2014 Volume 83 Issue 1 Pages 44-51
2014 Volume 83 Issue 1 Pages 44-51
The absorption of NO3− and growth of radish (Raphanus sativus L. ‘Yukikomachi’), and the timing and interval of NO3− supply were examined to evaluate quantitative nutrient management (QNM) of nutrient solution in a hydroponic culture of radish plants. In experiment 1, the amount of NO3− required for growth to a marketable size (30–35 g FW of thickened axis) was presumed to be approximately 1000 mg/plant by the direct measurement of NO3− absorption of radish plants grown with the EC-based control management method (EC-based control method) of nutrient solution containing different concentrations (2, 4, 6, 8, and 10 me·L−1) of NO3−. In Experiment 2, plants were supplied with the total amount of NO3− (1000 mg/plant) at the beginning of the experiment or with 1/5 of the total amount of NO3− (1000 mg/plant) repeatedly 5 times every 4 days, and then their fresh weight and nutrient absorption were compared with the plants grown with the EC-based control method. Significant differences in the growth of thickened axes and leaves were not obtained among plants grown by three different methods. However, plants appeared to be supplied with an excess amount of nutrients because EC and NO3− levels were high at the end of cultivation. From the experiment in which plants were supplied with the whole amount of mineral nutrients containing 900, 800, and 700 mg/plant of NO3− at the beginning of the experiment in December, it became apparent that 800 mg/plant of nitrate would be sufficient for radish growth in the cold season. In conclusion, we propose the QNM method supplying the whole amount of nutrients required for crop growth at the beginning of cultivation so that radish plants could be produced without draining nutrient solution containing a large amount of NO3− from the hydroponic system into the environment.
Among the various types of radish plants, small, quickly maturing types have become increasingly important root vegetable crops with the increasing demand for vegetable crops for salad. They mature to a marketable size from 20 to 30 days after seeding and can be cultivated in a greenhouse throughout the year. Moreover, they and many other small root or fresh leaf vegetables are usually consumed raw or with minimal processing in salads, and thus these plant foods are required to be clean and free of unfavorable contaminants. At present, a wide variety of vegetables are being cultivated with the hydroponic growing technique, which is also being successfully applied for the cultivation of small vegetables for salad. However, some vegetables hydroponically grown with higher concentrations of nutrient solution are reported to contain higher levels of nitrate (Aworth et al., 1980; Ikeda and Osawa, 1980), which is believed to contribute to some types of cancer (Eichholzer and Gutzwiller, 1998). This problem implies that vegetables for salad should be cultivated so as not to accumulate nitrate during the period of cultivation.
As an approach to an alternative method to conventional nutrient solution management, in which the levels of nutrients in nutrient solution are controlled to be maintained at optimum EC (EC-based control method) values, the quantitative nutrient management (QNM) method was proposed by several researchers in the hydroponic culture of tomatoes (Kageyama, 1991; Kidono and Suzuki, 2006; Nakano et al., 2006, 2010; Terabayashi et al., 1996), spinach (Maruo et al., 2001; Takei and Suzuki, 2013), melons (Pardossi et al., 2002), and chrysanthemums (Kageyama and Konishi, 1992, 1996; Kageyama et al., 1987, 1993, 1995; Shima et al., 1995). In spinach plants, Maruo et al. (2001) proposed a QNM method by which spinach plants were grown with a low concentration of nutrient solution by the daily supply of a limited amount of nutrients estimated from the measurement of daily nutrient uptake. They also proposed the advantages of their QNM method in hydroponics and summarized as follows: (1) improved efficiency of nutrient usage, (2) reduction of negative environmental impacts, (3) facilitation of growth control in fruit vegetable production, (4) reduction of nitrate content in leaf vegetables, and (5) reduction of physiological disorders through effects on the ionic balance. These advantages are of great interest for the production of vegetable crops of higher quality by the hydroponic technique, leading to conservation of water and nutrients and to the reduction of environmental pollution.
In the QNM method of Maruo et al. (2001), nutrients were supplied every day according to their nutrient supply program, in which daily uptake of nutrients were estimated from the growth pattern, nutrient composition, and days to harvest of spinach plants. Likewise, tomato plants were reported to be hydroponically grown by the QNM method with the daily supply of a small amount of nitrogen to regulate the leaf area index (LAI) at optimum (Hosoi and Hosono, 2005) and with the daily supply of nutrients according to the average amount of water absorbed daily during the previous three days (Nakano et al., 2010). Thus, daily or shorter interval supply of nutrients appears to be highly applicable to the long-term culture of tomato plants. However, Terabayashi et al. (2004a, b) reported that a weekly supply of NO3− and PO43− during the fruiting stage did not decrease fruit yield of tomato plants, and Kageyama (1991) reported that the yield and nitrogen content of tomato plants grown hydroponically in a two-truss culture were unaffected by supplying the total amount of nitrogen required for tomato plants at the beginning of the experiment. Similarly, quantitative supply of potassium at 12-day intervals during the fruiting stage did not affect fruit growth of tomato plants in a two-truss culture (Kidono and Suzuki, 2006). These results indicate the possible short-term production of tomato fruits with the QNM method by which nutrients are supplied at longer intervals or only once at the beginning of culture.
Previously, we reported that the marketable size of spinach plants containing lower levels of NO3− could be cultivated by the QNM method, supplying the total amount of NO3− required for their growth at the beginning of cultivation (Takei and Suzuki, 2013). This is a simple method in which complex management practices of nutrient solution are not needed, except for pH adjustment, after nutrient supply at the beginning of cultivation. Thus, this QNM method can be widely applied in the short-term hydroponic culture of small leaf vegetables such as spinach. Likewise, this method is expected to be applied in the hydroponic culture of radishes. However, it is well known that root vegetables such as radishes have different nutrients requirements from those of leaf vegetables such as spinach. For example, the requirements for phosphorus and sulfur are high in radishes, while those for nitrate and potassium are high in spinach (Takano and Kawazoe, 1973). These findings indicate the importance of studying the possible hydroponic culture of radish plants with the QNM method for the purpose of applying this method to a variety of vegetable crops.
