2014 Volume 83 Issue 1 Pages 81-89
2014 Volume 83 Issue 1 Pages 81-89
Japanese apricot (Prunus mume Siebold & zucc.) fruits of ‘Nanko’ have softer flesh than those of ‘Gojiro’. Therefore, there are differences in the processability of these cultivars. We investigated the characteristics of cell-wall polysaccharide degradation that significantly affects fruit firmness in these 2 cultivars at unripe, ripe, and drop stages. Fruit firmness of ‘Gojiro’ was maintained during unripe and ripe stages and decreased after the ripe stage, while that of ‘Nanko’ decreased constantly and was lower than that of ‘Gojiro’ at ripe and drop stages. Amount of the pectin and the hemicellulose substances decreased commonly in ‘Gojiro’ and ‘Nanko’ in the process of fruit softening, in addition, decrease in mol wt of these polysaccharides significantly synchronized with decrease in fruit firmness, suggesting that degradation of the pectin and the hemicellulose cause the softening of Japanese apricot fruits. The amount of neutral sugar (NS) and uronic acid (UA) in the pectin fraction and NS in the hemicellulose fraction in ‘Nanko’ decreased more than in ‘Gojiro’. Moreover, the cellulose content of ‘Nanko’ decreased during the fruit-softening process. These results suggest that higher degradation of pectin, hemicellulose, and cellulose in ‘Nanko’ fruits would result in softer fruits than ‘Gojiro’.
In Wakayama prefecture, the yield of apricot fruits is the largest in Japan (MAFF statistical report, 2012). Japanese apricot fruits are mainly processed as pickled ume or ume liquor. As the raw material fruit for pickled ume, softness of the flesh is one of the most important characteristics. The mature fruit of ‘Nanko’, the major cultivar in Wakayama Prefecture, has soft flesh. Eighty percent yield of ‘Nanko’ are harvested at the dropping stage at full maturity and processed for pickled ume. On the other hand, ‘Gojiro’ fruits, the secondary major cultivar in Wakayama prefecture, still have a hard flesh at the full mature stage and are not suitable for pickled ume but are used for producing ume liquor. This cultivar starts to bloom later than ‘Nanko’; however, the fruits grow faster than those of ‘Nanko’; hence, the harvesting time is earlier than ‘Nanko’.
The characteristics of fruit flesh are known to be associated with the cell-wall polysaccharide components, which lead to loosening of the structure and softening of the flesh (Dey and Brinson, 1984; Gross and Sams, 1984). Decrease in the neutral sugar content of the cell wall (Ahmed and Labavitch, 1980; Jermyn and Isherwood, 1956; Knee, 1973; Knee et al., 1977; Sakurai and Nevins, 1997; Tsuchida et al., 2003; Wallner and Bloom, 1977) and depolymerization of polysaccharides (Rose et al., 1998; Sakurai and Nevins, 1993, 1997; Terasaki et al., 2001; Tsuchida et al., 2004; Yakushiji et al., 2001) have been described for softening fruit; hence, clarifying cultivar differences in cell-wall-degrading characteristics would contribute to the processability of Japanese apricot fruits.
Several reports have clarified that pectic polysaccharides decrease during the softening process of Japanese apricot fruits (Kaneko et al., 1983, 1995; Otoguro and Kaneko, 1993; Otoguro et al., 1993, 1995). However, they did not fully investigate other polysaccharides such as hemicellulose and cellulose. Hence, there are still unknown characteristics of overall cell-wall degradation during the softening process of Japanese apricot fruits. In this report, we investigated changes in the sugar composition and molecular distribution of cell-wall polysaccharides during the softening process in Japanese apricot fruits of ‘Gojiro’ and ‘Nanko’.
Four fruits of 8-year-old Japanese apricot trees of ‘Gojiro’ and ‘Nanko’ in the open field of the Japanese apricot laboratory in Minabe, Wakayama prefecture were obtained according to the fruit softening stages of unripe, ripe, and drop at full maturity. Unripe fruits were obtained when the surface of fruit showed no luster because of remaining trichome, and fruit firmness measured by a penetrometer (Fujiwara Factory Co., Ltd., Tokyo, Japan) indicated approximately 1.5 kg. Fruit firmness was judged by introducing a column probe (1.9 mm in diameter) to a depth of 4.5 mm at one point on the equatorial plane of the fruit through the peel and the maximum stress perceived by the probe was recorded.
Ripe fruits, at the stage of harvesting for processing ume liquor, were obtained when 30% surface of the fruit skin showed luster caused by falling trichome based on visual assessment, demonstrated by Oe et al. (2012). Drop fruits were obtained just after dropping at full maturity. ‘Gojiro’ (full bloom February 17, 2009) fruits were harvested on May 21 (unripe), June 1 (ripe) and 12 (drop), 2009. ‘Nanko’ (full bloom February 2, 2009) fruits were harvested on June 2 (unripe), 16 (ripe), and 24 (drop), 2009. Fruit firmness of each sample was measured as mentioned above.
