-X-S/T (target for CDPK), ♦ S/T-X-X-E/D (target for CK II).
Leu zipper region, ♢ R-X-X-S/T (target for CDPK), ♦ S/T-X-X-E/D (target for CK II).
In ‘Light Pink Barbara’, the flower-opening process was separated into 6 stages (Os 1 to Os 6), and the flower senescence process into 4 stages (Ss 1 to Ss 4) according to Harada et al. (2010) and Morita et al. (2011), respectively. Photographs of the respective stages are shown in Figure 3. Os 6 and Ss 1 could not be separated morphologically, but the two stages are shown separately for convenience in Figure 3. Ethylene production in whole ‘Light Pink Barbara’ flowers during senescence is shown in Morita et al. (2011). Also, we measured ethylene production from ‘Light Pink Barbara’ flowers at the respective stages, showing almost the same ethylene production profile as reported previously, and then used the flowers for determination of ABA content (data not shown).
Profile of opening and senescence of ‘Excerea’ was similar to that of ‘Light Pink Barbara’ (Fig. 3). ‘Excerea’ was Ss 2 at 6 days after Os 6; the progress of senescence was similar to that in ‘Light Pink Barbara’, Ss 2 at 5 days after Os 6. Cut ‘Excerea’ flowers started to produce ethylene 4 days after full opening of flowers and exhibited the highest ethylene production at day 6 (Onozaki et al., 2006); however, the progress of ethylene production in ‘Excerea’ flowers was delayed by a couple of days depending on harvest lots (Satoh et al., unpublished).
‘Miracle Rouge’ showed almost the same profile of flower opening as ‘Light Pink Barbara’ and ‘Excerea’, but a totally different senescence profile (Fig. 3). ‘Miracle Rouge’ did not show petal wilting even 18 days after Os 6. Although the flowers actually remained at Ss 1 during the senescence period, the flowers were separated into Ss 1 (3 days after Os 6), Ss 2 (6 days), Ss 3 (12 days), and Ss 4 (18 days) for convenience in the analyses shown below.
Changes in content of ABA in floral tissues of ‘Light Pink Barbara’, ‘Excerea’, and ‘Miracle Rouge’ during flower opening and senescenceABA contents during flower opening and senescence were determined (Fig. 4). In ‘Light Pink Barbara’, the ABA content was much higher in the ovary than in the petals, stigma + style, receptacle, and calyx. In the ovary, the ABA was 550 pmol·g−1 FW already at Os 2, and fluctuated thereafter, showing a maximum of 700 pmol·g−1 FW at Os 4 and minimum of 300 pmol·g−1 FW at Ss 2. The ABA content was < 70 pmol·g−1 FW in the stigma + style at Os 4 through Ss 2, and < 40 pmol·g−1 FW in petals at Os 2 through Ss 2, although they increased to about 200 pmol·g−1 FW at Ss 3 in both tissues. The ABA content was < 50 pmol·g−1 FW in the receptacles and calyx at Ss 2 and Ss 3.

Changes in the content of ABA in the floral tissues in (A) ‘Light Pink Barbara’, (B) ‘Excerea’, and (C) ‘Miracle Rouge’ flowers during flower opening and senescence. ■, ovary; ●, petal; ♦, stigma + style; ▲, receptacle; ▼, calyx. Data are the mean ± SE of three separate samples, but SEs are shown when they were bigger than the sizes of symbols. Vertical bars with different letters are significantly different by Tukey’s multiple range test (P < 0.05). Results of statistical analysis are shown only for the ovary and petals.
In ‘Excerea’, the ABA content was about twice as high in the ovary as in other tissues during the flower opening stage. In the ovary, the ABA content was 200 pmol·g−1 FW at Os 2, 370 and 380 pmol·g−1 FW at Os 4 and Os 6, respectively, 940 pmol·g−1 FW at Ss 2. On the other hand, in petals, the ABA content increased gradually from 100 pmol·g−1 FW at Os 2 to 220 pmol·g−1 FW at Ss 2. The ABA content was < 200 pmol·g−1 FW in the stigma + style, receptacle and calyx at Ss 2.
In the ovary of ‘Miracle Rouge’, the ABA content was 70 pmol·g−1 FW at Os 2 and gradually increased to 150 pmol·g−1 FW at Ss 4. In the petals, the ABA content remained < 50 pmol·g−1 FW during the opening stages, and slightly increased during the senescence stage, attaining 100 pmol·g−1 FW at Ss 4. In the stigma + style, receptacle and calyx, the ABA content was < 50 pmol·g−1 FW at Ss 2.