In the QNM method, the concentration of nutrient solution is high at the beginning of cultivation, followed by its continuous decline during the period of cultivation. However, the thickened axis of root vegetables such as radishes quickly swells in the later growth period. The effect of these conditions of nutrient solution on the growth and yield of radishes has not been studied. In this paper, we report the possible mass production of radishes by hydroponics with the QNM method without draining nutrient solution containing a large amount NO3− from the hydroponic system into the environment, and identify the minimum amount of NO3− required to obtain radish plants of marketable size.
Radish (Raphanus sativus L., ‘Yukikomachi’, Sakata Seed Co., Kanagawa, Japan) seeds were sown on wet filter paper in a Petri dish (9 cm in diameter) and incubated in the dark at 25°C. After 24 h, germinated seeds were selected for uniformity and planted in pots (3 seeds per pot) of black polypropylene mesh (6 cm in diameter and 10 cm in height) filled with a mixture of granulated rock wool and carbonated rice hulls (8 : 2, v/v). The pots were placed in a plastic tray (505 mm in length, 350 mm in width, and 85 mm in depth). Seedlings were grown in a glasshouse on the Tempaku Campus of Meijo University and supplied with tap water by bottom irrigation. After seedling emergence, plants were supplied with half–strength nutrient solution containing 2.25 me·L−1 MgSO4·7H2O, 1.5 me·L−1 NH4H2PO4, 0.75 me·L−1 Ca(H2PO4)2·H2O, 3 me·L−1 Ca(NO3)2·4H2O, 7.5 me·L−1 KNO3, and microelements (Fe, 3; Mn, 0.5; B, 0.5; Zn, 0.05; Cu, 0.02; Mo, 0.01 ppm). These concentrations of microelements were also added to the nutrient solutions used in Experiment 1 and 2. After cotyledons fully expanded, one seedling per pot remained for hydroponic cultivation.
Sixteen pots were placed with 10 cm spacing in a closed, circulating deep flow technique (DFT) hydroponic system consisting of a nutrient solution pool (60 cm long, 60 cm wide, and 5 cm deep) and a nutrient solution tank (45 L) placed under the pool. Nutrient solution, which is pumped at the rate of 5 L·min−1 from the tank, overflowed the pool and flowed down into the tank. The total amount of nutrient solution in the system was 35 L, being maintained by adding ground water. Temperature in the solution tank was controlled at about 20°C in the cold season with an electric heater or below 25°C in the hot season with a stainless cooling tube through which ground water flowed.
When the plants reached marketable size (approximately 2 cm in diameter and 30–35 g FW of thickened axis) after 18–32 days of cultivation, plants were harvested and separated into organs for the measurement of fresh weight, dry weight, and content of mineral elements. Fresh samples were dried in a ventilated oven at 70°C for 3 days and ground to powder with a pestle and mortar to pass through a 0.35 mm sieve. After being digested by the standard method of wet-acid digestion with nitric acid and perchloric acid (Jones, 2001), the mineral contents of the samples were analyzed using a flame photometer (ANA-10KL; Photo-electric, Tokyo, Japan) for K and Na.