Extraction of cell-wall fractionCell-wall fractions were extracted according to the method described by Sakurai and Nevins (1997). A 10-g sample of flesh (mesocarp) was homogenized in 80% EtOH successively with a homogenizer (model AM-3; Nihonseiki, Niigata, Japan), a polytron homogenizer (model PB95; SMT, Tokyo, Japan) and a glass homogenizer (model HOM; As one, Osaka, Japan). The homogenate was boiled for 15 min and then centrifuged for 15 min at 4000 × g. The supernatant was designated as the EtOH fraction. The precipitate was dispersed in Na-acetate buffer (pH 6.84) and the residue was incubated for 2 hr at 80°C with 500 units of α-amylase (Bacillus amyloliquefaciens α-amylase; Sigma Aldrich, St. Louis, MO, USA). The mixture was centrifuged (15 min at 4000 × g) and the supernatant was designated as the starch fraction. The precipitate was dispersed in distilled water, heated for 10 min at 100°C, and then centrifuged (15 min at 4000 × g). This treatment was repeated three times. The combined supernatant was designated as the water-soluble (WS) fraction. The precipitate was dispersed in EDTA solution (50 mM, pH 6.8), heated for 15 min at 100°C, and then centrifuged (15 min at 4000 × g). This treatment was repeated three times. The combined supernatant was designated as the pectin fraction. The precipitate was treated with 17.5% NaOH containing 0.02% NaBH4 and centrifuged (10 min, 4000 × g). This treatment was repeated three times for a total of 24 hr. The combined supernatant neutralized with glacial acetic acid was designated as the hemicellulose fraction. Finally, the precipitate was washed twice successively with 30 mM acetic acid, EtOH, EtOH: diethylether (1:1, v/v), and dietylether, then dried at room temperature for 3 days and designed as the cellulose fraction.
Total sugar and uronic acid analysisTotal sugar (TS) content of the EtOH-soluble, starch, WS, pectin, hemicellulose, and cellulose fractions was estimated by the phenol-sulfuric acid method, using glucose as a standard (Chaplin, 1986). The dried cellulose fraction was dissolved in 1.5 mL 72% H2SO4 (w/w), kept for 1 hr at room temperature, diluted with 40.0 mL distilled water, then subjected to the phenol-sulfuric acid method. Uronic acid (UA) of the WS, pectin, and hemicellulose fractions was estimated by the m-hydroxydiphenyl method (Blumenkranz and Asboe-Hansen, 1973). After analysis, the WS fraction was consequently lyophilized. The pectin and hemicellulose fractions were dialyzed for 48 hr against distilled water and then lyophilized.
Sugar composition analysisThe lyophilized samples (equivalent to ca. 1 mg TS) of the WS, pectin, and hemicellulose fractions were analyzed for neutral sugar (NS). The samples were hydrolyzed by 2 M trifluoroacetic acid containing 300 μg myo-inositol as an internal standard. The hydrolyzed monomeric sugars were reduced by treating with 10 mg NaBH4 in 0.5 mL of 2 M NH4OH, then acetylated by adding 200 μL acetic anhydride with 40 μL of 1-methylimidazole as a catalyst. The acetylated sugars were dissolved in 200 μL acetone. An aliquot of the acetone solution was introduced into GLC (model 1700; Shimadzu, Kyoto, Japan), equipped with a capillary column (SP-2340, 0.25 mm i.d. × 30 m; Supelco, St. Louis, MO, USA). The injection temperature was 240°C and the column temperature 220°C. One minute after introduction of the acetone solution, the column temperature was raised at a rate of 2°C per min to 245°C, and maintained for 8 min.
Molecular weight analysis for the cell-wall polysaccharidesThe lyophilized samples (equivalent to ca. 1 mg TS) of WS, pectin, and hemicellulose fractions were dissolved in 50 mM Na-phosphate buffer (pH 7.2) and introduced into an HPLC system (model LC-10AD; Shimadzu), equipped with a gel-filtration column (TSK-Gel G5000 PW, 7.5 mm × 30 cm; Tosoh, Yamaguchi, Japan). The samples were eluted with 50 mM Na-phosphate buffer (pH 7.2) at a flow rate of 0.5 mL·min−1. Fractions were collected at 0.5 min intervals and then TS and UA contents at each retention time (Rt) were estimated by the method mentioned above. Standard dextrans of known molecular weights were run on the column for molecular calibration.
Fruit firmness of ‘Nanko’ linearly decreased as the softening process progressed, whereas that of ‘Gojiro’ fruits was maintained between the unripe and ripe stages then decreased (Fig. 1). Fruit firmness of ‘Gojiro’ throughout the ripe and drop stages was significantly higher than that of ‘Nanko’.