Preliminary screening by RT-PCR analysis of specific genes involved in ABA-related events in ‘Light Pink Barbara’, ‘Excerea’, and ‘Miracle Rouge’ flowers undergoing opening and senescenceFor this analysis, the expressions of DcACS1 and DcACO1, their translation products being the key enzymes in ethylene biosynthesis, in the ovary and petals of carnation flowers undergoing flower opening and senescence were investigated by reverse transcription (RT)-PCR (Fig. 5). The transcript level of DcUbq3-7 was almost constant throughout the stages and tissues, and was used to normalize the transcript level (Nomura et al., 2012).

RT-PCR analysis of the transcripts of genes for ABA-related events in ‘Light Pink Barbara’, ‘Excerea’, and ‘Miracle Rouge’ carnation. Ubiquitin (DcUbq 3-7) transcripts were used to ensure equal loading of DNA samples (Nomura et al., 2012).
In ‘Light Pink Barbara’, transcripts of genes responsible for ABA synthesis (DcZEP1, DcNCED1a/b, and DcNCED2a/b) and catabolism (DcCYP707A1) were detectable in both the ovary and petals at almost all stages of opening and senescence, although the transcript levels varied during flower opening and senescence. Transcripts of genes for ABA signaling (DcPP2C1 and DcSnRK2.1) and action (DcAREBF1) were detected in the ovary at nearly constant levels, except those of DcPYR1 and DcSnRK2.1, whose transcript levels were low from Os 1 to Os 4 and from Os 4 to Os 6, respectively. In petals, transcripts of genes for ABA signaling and action were detected at similar levels during flower opening and senescence. Of the genes for ethylene biosynthesis, DcACS1 and DcACO1, showed detectable transcript levels at Ss 2 and Ss 3 in the ovary, but DcACO1 transcript at Os 6 and DcACS1 at Ss 1 in the petal.
In ‘Excerea’, transcripts of DcZEP1, DcNCED1a/b, and DcNCED2a/b were detectable in the ovary during flower opening and senescence (Os1 to Ss 2). The transcript level of DcNCED1a/b was lower than that of DcNCED2a/b, but its level increased evidently at Ss 2. DcCYP707A1 transcript was detected during flower opening and senescence, but its level was high at Os 2 and Os 3. On the other hand, in the petals, the transcript of DcNCED2a/b was detected, but not those of DcZEP1, DcNCED1a/b, and DcCYP707A1 during flower opening and senescence. On the other hand, transcripts of DcPP2C1, DcSnRK2.1, DcSnRK2.2, and DcAREBF1 were detected in the ovary throughout flower opening and senescence, as in the petals. In the ovary, DcACS1 transcript became detectable at Ss 2, whereas DcACO1 transcript became faintly detectable at Os 1 through Ss 1, then markedly at Ss 2; however, transcripts of DcACS1 and DcACO1 were not detectable in the petals, even at Ss 2.
In ‘Miracle Rouge’, transcripts of DcZEP1, DcNCED2a/b, and DcCYP707A1 were detectable at various levels in the ovary during flower opening and senescence, whereas the transcript of DcNCED1a/b was faintly detectable in the ovary at Os 1 through Ss 2, although its level increased at Ss 3 and Ss 4. In the petals, only DcNCED2a/b transcript accumulated during flower opening. Transcripts of DcZEP1, DcNCED1a/b, and DcCYP707A1 became detectable in the petal at the late stage of senescence, 6 days after Os 6 for DcCYP707A1 and 18 days after Os 6 for DcZEP1 and DcNCED1a/b. Transcripts of genes for ABA signaling and action were detected in both the ovary and petals during flower opening and senescence, but the DcPYR1 transcript was not detected in the ovary at Os 1 through Ss 2. DcACS1 transcript was detected in a small amount in the ovary at Ss 3 and Ss 4, and that of DcACO1 at Ss 2 and Ss 3.
Real-time RT-PCR analysis of transcript levels of DcNCED1a/b, DcNCED2a/b, and DcPYR1 in the ovary during flower opening and senescenceIn ‘Light Pink Barbara’, the level of transcript of DcNCED1a/b in the ovary was ≃ 0.07 relative expression level (r.e.l.) at Os 1 and Os 2, decreased gradually until the late stages of flower opening, and increased at Ss 2 and Ss 3 (Fig. 6A). In ‘Excerea’, it remained at 0.02 r.e.l. with little fluctuation at Os 1 through Os 6, and then increased markedly at Ss 1 and Ss 2. In ‘Miracle Rouge’, the transcript level of DcNCED1a/b was negligible at Os 1 through Ss 1, although it increased at Ss 3 and Ss 4.