Experiment 1. Amount of NO3− required for commercial production of radishesSeeds were sown on Sept. 16, 2010 and Nov. 12, 2010. After cotyledons had fully expanded, the pots were transferred to the DFT hydroponic system and plants were grown by the EC-based control method with nutrient solutions containing different levels of NO3−; i.e. 2, 4, 6, 8, and 10 me·L−1 (Table 1). The levels of NO3− in the solution were measured with a compact ion meter (Caddy Ion C-141; Horiba, Kyoto, Japan) and adjusted to 2, 4, 6, 8, and 10 me·L−1 by adding NaNO3 every 3 days. Other elements were also replenished every 3 days with the nutrient solution containing no NO3− until the EC values returned to the original level. The pH values were adjusted to 5.5–6.0 every 3 days with 4.0 N sulfuric acid. Daily average temperatures in the glasshouse in Sept. and Nov. were 27°C (maximum of 32°C and minimum of 23°C) and 21°C (maximum of 25°C and minimum of 18°C), respectively.
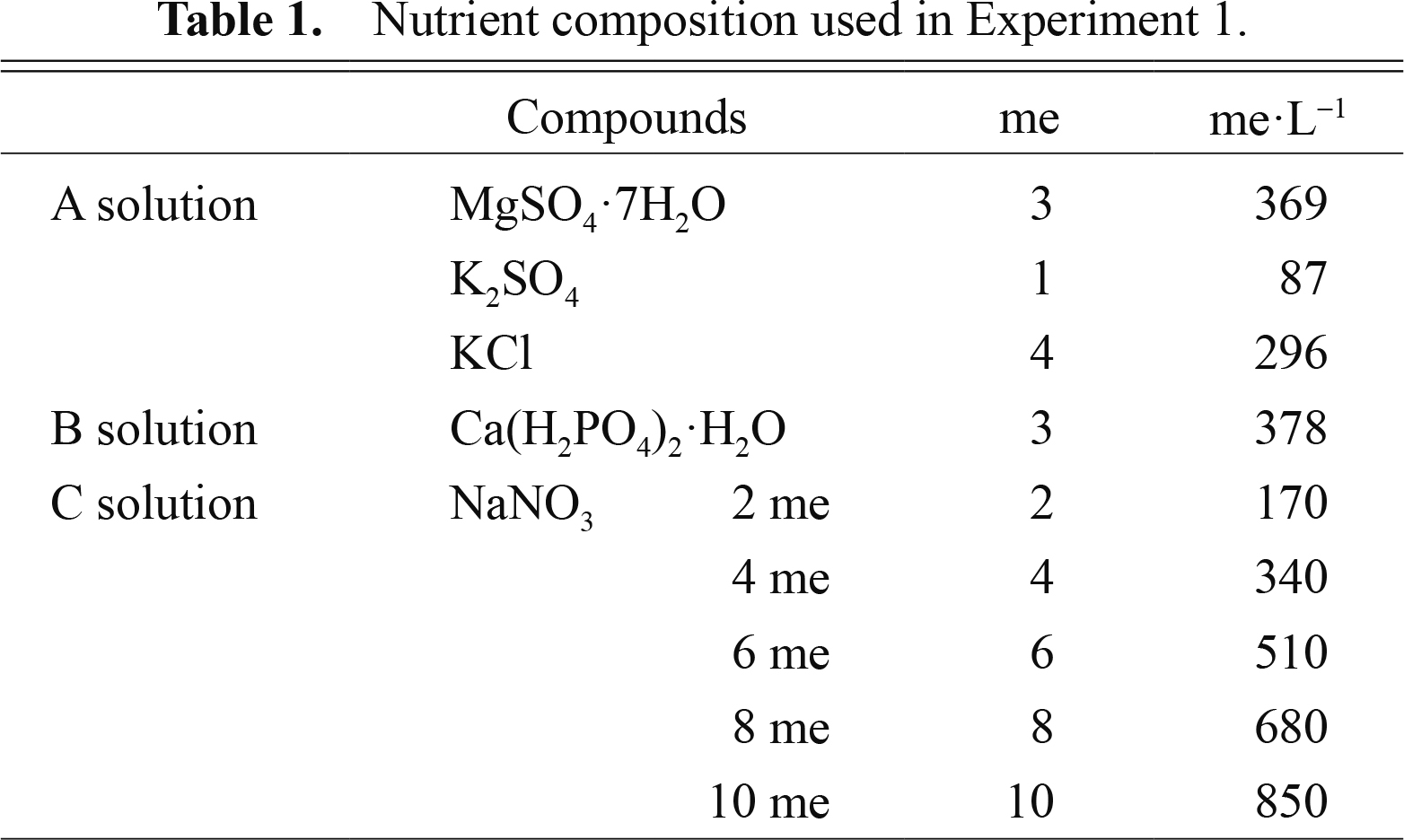
Nutrient composition used in Experiment 1.
Seeds were sown on Sept. 8 and Dec. 9, 2011. After cotyledons had fully expanded, the pots were transferred to the DFT hydroponic system and plants were supplied with nutrient solutions by three different methods. In the first method, plants were supplied with nutrient solution containing 4 me·L−1 NO3− and the other elements (Table 2); thereafter, nutrient solution of the same composition was added to the nutrient tank to maintain the volume of the solution at the original level. In this case, EC was maintained at approximately 0.7–0.8 dS·m−1 (EC-based control treatment). In the second method, plants were supplied with 1/5 of the whole amount of mineral nutrients containing 1000 mg/plant of NO3−, which is required for growth to marketable size, at the beginning of cultivation and this application was repeated 5 times every 4 days (AWAN-supplied treatment). In the third method, the whole amount of mineral nutrients containing 1000 mg/plant of NO3− was supplied at the beginning of the experiment (WAN-supplied treatment). Adjustment of pH values to 5.5–6.0 and of the volume of nutrient solution were carried out every 2 days in the EC-based control treatment and every 4 days in AWAN-supplied and WAN-supplied treatments. At the same time, a small amount of nutrient solution was sampled for the measurement of mineral elements. The levels of NO3− in the solution was determined by means of optical reflectometry (RQflex plus 10; Merck, Darmstadt, Germany). Five plants per treatment were selected for the measurement of leaf growth and the length of the second foliage leaf. Leaf growth was evaluated by the leaf elongation rate (LER) calculated according to Hunt (1990). In the cultivation in Dec., plants were supplied with the whole amount of mineral nutrients containing 900, 800, and 700 mg/plant of NO3− at the beginning of the experiment. Daily average temperatures in the glasshouse in Sept. and Dec. were 27°C (maximum of 33°C and minimum of 24°C) and 18°C (maximum of 26°C and minimum of 14°C), respectively.
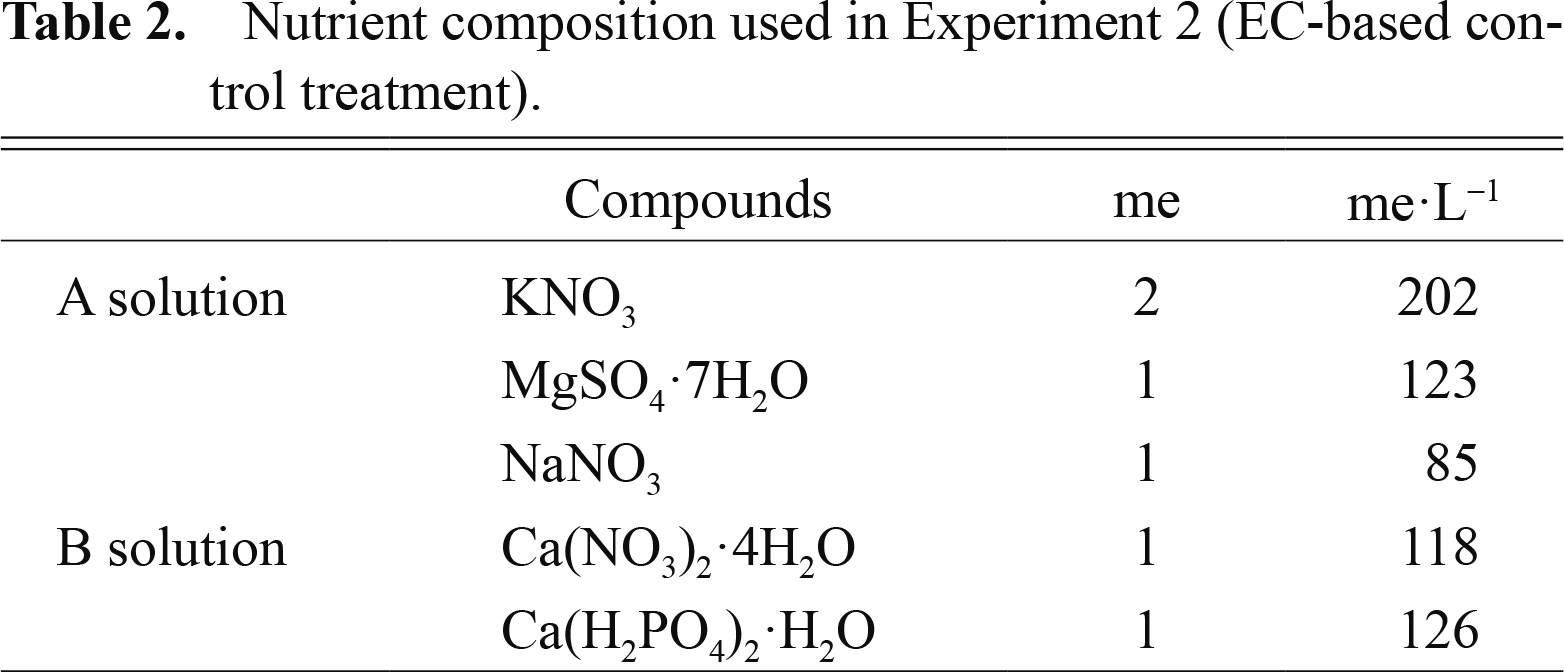
Nutrient composition used in Experiment 2 (EC-based control treatment).