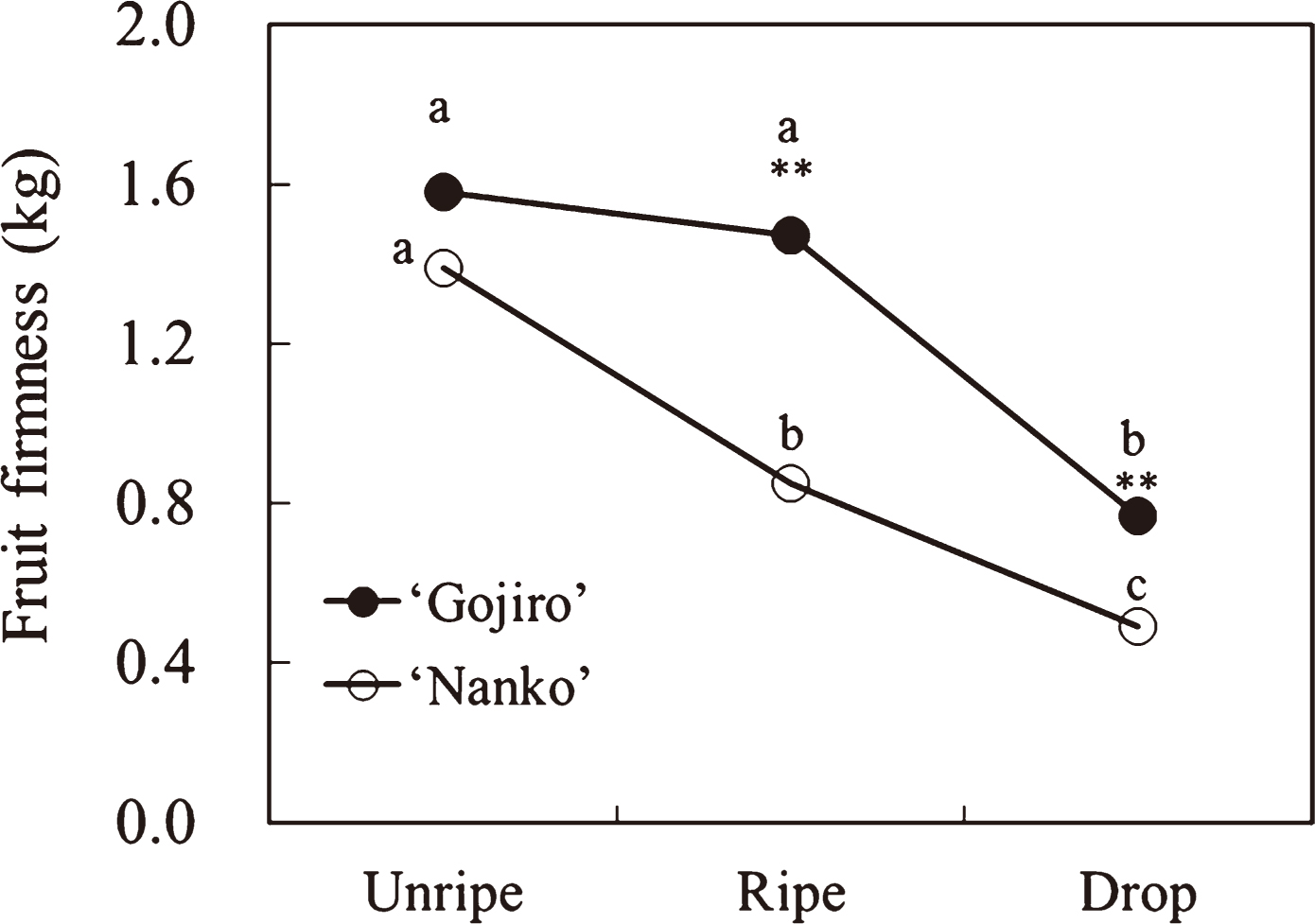
Changes in fruit firmness of Japanese apricot ‘Gojiro’ and ‘Nanko’. Different letters indicate significant differences among unripe, ripe, and drop fruit at the 5% level by Tukey’s multiple-range test (n = 4). **represents values significant higher than that of ‘Nanko’ at 1% level by t-test (n = 4).
The amount of EtOH-soluble fractionation increased with the progression of fruit softening. The amount of ‘Gojiro’ specifically increased between the ripe and drop stages and that of ‘Nanko’ increased between the unripe and ripe stages (Table 1).

EtOH-soluble fractionation and starch content of Japanese apricot ‘Gojiro’and ‘Nanko’flesh at different softening stages (mg·g−1 FW).
Starch content did not change in ‘Gojiro’ during the softening process (Table 1), while that of ‘Nanko’ decreased slightly. Starch content in the drop fruits of ‘Nanko’ was lower than that of ‘Gojiro’.
Polysaccharide contents in the cell-wall fractionsFigure 2 shows the polysaccharide contents in each cell-wall fraction. The hemicellulose fraction was the major polysaccharide, followed by the cellulose, pectin, and WS fractions. UA content in the hemicellulose fraction was significantly low.
