2014 Volume 83 Issue 1 Pages 59-63
2014 Volume 83 Issue 1 Pages 59-63
Cut Eustoma grandiflorum (Raf.) Shinn. flowers are produced year-round in Japan; however, winter conditions are not favorable for flower production due to low sunlight levels. Here, we investigated the effect of CO2 enrichment after the flower budding stage on the growth and flowering of Eustoma ‘Bolero White’, which were grown under forcing culture for winter shipping. CO2 enrichment increased the fresh weight of plants, in addition to increasing the dry weights of leaves, stems, flower buds and open flowers, and roots. CO2 enrichment also increased the relative growth rate (RGR) by 32%, due to the net assimilation rate (NAR) being stimulated. However, CO2 enrichment had no effect on plant height or the leaf area ratio (LAR). Furthermore, CO2 enrichment increased the total number of flower buds and open flowers, in addition to accelerating flower bud development and the promotion of flowering. During the period of enrichment, the vegetative organs continued to grow in CO2-enriched plants, but not in the control plants. In conclusion, CO2 enrichment promoted flowering and improved the quality of cut flowers (i.e., increasing plant fresh and dry weight and the total number of flower buds and open flowers) of Eustoma under low-sunlight winter conditions.
Eustoma grandiflorum (Raf.) Shinn. is a popular ornamental cut flower because of its long vase life and range of flower colors. In Japan, Eustoma flowers are produced year-round; however, fewer flowers are produced in winter than in summer. For example, the market wholesale quantity of Eustoma in January 2008 was 22% of that in August 2007 (Ministry of Agriculture, Forestry and Fisheries, http://www.maff.go.jp/j/tokei/kouhyou/kaki_orosi/index.html, June 8, 2013).
Eustoma is a summer-blooming plant. In this species, flowering is promoted by long day length (Islam et al., 2005; Tsukada et al., 1982; Zaccai and Edri, 2002) and high temperature (Halevy and Kofranek, 1984; Tsukada et al., 1982; Zaccai and Edri, 2002). In addition, a high daily light integral is crucial for promoting flowering and for improving flower stem quality (i.e., increasing shoot dry weight and the number of flower buds and open flowers; Islam et al., 2005). In contrast, short day length, low temperature, and low light integral, which occur during winter, are unsuitable for the production of flowers of this species, leading to delayed flowering. Moreover, low sunlight winter conditions in combination with high nitrogen concentrations promote the abortion of flower buds in Eustoma (Ushio and Fukuta, 2010).
CO2 enrichment benefits the production of horticultural crops (Gruda, 2005; Mortensen, 1987) by reducing the negative effects of other environmental factors, such as low light conditions (Gruda, 2005). For example, rose plants are produced commercially under CO2 enrichment in greenhouses to increase yield and to improve cut flower quality (Pandey et al., 2007). Although a preliminary study has examined the use of CO2 enrichment for Eustoma (Sato et al., 2005), it focused on retarding culture for shipping in December.
At present, the winter production of Eustoma in Japan primarily occurs in regions with high sunlight levels to facilitate winter shipping. When Eustoma is produced for harvesting in January, flower bud development and flowering occur under winter climatic conditions, i.e., low sunlight, short day length, and low temperature. These environmental conditions cause a reduction in photo-synthetic rates and carbohydrate production, which are required for plant growth and organ development. Since CO2 enrichment increases the net photosynthesis of plants, its application during flower development under winter climatic conditions might improve the growth and flower quality of Eustoma.
In the present study, we investigated the effect of CO2 enrichment, after the flower-budding stage, on the growth, flowering, and cut flower quality of a double-and early-flowering variety of Eustoma grown in a region of Japan with low sunlight levels during winter, under forcing culture for winter shipping.