Fresh weight of the thickened axis appeared to be slightly higher at 4 me·L−1 in Sept. (Fig. 1a) and apparently higher at 4–8 me·L−1 in Nov. (Fig. 1b). However, significant differences were not obtained in Sept. or in the range of 4–8 me·L−1 in Nov. The amounts of NO3− absorbed (Fig. 2a, b) by radishes supplied with 2 and 10 me·L−1 of NO3− were 395–625 and 1726–1947 mg/plant, respectively. The relationship between the fresh weight of the thickened axis and NO3− absorption indicated that the fresh weight of the thickened axis was unaffected by the broad range of NO3− absorption in Sept. (Fig. 2a). However, the data from the experiments in Nov. showed that the fresh weight of the thickened axis appeared to decrease below 1000 mg/plant (4 me·L−1) of NO3− (Fig. 2b). Moreover, our analytical results of major elements showed that Na+ accumulated and K+ decreased with increasing NO3− (data not shown). In thickened axes, more than 1.3% of Na+ (dry weight %) accumulated in the plants supplied with 6–10 me·L−1. According to Osawa (1961), the growth of radish plants was inhibited above 1.3% sodium ions in thickened axes, indicating that these plants appeared to accumulate sodium ions above the toxic level.
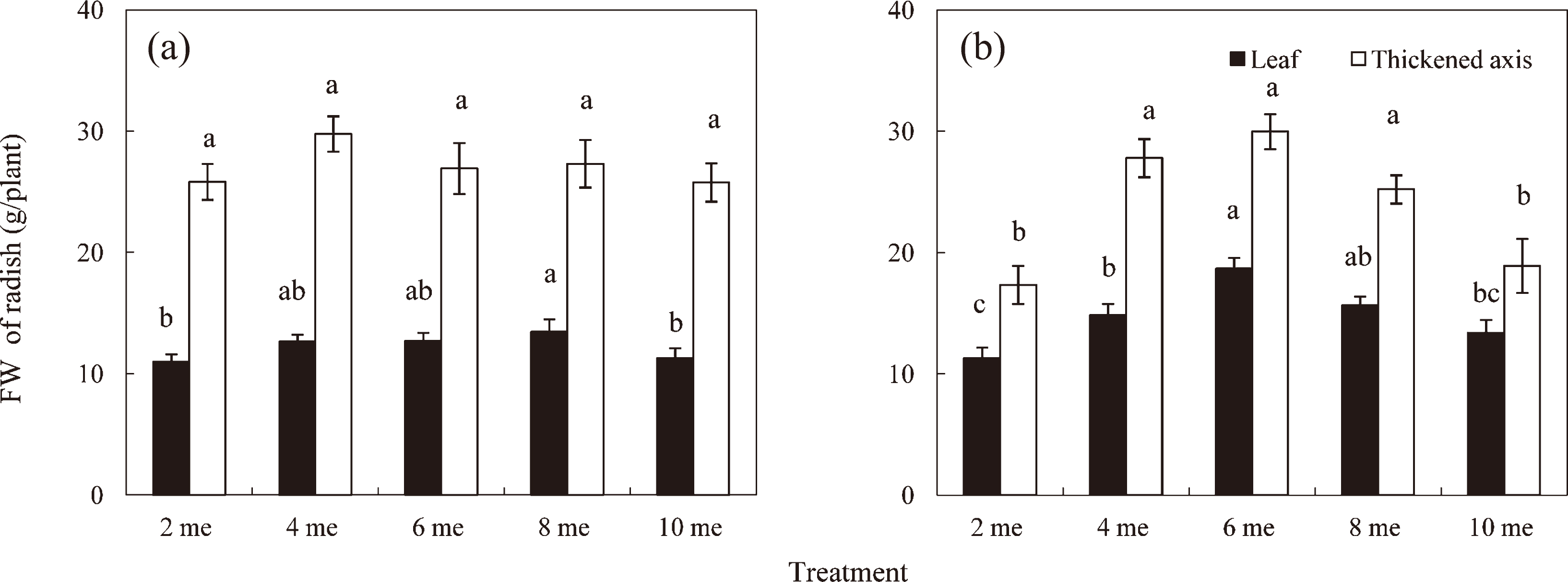
Effects of different levels of nitrate (2, 4, 6, 8, and 10 me·L−1) on fresh weight of leaves and thickened axes of radish plants. The data from Experiment 1 in Sept. and Nov. are shown in (a) and (b), respectively. Vertical bars indicate SD (n = 9). Means with the same letter are not significantly different by Tukey’s test at P < 0.05.