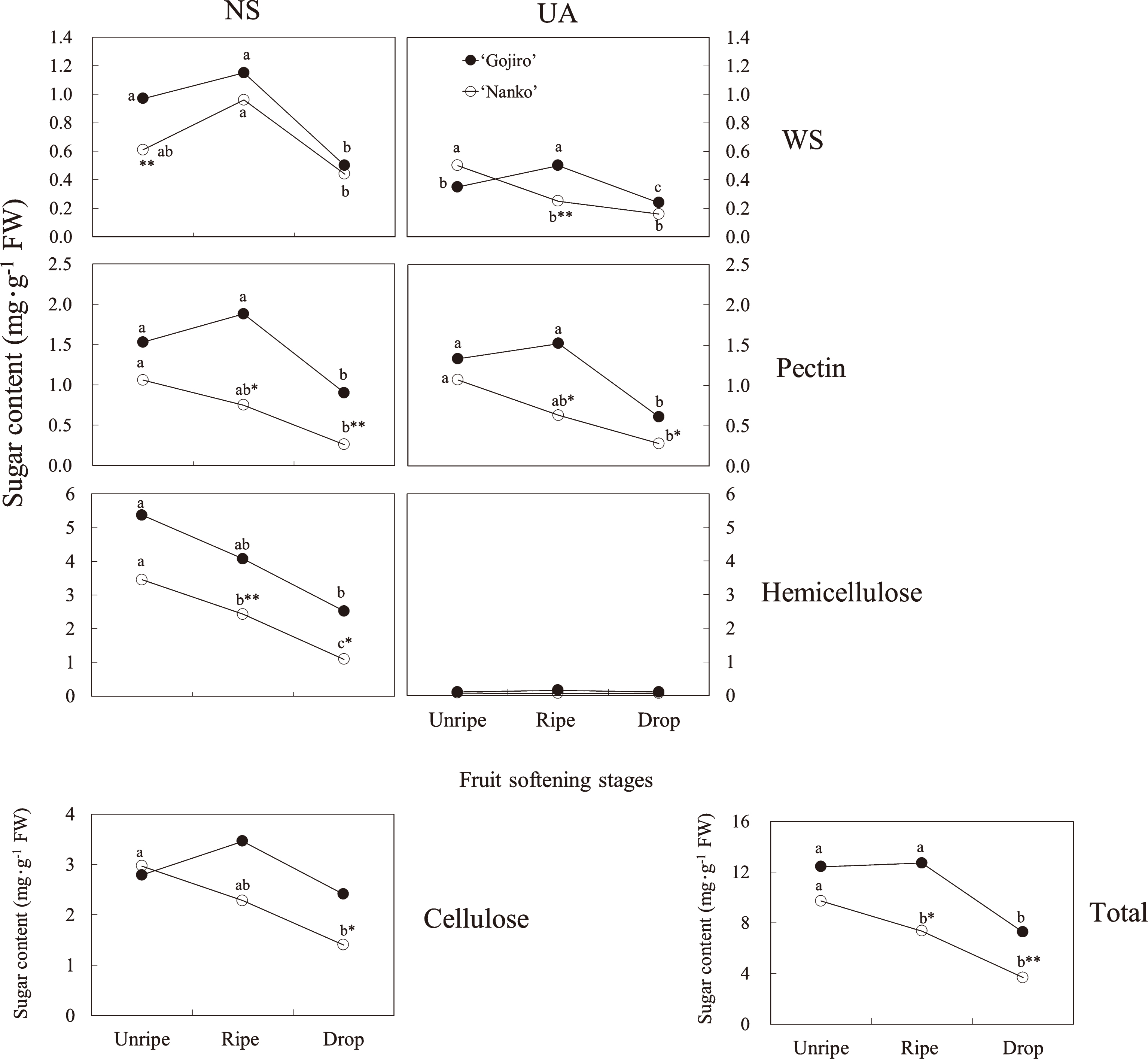
Sugar content of cell-wall polysaccharide fractions in Japanese apricot ‘Gojiro’ and ‘Nanko’ at different softening stages (mg·g−1 FW). Total neutral sugar (NS) content was determined by GLC. Cellulose content was determined by a phenol sulfuric acid method. Uronic acids (UA) were determined by a m-hydroxydiphenyl method. Different letters indicate significant differences among unripe, ripe, and drop fruit at the 5% level by Tukey’s multiple-range test (n = 4). *,**significantly lower than ‘Gojiro’ at 5 and 1%, respectively by t-test (n = 4).
In the WS fraction, NS of ‘Gojiro’ decreased by 48.5% during the fruit softening process, while that of ‘Nanko’ decreased by 27.9%. UA in the WS fraction of ‘Gojiro’ decreased by 31.4% during the fruit-softening process, while that of ‘Nanko’ decreased by 68.0%.
In the pectin fraction, NS and UA contents, and in the hemicellulose fraction, NS content decreased in both cultivars during the softening process. However, a difference in the rate of reduction between the 2 cultivars, i.e., in ‘Gojiro’, NS and UA contents decreased by a maximum of 54.1% during the softening process, whereas in ‘Nanko’, the reduction was more than 68.4%. The cellulose content in ‘Gojiro’ was constant during the softening process, while that in ‘Nanko’ decreased by 52.9%.
In ‘Gojiro’, total sugar content of the cell walls decreased by only 41.5% during the softening process, while that in ‘Nanko’ decreased by 62.1%. Consequently, between the ripe and drop stages, the total polysaccharide content was lower in ‘Nanko’ fruits than in‘Gojiro’ fruits.
NS composition in the WS, pectin, and hemicellulose fractionsIn the WS fraction, galactose (Gal) was the major constituent of the fruits of both the cultivars, followed by arabinose (Ara), glucose (Glc), mannose (Man), and xylose (Xyl) (Table 2). NS contents of the WS fraction did not change from the unripe stage to the ripe stage for the 2 cultivars, but subsequently, Gal content decreased markedly from the ripe stage to the drop stage. Fucose (Fuc), Xyl, Man, Gal, and Glc contents in the WS fraction in ‘Nanko’ were lower than those in ‘Gojiro’, mainly at the unripe stage.