The experiment was performed in a multi-span greenhouse in Miyawaka City, Fukuoka Prefecture, Japan (latitude 33°43′47″N, longitude 130°36′34″E). Fukuoka is a region of Japan with low sunlight during winter. Specifically, this region had a global solar radiation of 6.2 MJ·m−2·day−1 and 6.7 MJ·m−2·day−1 in December 2010 and January 2011, respectively (Japan Meteorological Agency, http://www.data.jma.go.jp/obd/stats/etrn/index.php, June 8, 2013). The greenhouse was divided into a CO2-enriched area and a control area using a plastic sheet to prevent the diffusion of CO2 into the control area. The plastic sheet was attached to an eave and extended from the floor to the roof. In the CO2-enriched area, CO2 gas was supplied and controlled with a CO2 gas generator (CG-554T2; NEPON Inc., Tokyo, Japan).
E. grandiflorum ‘Bolero White’ (Miyoshi Co., Yamanashi, Japan) seeds were sown in plastic germination trays with 288 cells (Plant Plug; Sakata Seed Co., Kanagawa, Japan) on June 17, 2010. The seeded trays were maintained in a dark cool-room at 10°C for 30 days, after which the trays were transferred to a greenhouse and grown under a constant day/night temperature regime of 25/15°C until planting.
A commercial fertilizer containing 1.9% N, 6.0% PO4, and 2.9% K (Biotech-Bioace; Sakata Seed Co., Kanagawa, Japan) was applied at 0.23 kg·m−2 in the multi-span greenhouse fields during late August 2010.
Seedlings were planted on beds (width = 0.6 m, length = 36 m) in the CO2-enriched area and the control area on September 10, 2010, at a planting density of 66.7 plants·m−2. Long-day treatment was applied to the plants from September 15 to December 25, 2010, using incandescent bulbs, with illumination times from 2:00 to 8:00 and 17:30 to 22:00. These incandescent bulbs only affected the photoperiod, with a photosynthetic photon flux density (PPFD) of 2 μmol·m−2·s−1 at plant level.
The first flower buds became visible on the main stems of plants from October 23 to October 27, 2010. The first flower buds on the main stems and on the primary inflorescence branches were removed after the flower buds became visible (Fig. 1).

Debudding method in inflorescence. F0, F1, F2, and F3 denote flower buds.
The greenhouse was heated from November 18, 2010, onwards. In both the CO2-enriched area and the control area, the minimum nighttime temperature was set to 5°C until January 10, 2011, and 10°C from January 11, 2011. The minimum daytime temperature was set to 20°C until December 22, 2010, and 15°C from December 23, 2010. Here, in relation to environmental control in the greenhouse, “daytime” was defined as 8:00–14:00 from November 18 to December 5, 2010, and 8:00–13:00 from December 6, 2010 to January 22, 2011.
CO2 enrichment was performed from November 18, 2010 to January 22, 2011, and was applied during the daytime only. When the roof vents were closed during the daytime, the CO2 concentration was set to 1000 μmol·mol−1; however, when the roof vents were opened during the daytime, the CO2 gas generator was stopped. The greenhouse was ventilated at 28°C. On rainy or cloudy days, the temperature in the greenhouse did not increase above the ventilation temperature; therefore, the greenhouse was manually ventilated after CO2 enrichment to prevent excessive humidity. Since the greenhouse was multi-span, both the CO2-enriched area and the control area were ventilated simultaneously.
A data logger (3671; HIOKI Co., Nagano, Japan) equipped with a light sensor (LI-190SL; LI-COR Inc., Lincoln, NE, USA) was used to measure the PPFD in the greenhouse throughout the experiment. The daily integral of PPFD inside the greenhouse was calculated on a monthly basis, and was 6.0, 12.0, 11.1, 7.6, and 6.6 mol·m−2·day−1 from September 2010 to January 2011.
A thermo recorder (TR-52; T&D Co., Nagano, Japan) was used to measure air temperature inside the greenhouse from September 14, 2010, to the end of the experiment. The average daily temperature inside the greenhouse was calculated on a monthly basis, and was 23.1, 19.1, 14.7, 12.6, and 12.0°C from September 2010 to January 2011.
CO2 concentrations in the CO2-enriched and control areas were measured using CO2 meters (GM70; VISALA Oyj., Helsinki, Finland).
2. Measurements and growth analysisSeedling biomass at the time of planting on September 10, 2010, was determined by randomly selecting five seedlings from the trays. The roots were washed, oven-dried at 80°C for 4 days, and then the dry weight was measured.