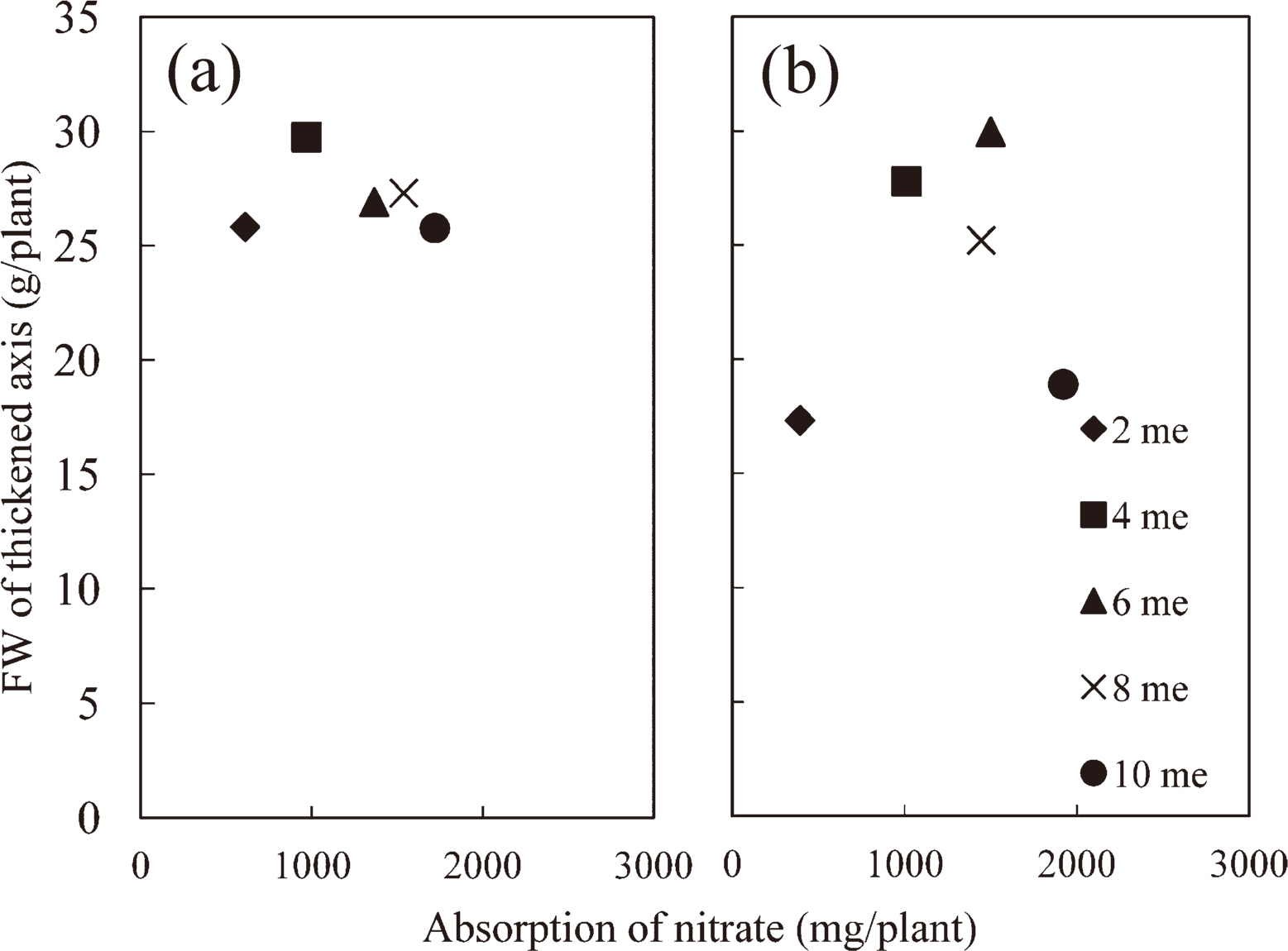
Relationships between shoot fresh weight and NO3− absorption in Experiment 1. The data from Experiment 1 in Sept. and Nov. are shown in (a) and (b), respectively.
EC values (dS·m−1) of the nutrient solution decreased from 1.3 to 0.6 in WAN-supplied solution, whereas they increased from 0.3 to 1.1 for 16 days after transplanting in AWAN-supplied solution, followed by their decrease to 0.9 (Fig. 3a). At the end of the experiment, EC values were in the range of 0.6 to 0.9, suggesting that a large amount of nutrients remained unabsorbed in the AWAN-and WAN-supplied nutrient solutions. Moreover, similar trends of changes in the levels of NO3− (Fig. 3b) and the other macro elements (data not shown) to that in EC also suggest that an excess amount of nutrients was supplied by these three different methods of nutrient supply. The level of sodium ions was below 0.4% (data not shown), indicating that the toxic effect of sodium ions on the plants is negligible. Significant differences in the growth of thickened axes and leaves were not obtained among plants grown by the three different methods of nutrient supply (Table 3). The values of LER were higher in the early stage of growth and thereafter decreased during the experimental period (Fig. 4). Compared with AWAN-supplied plants, EC-control plants appear to have significantly higher LER in the early stage. In the later stage, moreover, no differences among treatments were observed in LER.

Changes in EC values (a) and NO3− concentration (b) of nutrient solution in Experiment 2.