Real-time RT-PCR analysis of the expression of (A) DcNCED1a/b and (B) DcNCED2a/b genes in the ovary of ‘Light Pink Barbara’, ‘Excerea’, and ‘Miracle Rouge’ flowers during flower opening and senescence. Relative expression levels were calculated by dividing the transcript copy number of respective genes with that of DcUbq3-7 transcripts. Data are the means ± SE of three separate samples. Empty bars, ‘Light Pink Barbara’; Grey bars, ‘Excerea’, Enclosed bars, ‘Miracle Rouge’. Vertical bars with different letters are significantly different by Tukey’s multiple range test (P < 0.05). Statistical analysis was conducted separately for each of three cultivars, and the results are shown by small letters without and with single and double apostrophes, respectively. At the given flower-opening and senescence stages, where no histograms are shown, analysis was not conducted.
In ‘Light Pink Barbara’, the transcript level of DcNCED2a/b was 0.03 r.e.l. at Os 1, became 0.06–0.08 r.e.l. at Os 2 through Os 4, then declined to the initial level and remained at a similar level until Ss 3 (Fig. 6B). In ‘Excerea’, it remained at 0.02–0.04 r.e.l. during flower opening and senescence. Similarly, in ‘Miracle Rouge’, it remained at 0.01–0.02 r.e.l. during flower opening and senescence, although it increased at Ss 3.
Figure 7 shows changes in the transcript level of DcPYR1 in the ovary during flower opening and senescence. In ‘Light Pink Barbara’, it was 0.004 r.e.l. at Os 1, increased gradually to Os 3, increased rapidly until Ss 1 attaining 0.029 r.e.l. at Ss 1, and then remained at the elevated level thereafter. In ‘Excerea’, it was as high as 0.025 r.e.l. at Os 1 and maintained the high level of 0.03–0.04 r.e.l. until Ss 2. In ‘Miracle Rouge’, it was 0.002 r.e.l. at Os 1, remained at almost the same low level until Ss 2, and then increased slightly at Ss 3 and Ss 4.

Real-time RT-PCR analysis of the expression of the DcPYR1 gene in the ovary of ‘Light Pink Barbara’, ‘Excerea’, and ‘Miracle Rouge’ flowers during flower opening and senescence. Data presentation is the same as that for Figure 6. Empty bars, ‘Light Pink Barbara’; Grey bars, ‘Excerea’, Enclosed bars, ‘Miracle Rouge’. Statistical analysis and presentation of the results are similar to Figure 6. At the given flower-opening and senescence stages, where no histograms are shown, analysis was not conducted, mainly because of the shortage of flower materials.
As the first step of the present study, cDNAs of putative genes involved in ABA biosynthesis and signaling were cloned from flower tissues of carnation (Dianthus caryophyllus L. ‘Light Pink Barbara’) and characterized by comparing with their counterparts from other plant sources. The cDNAs isolated and characterized were those for genes for ABA biosynthesis [DcNCED1a and DcNCED1b, and DcNCED2a and DcNCED2b (9-cis-epoxycarotenoid dioxygenases), and DcZEP1 (zeaxanthin epoxidase)], ABA catabolism [DcCYP707A1 (ABA 8′-hydroxylase)], ABA signaling [DcPYR1 (ABA receptor), DcPP2C (type 2C protein phosphatase), and DcSnRK2.1 and DcSnRK2.2 (subfamily 2 of SNF1-related kinases)], and ABA action [DcAREBF1 (ABA responsive element binding factor)].
ABA contentABA has been expected to act as an inducer of the expression of genes for ethylene biosynthesis (DcACS1 and DcACO1) in the ovary of carnation flowers undergoing senescence. This was partly supported by the results that administration of exogenous ABA to carnation flowers enhanced ethylene production in the flowers (Onoue et al., 2000). To gain further support for the role of ABA, we examined changes in the ABA content of floral tissues during flower opening and senescence (Fig. 4). Since our major concern was ethylene production in the gynoecium induced by ABA, we focused our discussion below on the ABA content of the gynoecium, especially the ovary. In the ovary of ‘Light Pink Barbara’, the ABA content was as high as 500–700 pmol·g−1 FW from Os 2 to Ss 1, earlier than DcACO1 and DcACS1 transcripts accumulated at Os 6 and Ss 1, respectively. In the ovary of ‘Excerea’, the ABA content was 200–400 pmol·g−1 FW, but increased markedly in the early stage of senescence; 600 and 900 pmol·g−1 FW at Ss 1 and Ss 2, respectively (Fig. 4). In the ‘Excerea’ ovary, DcACS1 and DcACO1 transcripts accumulated abundantly at Ss 2 (Fig. 5). On the other hand, in the ovary of ‘Miracle Rouge’, the ABA content remained at 50–150 pmol·g−1 FW at Os 2 through Ss 4, and abundant accumulation of DcACS1 and DcACO1 was not observed (Fig. 5).