The dry weights of the leaves, stems, flower buds and open flowers, and roots were determined four times during the experiment: (1) on October 10, 2010, which was 1 month after planting; (2) on November 17, 2010, which was 1 day before the initiation of CO2 enrichment; (3) on December 20, 2010, which was 33 days after the initiation of CO2 enrichment; and (4) on January 22, 2011, when CO2 enrichment ended (66 days after the initiation of CO2 enrichment). For plant sampling, each bed in the CO2-enriched and control areas was subdivided into three plots of 0.6 × 12 m, with three plants per plot being randomly selected; that is, nine plants from each treatment were sampled on each of the four occasions. Sampled plants were separated into aboveground parts and the roots. After the plant height and leaf area were measured, the aboveground parts were then further separated into the leaves, stems, and flower buds and open flowers. The roots were washed with water. The leaves, stems, flower buds and open flowers, and roots were oven-dried at 80°C for at least 4 days, and the dry weight of each plant part was measured.
For plants sampled on January 22, 2011, the fresh weight of the aboveground parts and the number of flower buds and open flowers were also measured.
Growth analysis was used to calculate the relative growth rate (RGR), net assimilation rate (NAR), and leaf area ratio (LAR) of plants by using the total dry weight and leaf area of the plants sampled on November 17, 2010 (1 day before CO2 enrichment was initiated), and those sampled on January 22, 2011 (when CO2 enrichment ended), according to the equation of Nagai and Makino (2009).
3. Statistical analysesData were analyzed using t-tests and JMP (SAS Institute Inc., Cary, NC, USA).
There was no significant difference in plant height between the CO2-enriched and control plants at the initiation (November 17, 2010) and end (January 22, 2011) of CO2 enrichment (Table 1; Fig. 2A). However, the fresh weight of the aboveground parts was 32% greater and the leaf area was significantly higher in CO2-enriched plants than control plants (Table 1). The total dry weight of CO2-enriched plants was also significantly higher than the control plants on the 33rd day (December 20, 2010), and was 1.37 times higher at the end of the CO2 enrichment (January 22; Fig. 2B).

Effects of CO2 enrichment treatment of 66th day (Jan 22) on the growth of Eustoma ‘Bolero White’.
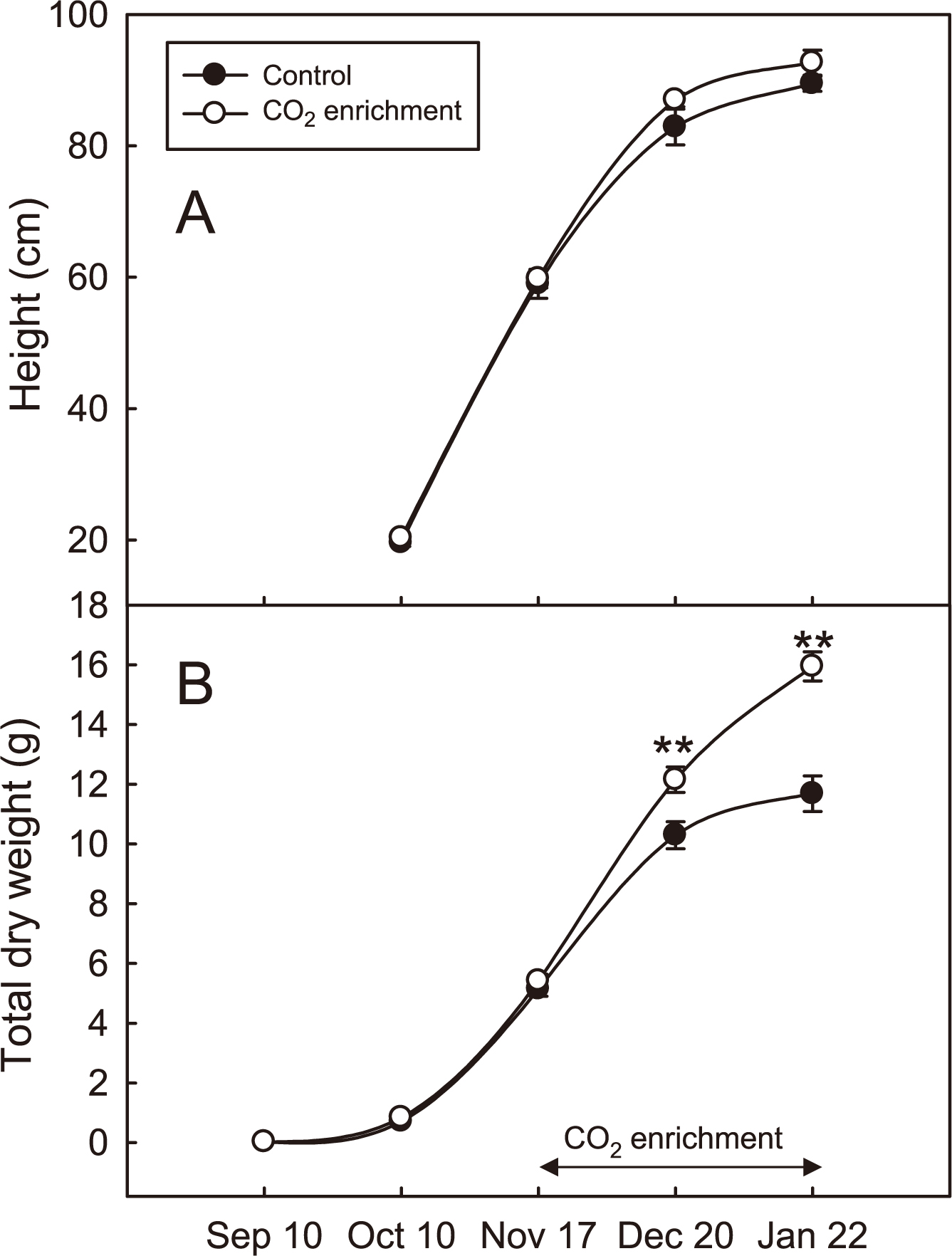
Changes in the height (A) and total dry weight (B) of CO2-enriched and control Eustoma plants. Values are the means ± SE (n = 9). **: P < 0.01 (t-test).