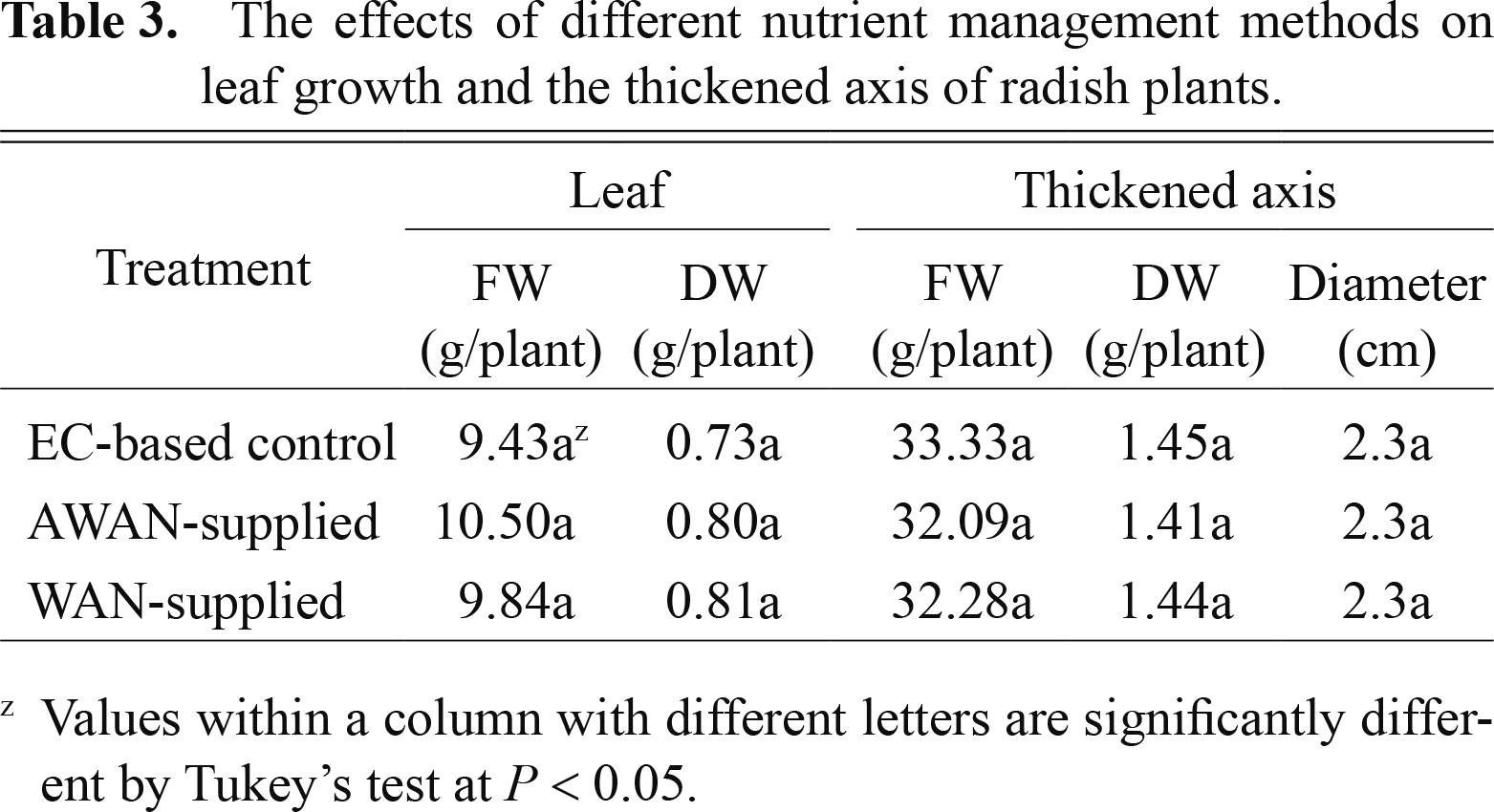
The effects of different nutrient management methods on leaf growth and the thickened axis of radish plants.
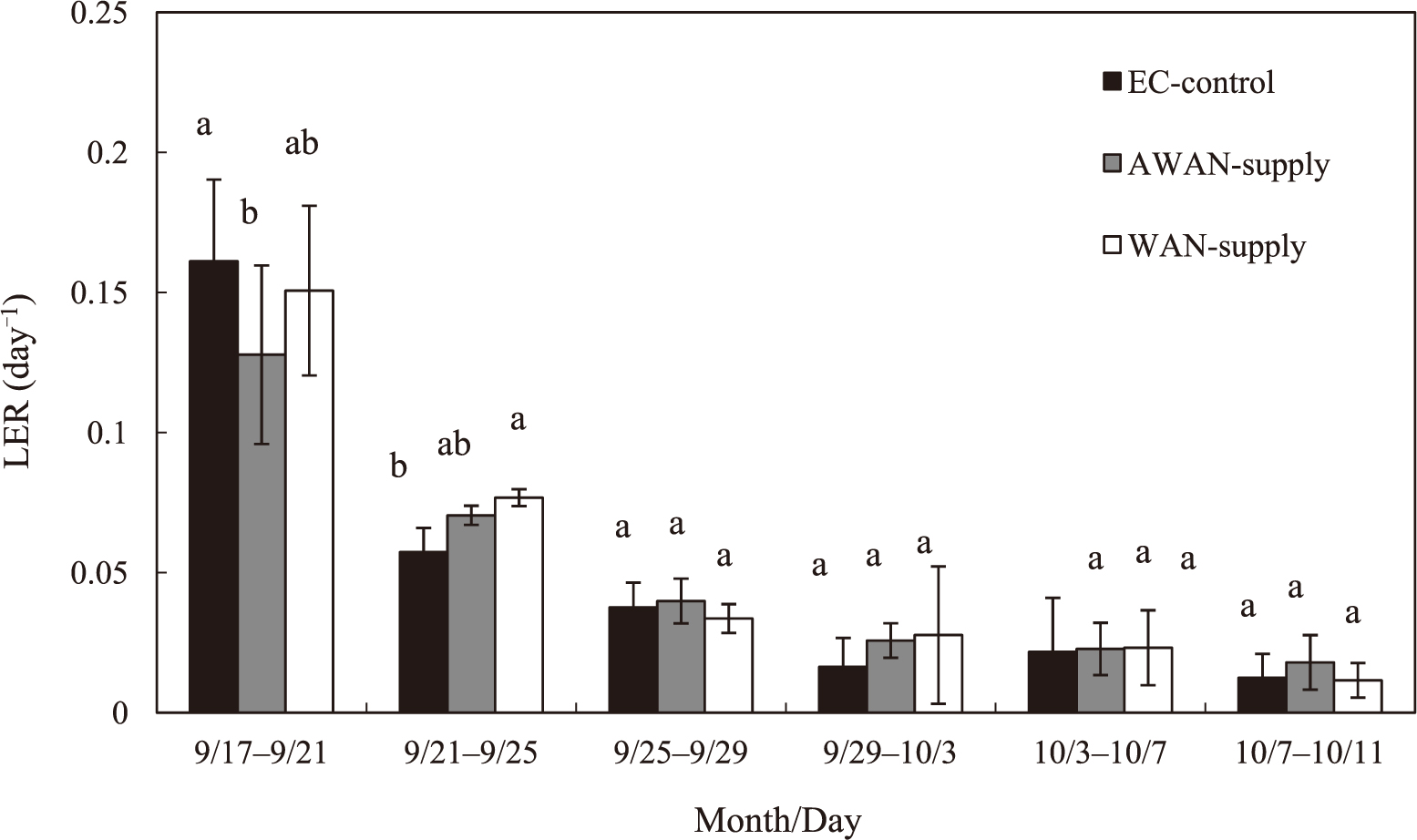
Changes in leaf elongation rate (LER) of the second foliage leaves of radish plants grown by EC-based control treatment, AWAN-supply treatment and WAN-supply treatment. Vertical bars indicate SD (n = 5). Means with the same letter are not significantly different by Tukey’s test at P < 0.05.