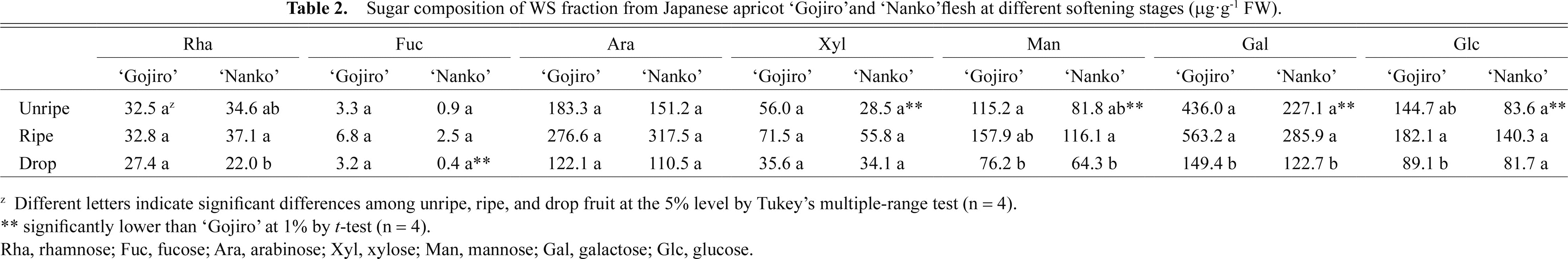
Sugar composition of WS fraction from Japanese apricot ‘Gojiro’and ‘Nanko’flesh at different softening stages (μg·g−1 FW).
In the pectin fraction, Ara was the major NS constituent, followed by rhamnose (Rha) and Gal (Table 3). The Ara content in ‘Gojiro’ decreased from the ripe to drop stage, while in ‘Nanko’ it decreased from the unripe to drop stage. Rha and Gal contents decreased markedly during the softening process in ‘Nanko’ fruits, whereas they did not change in ‘Gojiro’ fruits. Rha, Ara, Xyl, Man, and Gal contents in the pectin fraction of ‘Nanko’ fruits were lower than those of ‘Gojiro’ fruits at all or some stages.
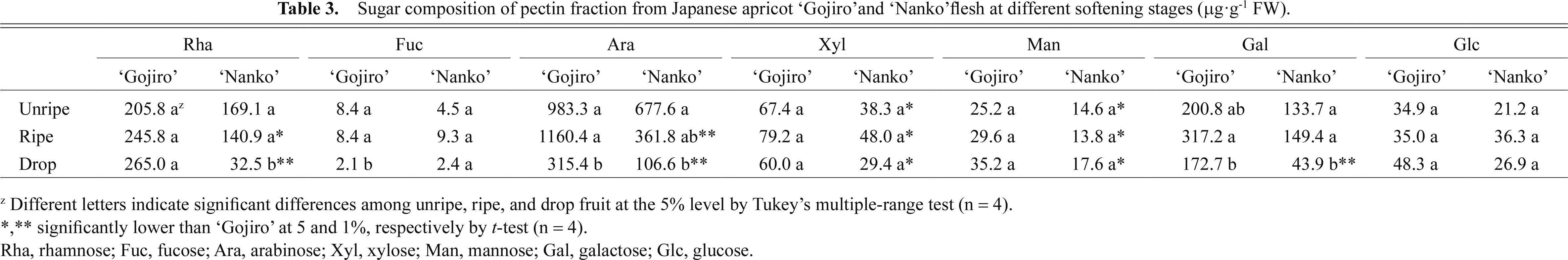
Sugar composition of pectin fraction from Japanese apricot ‘Gojiro’and ‘Nanko’flesh at different softening stages (μg·g−1 FW).
In the hemicellulose fraction, Glc was the major constituent, followed by Xyl, Gal, and Man (Table 4). Xyl content decreased by 71.8% in ‘Gojiro’ fruits and by 66.2% in ‘Nanko’ fruits during the softening process. Further, in ‘Nanko’ fruits, the Rha, Fuc, Man, Gal, and Glc contents decreased. All NS contents in the hemicellulose fraction of ‘Nanko’ fruits were lower than those of ‘Gojiro’ fruits, at mainly ripe and drop stages.
Sugar composition of hemicellulose fraction from Japanese apricot ‘Gojiro’and ‘Nanko’flesh at different softening stages (μg·g−1 FW).
The molecular weight distribution in the WS fraction of Japanese apricot fruits was determined by gel-filtration chromatography. The area of each chromatogram was drawn to represent the amount of total sugar (TS) and UA extracted from 1 g fresh weight of the fruit (Fig. 3). The WS fraction always contained a small amount of high molecular weight polymer and a large amount of small molecular weight polymer that were eluted at a single TS and UA peak (retention time [Rt] 17.25–18.75 min for TS and 17.75 min for UA) in both the cultivars. The decrease in TS and UA small molecular weight polymers was marked in both the cultivars.