The dry weight of all plant parts was higher in CO2-enriched plants than the control plants (Fig. 3). These differences became evident on the 33rd day of the CO2 enrichment (Fig. 3). Although the dry weights of leaves, stems, and roots of the control group were similar on the 33rd day (December 20) and the 66th day (January 22), those of the CO2-enriched group were slightly higher on the 66th day (January 22) compared to the 33rd day (December 20). However, there was an increase in the dry weights of the flower buds and open flowers in both the control and CO2-enriched groups between the 33rd day (December 20) and the 66th day (January 22), although the rate of increase was greater in the CO2-enriched plants (as shown by the gradient of the lines in Figure 3A).

Changes in the dry weight of flower buds and open flowers (A), stems (B), leaves (C), and roots (D) in CO2-enriched and control Eustoma plants. Values are the means ± SE (n = 9). *: P < 0.05 (t-test). **: P < 0.01 (t-test).
Growth analysis showed that the RGR and NAR of CO2-enriched plants were significantly higher than the control plants (1.32-fold difference; Table 2). However, there was no difference in LAR between the control and CO2-enriched groups.

Effects of CO2 enrichment treatment on the relative growth rate (RGR) and net assimilation rate (NAR), and leaf area ratio (LAR) of Eustoma ‘Bolero White’ between Nov 17, 2010 and Jan 22, 2011.
CO2-enriched plants had a greater number of open flowers than the control plants (on average, approximately three flowers on CO2-enriched plants vs. one flower on control plants; Table 1). Further, CO2-enriched plants had a greater total number of flower buds and open flowers than the control plants (Table 1). Flower bud abortion was not observed in either group.
CO2 enrichment is widely used in greenhouses to improve the marketable yield and quality of flowers and vegetables. CO2 enrichment has been reported to have a positive effect on many species of flowering plants grown in greenhouses (Mortensen, 1987). For example, CO2 enrichment significantly increased the weight and height of chrysanthemum (Mortensen, 1986; Tanigawa et al., 1993) and rose (Mortensen and Moe, 1992). In contrast, CO2 enrichment had no evident effect on the growth and flowering of campanula (Niu et al., 2001). In the present study, we found that CO2 enrichment increased plant weight, but had no effect on plant height in Eustoma (Table 1; Fig. 2). Furthermore, CO2 enrichment accelerated flowering and increased the total number of flower buds and open flowers (Table 1; Fig. 3).