In WAN-supplied treatments of different amounts of nutrients containing 900, 800, and 700 mg NO3−·per plant conducted on the lower temperature season (in Dec.), EC values decreased to 0.5–0.4 dS·m−1 (Fig. 5a) and levels of NO3− decreased to 60–15 ppm during the 32 days of the experiment (Fig. 5b). Although the fresh weight of leaves was unaffected by these treatments (Fig. 6), the fresh weight of thickened axes decreased at 700 mg/plant NO3−.

Changes in EC values (a) and NO3− concentration (b) of nutrient solution in Experiment 2. Plants were supplied with the whole amount of mineral nutrients containing 900, 800, and 700 mg/plant of NO3− at the beginning of the experiment.
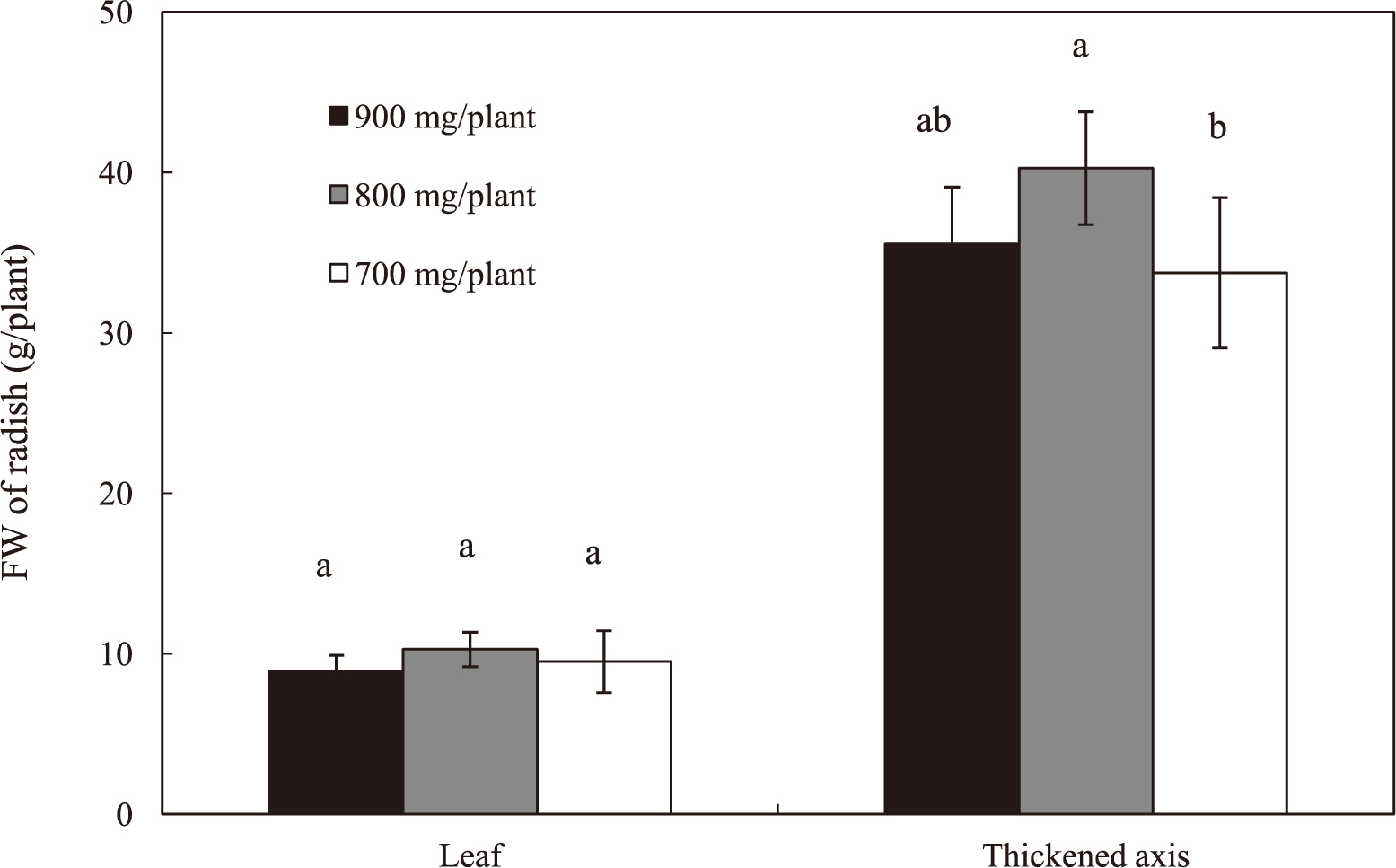
Effects of different amounts of nitrate on fresh weight of leaves and thickened axes of radish plants. Plants were supplied with the whole amount of mineral nutrients containing 900, 800, and 700 mg/plant of NO3− at the beginning of the experiment. Vertical bars indicate SD (n = 5). Means with the same letter are not significantly different by Tukey’s test at P < 0.05.