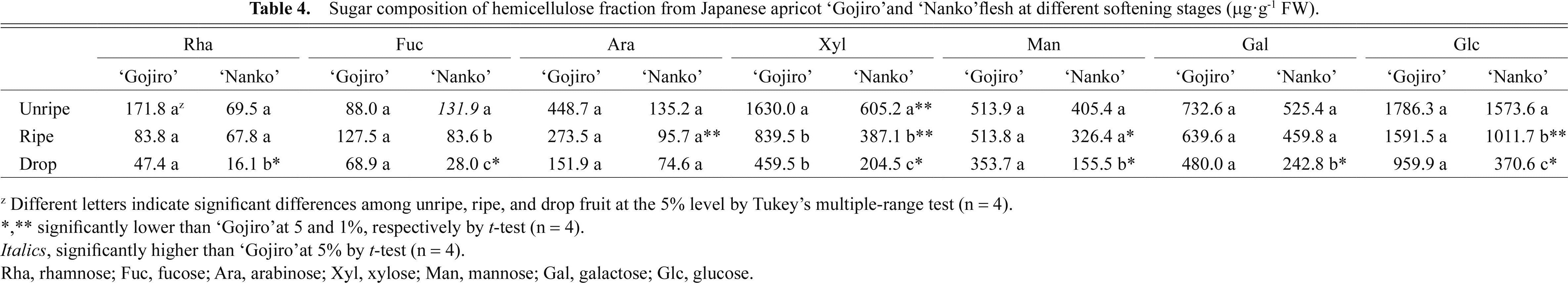
Molecular distribution profile of WS fraction from flesh of Japanese apricot ‘Gojiro’ and ‘Nanko’ fruits. An aliquot of the lyophilized powder of WS was subjected to HPLC with a gel-filtration column. The left row of panels shows the molecular distribution of TS estimated by phenol-sulfuric acid assay, and the right panels that of UA estimated by the m-hydroxydiphenyl assay. Total peak area represents the amount of sugar extracted from 1 g FW of tissue. The column was calibrated with dextrans of known molecular mass (413, 148, 65.5, 39, 9.9 kDa), as shown in the upper panel.
The pectin fraction contained 2 major TS peaks at high molecular weight (Rt, 12.25–12.75 min) and low molecular weight (Rt, 16.75–17.25 min) (Fig. 4). UA also showed 2 peaks at high molecular weight (Rt, 12.25–12.75 min) and low molecular weight (Rt, 16.75–17.25 min). In ‘Gojiro’, molecular distribution of TS and UA did not change between the unripe and ripe stages, after which a wide molecular range of these polymers decreased. In ‘Nanko’, a wide molecular range of TS polymers decreased between the unripe and ripe stages, after which only high molecular weight polymers decreased, while a wide molecular range of UA polymers decreased throughout the fruit-softening process.
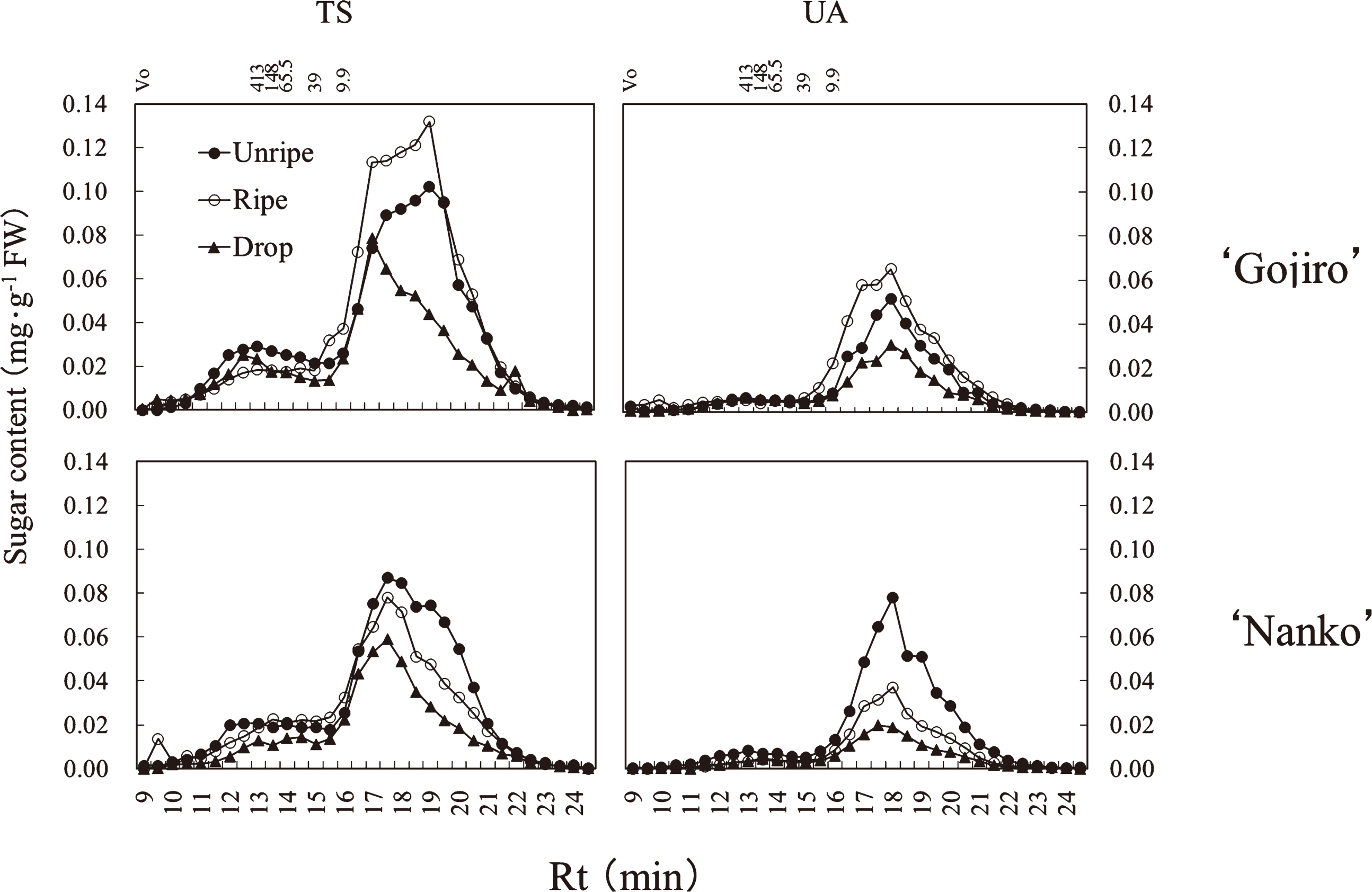
Molecular distribution profile of pectin fraction from flesh of Japanese apricot ‘Gojiro’and ‘Nanko’ fruits. An aliquot of the lyophilized powder of pectin was subjected to HPLC with a gel-filtration column. The left row of panels shows the molecular distribution of TS estimated by phenol-sulfuric acid assay, and the right panels that of UA estimated by the m-hydroxydiphenyl assay. Total peak area represents the amount of sugar extracted from 1 g FW of tissue. The column was calibrated with dextrans of known molecular mass (413, 148, 65.5, 39, 9.9 kDa), as shown in the upper panel.