In the present study, a continuous increase in plant dry weight during CO2 enrichment was observed (Fig. 2B). The RGR, which is a product of LAR and NAR, was 1.32 times greater in CO2-enriched plants than control plants (Table 2). It has been suggested that CO2 enrichment decreases LAR (Makino et al., 1997; Roden and Ball, 1996; Yoon et al., 2009). Since LAR remained unchanged in this study (Table 2), the increase in NAR, as a result of CO2 enrichment, effectively contributed to an increase in RGR.
The sunlight and temperature levels of the study region were lowest between late December 2010 and late January 2011. Even under such climatic conditions, CO2 enrichment caused a noticeable increase in the dry weights of flower buds and open flowers. In addition, CO2 enrichment caused a slight increase in the dry weights of the leaves, stems, and roots (Fig. 3). These results indicate that CO2 enrichment maintained the growth of the vegetative organs. In contrast, although the dry weights of the flower buds and open flowers increased in the control plants, there was no increase in the dry weights of the leaves, stems, and roots. The control plants also had a lower NAR (Table 2), indicating that the production of carbohydrates for growth was restricted. Therefore, carbohydrates produced by photosynthesis appear to be mainly directed into flower development.
CO2 enrichment increased the total number of flower buds and open flowers (Table 1). However, Sato et al. (2005) reported that the total number of flower buds and open flowers did not increase under CO2 enrichment in Eustoma ‘Tsukushinoyuki’. This difference may be due to cultivar variation. An increase in the total number of flower buds and open flowers after CO2 enrichment has been reported for many other plants, including chrysanthemum (Mortensen, 1986), rose (Mortensen, 1987, 1995), and dianthus (Mortensen, 1987).
Springer and Ward (2007) summarized the results of 60 studies reporting the flowering-time responses of both crops and wild species at elevated CO2 levels. This review indicates that elevated CO2 affects the flowering time of various species differently, being accelerated in some species, remaining constant in others, and being suppressed in yet others. However, Springer and Ward (2007) found that, in general, CO2 enrichment accelerates the flowering of crops. For example, CO2 enrichment has been shown to accelerate the time to flowering in rose (Mortensen, 1987; Springer and Ward, 2007) and dianthus (Mortensen, 1987). Similarly, CO2 enrichment accelerated flowering in Eustoma in the present study.
Flower bud abortion is a major problem for the winter production of Eustoma. CO2 enrichment reduces the levels of flower abortion in rose (Mortensen, 1987) and alstroemeria (Van Labeke and Dambre, 1998). Although in the present study, flower bud abortion was not observed in the presence or absence CO2 enrichment, a previous study reported that flower bud abortion occurred under winter conditions in the Eustoma ‘Piccorosa Snow’ (Ushio and Fukuta, 2010). This difference might be due to differences in the tolerance of Eustoma cultivars to flower bud abortion. In this study, we used the Eustoma ‘Bolero White,’ which is considered to be relatively tolerant to flower bud abortion. Therefore, the effect of CO2 enrichment on flower bud abortion in Eustoma could not be clarified in the current study.
In conclusion, the CO2 enrichment of Eustoma after flower buds became visible promoted plant growth and flowering during winter in a low sunlight region of Japan. CO2 enrichment increased the fresh and dry weight of plants and RGR, which was 32% higher in CO2-enriched plants because of increased NAR. CO2 enrichment of Eustoma also increased the number of open flowers, the total number of flower buds and open flowers, and the flower developmental rate, as well as accelerating flowering. These findings suggest that the CO2 enrichment of Eustoma not only improves the quality of cut flowers but also shortens the period to harvest during winter.
The authors thank Mr. Shunsaku Nakamura, Nakamura Engei, for managing the plant materials and for providing valuable suggestions regarding the experimental design.