In the QNM method, plants are supplied with a limited amount of nutrients needed for their growth to marketable size. Total amount of nutrients required during the period of cultivation can be estimated (1) by the direct measurement of nutrient absorption by plants grown to marketable size and (2) on the basis of their levels in plants at harvest. In the experiment using the EC-based control method with nutrient solutions containing different levels of NO3−, i.e. 2, 4, 6, 8, and 10 me·L−1, the fresh weight of thickened axes of plant was unaffected by these NO3− levels in Sept. Since more than 1.3% Na+ (dry weight %) accumulated in thickened axes of the plants supplied with 6–10 me·L−1 (data not shown), it is possible that the fresh weight of thickened axes of these plants decreased due to the toxic effect of accumulated Na+. Considering the effects of accumulated Na+, it is difficult to estimate the effect of NO3− absorbed at higher levels. However, the fresh weight of plants supplied with 4 me·L−1 reached marketable size (approximately 30 g), suggesting that the amount of NO3− required for radish growth was provisionally presumed to be approximately 1000 mg/plant (Fig. 2). However, unabsorbed NO3− remained in the nutrient solution at the concentration of approximately 115 mg·L−1 in WAN-supplied treatment supplied with 1000 mg/plant of NO3− (Experiment 2), suggesting that plants were supplied with an excess amount of NO3−. On the other hand, the levels of NO3− in the nutrient solutions of WAN-supplied treatments with 900, 800, and 700 mg/plant of NO3− decreased to lower levels at the end of the experiment (Fig. 5b) and the fresh weight of thickened axes decreased at 700 mg/plant (Fig. 6), indicating that 800 mg/plant of the total amount of NO3− is required to produce a marketable size of radish plants. According to Takahashi and Iijima (1956), nitrogen content of a small, quickly maturing type of radish plants, which is similar to the variety used in this experiment, was reported to be approximately 6.5% on the dry weight basis. Accordingly, the radish plant (thickened axes and leaves) of marketable size with an average dry weight of 2.35 g in this experiment (data not shown) was estimated to contain 153 mg/plant of nitrogen. Assuming that this amount of nitrogen is derived from NO3− supplied during cultivation, the radish plant is considered to absorb 677.6 mg NO3− from nutrient solution. This value is slightly smaller than 800 mg/plant in Experiment 2 in Dec., indicating the possibility that we overestimated the amount of NO3− required to produce a marketable size of radish plants.
Previous studies showed that higher temperature enhanced the absorption of NO3− (Kaminishi and Kita, 2006; Nieuwhof, 1995). Our results also showed that the responses of radishes to NO3− supplied in Sept. and Nov. were different, suggesting that temperature is an important factor affecting the radish response to NO3−. The relationship between the fresh weight of the thickened axis and NO3− absorption showed that the fresh weight of the thickened axis was unaffected by the broad range of NO3− absorption in Sept. (Fig. 2a), and the minimum level of NO3− absorption by plants without inhibiting the growth of the thickened axis might be between 395–1000 mg/ plant (2–4 me·L−1) in Nov. (Fig. 2b). From the experiment in which NO3− supply was limited to 900, 800, and 700 mg/plant in Dec., we concluded that NO3− supply of 800 mg/plant was needed to grow radishes without yield reduction of the thickened axes. However, these temperature effects suggest the possibility that the amount of NO3− could be reduced to less than 800 mg/plant when growth temperature is high.
The basic concept of the QNM methods is that crop plants are supplied with a limited amount of nutrients so that their growth or yield is not reduced at the time when they require adequate nutrients for their normal growth. Accordingly, the precise control of nutrient supply to crop plants with a computerized system equipped with a variety of sensors for specific mineral ions would be required over the whole cultivation period. On the other hand, the simplest QNM method, supplying the whole amount of nutrients required for crop growth at the beginning of cultivation, was reported to be highly applicable to the hydroponic cultivation of spinach plants (Takei and Suzuki, 2013). Likewise, it was shown in this experiment that radish plants could be cultivated hydroponically by the simplest QNM method. In the plant supplied with 800 mg/plant of NO3− in WAN-supplied treatment, nearly 91% NO3− supplied was absorbed and the highest biomass of the thickened axis was obtained at the end of cultivation (Fig. 5). This implies that the efficiency of NO3− application was very high. According to Wang and Ito (1998), the residual concentration of NO3− in the solution was decreased to lower levels by the supply of supplemental solution containing a higher level of potassium ion for 6 days prior to harvest in the hydroponic cultivation of spinach plants. Their result suggests that potassium ions play a significant role in the absorption of NO3− by spinach plants. Thus, further studies are required to clarify the interactions among the nutrient elements in NO3− absorption by radish plants.
A large change in the nutrient concentration during the growth period is considered to affect radish growth; higher concentrations of NO3− at the early stage of growth promoted leaf growth by excess absorption, whereas lower concentrations of NO3− at the late stage retarded the thickening growth by its depletion. Compared with AWAN-supplied plants, WAN-supplied plants did not appear to have significantly higher LER in the early stage (Fig. 4). In the later stage, moreover, no differences were observed. Thus, the yield of radish plants grown by the QNM method was unaffected by higher and lower concentrations of nutrient solution at the early and late stages of plant growth, respectively. The growth phase of radish plants can be divided into two: Phase 1 and Phase 2 (Suzuki, 1976). The active thickening growth in Phase 2 is supported by the photosynthesis of foliage leaves fully developed in Phase 1. Under higher temperature conditions, however, thickening growth is retarded by the stimulation of leaf growth (Suzuki, 1978). The results of this experiment suggest that growth stimulation of foliage leaves by the higher nutrient concentration in the QNM method was not observed in the early stage of growth, resulting in no inhibitory effect on the thickening growth in the late stage. Further studies are needed on (1) the effect of higher and lower nutrient concentrations on nutrient absorption by radish plants and (2) the role of nutrients absorbed excessively during the early stage of growth in the later thickening growth of radish plants.
In conclusion, we propose that the QNM method, supplying the whole amount of nutrients required for crop growth at the beginning of cultivation, is the simplest method with which radish plants of marketable size could be produced without draining nutrient solution containing a large amount of NO3− from the hydroponic system into the environment. In this experiment, we did not estimate the NO3− level in thickened axes, because the accumulation of nitrate in root vegetables was reported to be less than in leaf vegetables (Murata et al., 2010). Considering the usefulness of foliage leaves of radishes for salad, further experiments could be needed to estimate nitrate levels of radishes grown by this method.