In ‘Gojiro’ and ‘Nanko’, the hemicellulose fraction was composed of 2 TS peaks of high molecular weight (Rt, 13.75 min) and low molecular weight (Rt, 16.25 min) in the unripe fruit (Fig. 5). In ‘Gojiro’, high molecular weight TS polymers did not decrease from the unripe to ripe stages, however, it decreased between ripe and drop stages. In ‘Nanko’ fruits, high molecular weight TS polymers decreased from the unripe to ripe stages, followed by decrease in a wide range of TS polymers.
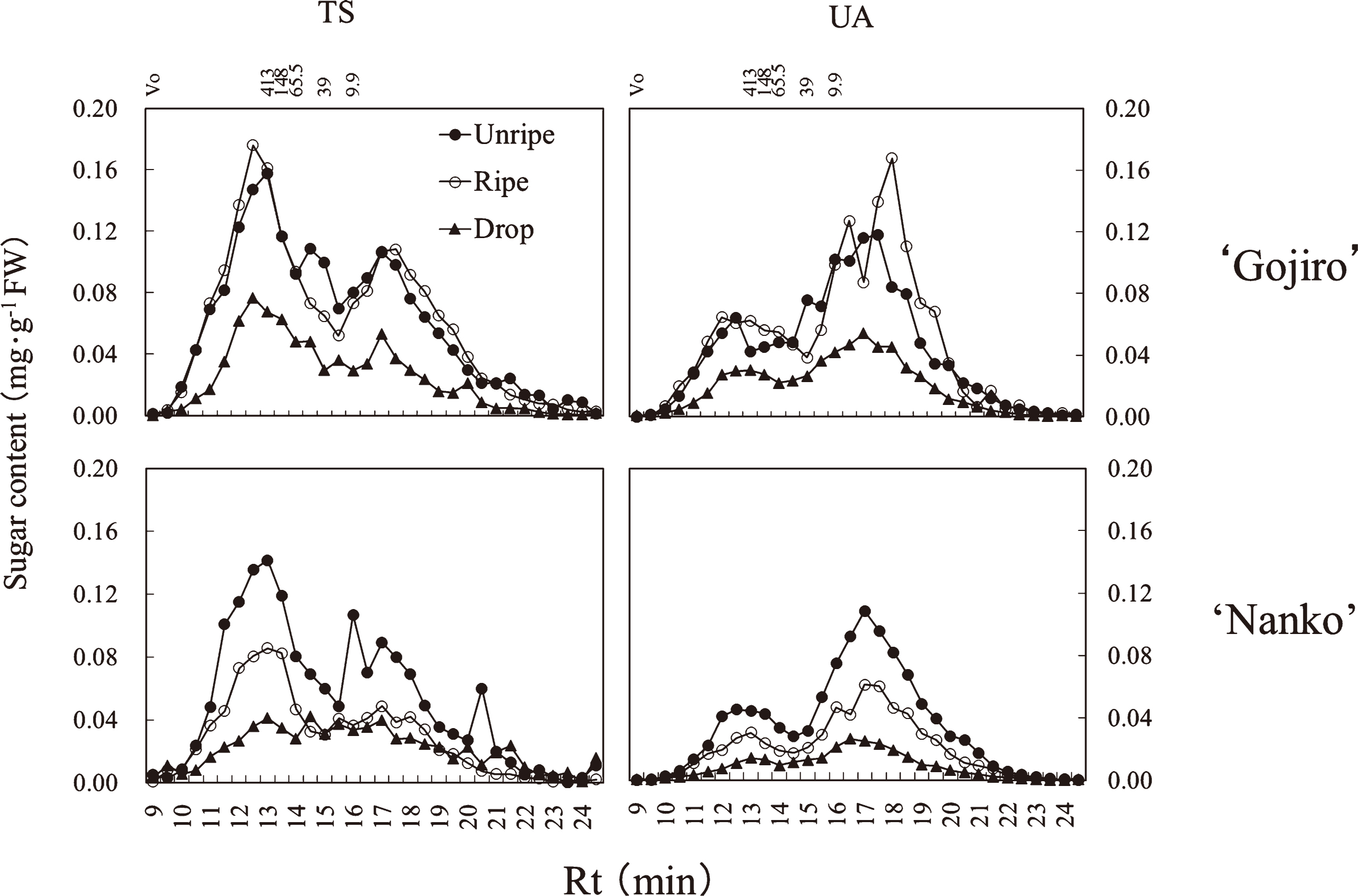
Molecular distribution profile of hemicellulose fraction from flesh of Japanese apricot ‘Gojiro’and ‘Nanko’ fruits. An aliquot of the lyophilized powder of hemicellulose was subjected to HPLC with a gel-filtration column. Panels show the molecular distribution of TS estimated by phenol-sulfuric acid assay. Total peak area represents the amount of sugar extracted from 1 g FW of tissue. The column was calibrated with dextrans of known molecular mass (413, 148, 65.5, 39, 9.9 kDa), as shown in the upper panel.