In ‘Light Pink Barbara’, the ABA content was 10–20 times higher in the ovary than in the petals during flower opening, and this was more than twice as high as in either ‘Excerea’ or ‘Miracle Rouge’.
In ‘Miracle Rouge’, the level of the transcript of DcACS1 in the ovary was low at Ss 3 and Ss 4 (12 and 18 days after full flower opening) and that of DcACO1 was low at Ss 2 (6 days after full flower opening) and Ss 3, although the flowers of this cultivar produced no detectable ethylene during senescence (Onozaki et al., 2006).
Based on these observations, we suggest that a given content of ABA is necessary to induce the expression of DcACO1 and DcACS1 in the ovary of carnation flowers. We speculate that the threshold ABA content might be 500 pmol·g−1 FW or higher.
Expression of genes for ABA biosynthesis and catabolismThe transcripts of DcZEP1, DcNCED1a/b, DcNCED2a/b, and DcCYP707A1 accumulated in the ovary during flower opening in ‘Light Pink Barbara’ and ‘Excerea’ flowers (Figs. 5 and 6); however, in ‘Miracle Rouge’, there was little accumulation of DcNCED1a/b transcript during flower opening and in the early senescence stages, although DcZEP1 and DcNCED2a/b transcripts accumulated.
Changes in the DcNCED1a/b transcript level correlated well with those in the contents of ABA in the ovary in the three cultivars during flower opening and senescence. In the ovary of ‘Light Pink Barbara’, the amount of DcNCED1a/b transcript was 0.06 r.e.l. at Os 1 and Os 2, and this high level coincided with or was preceded by the high ABA content at Os 2 through Os 6 (Figs. 4 and 6). DcNCED1a/b transcript in ‘Excerea’ ovary remained at < 0.03 r.e.l. from Os 1 to Os 6 and markedly increased to 0.06 and 0.23 r.e.l. at Ss 1 and Ss 2, respectively, and this change corresponded with the marked increase in ABA content, which showed a moderate change from Os 2 to Os 6, and then increased markedly at Ss 2 (Figs. 4 and 6). In ‘Miracle Rouge’, the DcNCED1a/b transcript was maintained at very low or negligible levels in the ovary, and ABA content was low and increased only slightly during flower opening and senescence (Figs. 4 and 6). On the other hand, the changes in the transcript levels of DcZEP1, DcNCED2a/b, and DcCYP707A1 were not correlated with the contents of ABA in the ovary during flower opening and senescence in all three cultivars. These results suggested that DcNCED1a/b, but not DcNCED2a/b, is involved mainly in the regulation of ABA biosynthesis, which leads to ethylene production, in the ovary of the three cultivars (Figs. 4–6). On the other hand, the expression of DcNCED2a/b is probably responsible for the steady state increase of ABA in the floral tissues during flower opening and senescence, which was evident in both the ovary and petals of ‘Miracle Rouge’ flowers and in petals of ‘Excerea’ flowers (Figs. 4 and 6).
Expression of genes for ABA perception and actionIn ‘Light Pink Barbara’ and ‘Excerea’ flowers, the ovary contained substantial amounts of ABA during flower-opening stages from Os 2 to Os 6, but DcACS1 and DcACO1 were not expressed in the ovary during this period, and were expressed at Ss 1 or Ss 2 (Fig. 5). The reason why the expression of ethylene biosynthesis genes, especially DcACS1, the key gene for ethylene biosynthesis, was not induced in the ovary during the flower-opening stages may be that the perception and signaling of ABA were repressed in the ovary during the flower-opening stages. To test this possibility, we examined the expression of the genes for core components in ABA perception and signaling pathway in the ovary of three cultivars.
In ‘Light Pink Barbara’, DcPYR1 transcript was not detectable at Os 1 through Os 3 by RT-PCR (Fig. 5), but it was 0.004–0.007 r.e.l., as revealed by real-time RT-PCR (Fig. 7). Thereafter, it gradually increased to 0.024 and 0.029 r.e.l. at Os 6 and Ss 1, respectively. On the other hand, the DcPP2C1 transcript was clearly detected by RT-PCR analysis during flower opening (Fig. 5). DcSnRK2.1 and DcSnRK2.2 transcripts were detected even at Os 1. The DcSnRK2.1 transcript level decreased at Os 3–Os 6, but the DcSnRK2.2 transcript level remained constant during the flower-opening period. Onoue et al. (2000) showed that ethylene was produced 3 days after the start of exogenous administration of ABA to carnation flowers at the full-opening stage. Therefore, it is reasonable to expect that the increase in DcPYR1 expression at Os 4 or later induced the expression of DcACO1 and DcACS1 at Os 6 and Ss 1, respectively (Fig. 5). Based on these findings, we suggest that the absence of the transcripts of ethylene biosynthetic genes, DcACS1 and DcACO1, during flower opening in ‘Light Pink Barbara’ flowers resulted from the absence of ABA action caused by the lack or shortage of its receptor protein DcPYR1.
DcACS1 and DcACO1 were not expressed in the ovary of ‘Excerea’ flowers during flower opening in spite of the presence of substantial amounts of ABA and expression of DcPYR1 at 0.025–0.038 r.e.l. In addition, there were no defects in the expression of genes for ABA signaling and action, i.e., DcPP2C1, DcSnRK2.1, DcSnRK2.2, and DcAREBF1 in this cultivar. The reason for the absence of ABA action in ‘Excerea’ ovary during flower opening may be that the ABA contents of the ovary did not exceed the threshold in spite of the presence of a sufficient amount of DcPYR1. At the early senescence stage (Ss 1), the ABA content exceeded its threshold and exerted its action by binding to DcPYR1.
In the ovary of ‘Miracle Rouge’ flowers, the ABA content never exceeded the putative threshold (Fig. 4), and the expression level of DcPYR1 also remained low (Figs. 5 and 7) during the flower-opening and senescence periods. This is probably why DcACS1 and DcACO1 were not expressed in the ovary, resulting in no production of ethylene and prolonged vase-life of the flowers in this cultivar.
Note that there were simultaneous defects in the expression of DcNCED1a/b in ABA biosynthesis and DcPYR1 in ABA signaling. These results suggested the presence of a common up-stream mechanism that regulates the expression of both DcNCED1a/b and DcPYR1 in the ovary of carnation flowers. This common mechanism is probably regulated by an unknown gene(s), which acts as a master gene(s) regulating the onset of senescence in carnation flowers.
In the present study we compared three carnation cultivars with distinct senescence characteristics to yield useful insights into the mechanisms underlying regulatory processes, i.e., induction of ethylene biosynthesis in the gynoecium; however this type of comparison may generate compelling correlations but not always the final proof. Keeping this notion in mind, we plan to test exogenous ABA and inhibitors of ABA biosynthesis on ethylene production in these cultivars. This would further demonstrate whether the hypothesis of the regulatory role of ABA holds true for the induction of ethylene biosynthesis in the gynoecium of senescing carnation flowers.
Concluding remarksABA probably plays a role in inducing ethylene production in the gynoecium (ovary) of carnation flowers at the early stage of flower senescence. The present work suggested that a certain level of both ABA content and expression of DcPYR1 (ABA receptor) is necessary to induce the expression of DcACS1 and DcACO1, leading to ethylene biosynthesis in the ovary at an early stage of flower senescence. In ‘Light Pink Barbara’, which exhibits ordinary ethylene production 6 days after full flower opening, ABA action was limited by the absence of the expression of DcPYR1, an ABA receptor, although ABA was present in a large amount in the ovary during flower opening. On the other hand, in ‘Excerea’ flowers, which also show ordinary ethylene production and senescence characteristics, DcPYR1 was expressed abundantly in the ovary during flower opening, but ABA action was limited by an insufficient content of ABA. In ‘Miracle Rouge’ flowers, which do not produce ethylene during senescence and show a prolonged vase-life, the ABA content was below the threshold level, and the gene expression of DcPYR1 was repressed during flower opening and senescence; therefore, the amount of ABA was not enough to induce ethylene production in the ovary. Overall, the present study gave further support of the role of ABA in ethylene production by inducing the expression of DcACS1 and DcACO1 in the ovary of carnation flowers undergoing senescence.