The fruit firmness of ‘Gojiro’ was maintained between the unripe and ripe stages and decreased after the ripe stage (Fig. 1). Conversely, that of ‘Nanko’ decreased linearly during the fruit-softening process. Total NS and UA contents in the pectin fraction and total NS content in the hemicellulose fraction in both cultivars, and the cellulose fraction in ‘Nanko’ decreased during the fruit-softening process, if not perfectly along with fruit firmness (Figs. 1 and 2). Instead of that, decrease in high molecular weight polysaccharides in the pectin and hemicellulose fractions of both cultivars synchronized with fruit firmness (Figs. 1, 4, and 5), suggesting that depolymerization of the polysaccharides of pectin and hemicellulose directly influences on Japanese apricot fruit firmness.
Previous studies have shown that only the pectin fraction decreased during the fruit-softening process of Japanese apricots (Kaneko et al., 1983, 1995; Otoguro and Kaneko, 1993; Otoguro et al., 1993, 1995). We cannot simply compare these different results because ammonium oxalate was used as the chelator in previous studies, whereas EDTA was used in our report. This difference may influence the composition and amount of extracted polysaccharides, although we consider that pectin and hemicellulose are important factors for the softening of Japanese apricot fruits, and in the case of ‘Nanko’, the cellulose fraction also influences fruit softening.
Even though an increase in the water-soluble pectin content of the walls during fruit softening has been shown in several studies (Ben-Arie et al., 1996; Carrington et al., 1993; Knee, 1978; McCollum et al., 1989; Muramatsu et al., 2004; Sakurai and Nevins, 1997; Siddiqui et al., 1996), suggesting that high molecular weight pectin polysaccharides are depolymerized into small water-soluble polysaccharides, our results were not in agreement. Instead, the content of EtOH soluble sugar increased during the fruit-softening process (Table 1). The EtOH soluble fraction may dissolve sugar oligomer but did not dissolve polymers. Terasaki et al. (2001) stated that sugars hydrolyzed from cell-wall polysaccharides during ripening metabolized to glucose, fructose, and sucrose in the EtOH-soluble fraction in kiwi fruits. Therefore, the pectin, hemicellulose, or in some cases, cellulose polysaccharides would be degraded directly into small oligomers or monomers of the sugar components during the softening process of Japanese apricot fruits.
The fruit firmness of ‘Nanko’ was less than that of ‘Gojiro’ between the ripe and drop stages (Fig. 1). This result may be attributed to the variety and amount of reduction in NS in the pectin and hemicellulose fractions during the softening process. In the pectin fraction of ‘Gojiro’ fruits, Fuc, Ara (Table 3), and UA (Fig. 2) contents decreased during the softening process, whereas in ‘Nanko’ fruits, Rha, Ara, Gal (Table 3), and UA (Fig. 2) contents decreased; further, their contents were lower than those in ‘Gojiro’ fruits during the softening process. The pectin fraction showed an increased rhamnogalacturonan backbone with side chains containing NS (i.e., l-arabinose, and d-galactose linked via l-rhamnosyl residues) (Aspinall, 1980; Gross and Sams, 1984; John and Dey, 1986). A decrease in Rha and UA contents in the pectin fraction in ‘Nanko’ indicates that the rhamnogalacturonan backbone degrades during the softening process. Moreover, the removal of galacturonan side chains, usually arabinans and galactans, directly influences fruit firmness (Huber, 1983). Thus, net loss of cell-wall Gal and Ara occurred in various fruit types during the softening process (Gross and Sams, 1984). In our results, both Gal and Ara content decreased in ‘Nanko’ fruits (Table 3), whereas only Ara content decreased in ‘Gojiro’. Therefore, degradation of the pectin fraction of ‘Nanko’ fruits would be more significant than that of ‘Gojiro’ fruit.
In the hemicellulose fraction of ‘Gojiro’ fruits, only the Xyl content decreased during the softening process (Table 4). Conversely, in ‘Nanko’ fruits, Rha, Fuc, Xyl, Man, Gal, and Glc contents decreased, and their contents were lower than those of ‘Gojiro’ during the fruit-softening process. Xyloglucan is the most abundant hemicellulose in the primary walls of spermatophytes, except for grasses (Scheller and Ulvskov, 2010). Xyloglucan consists of a backbone of β-1,4-linked d-glucosyl residues with single d-xylosyl residues bonded via α-1,6 linkages to the backbone (John and Dey, 1986). Additionally, some of the xyloglucan can be modified by the addition of Fuc (Campbell and Braam, 1999). Xyloglucan depolymerization was previously shown to be involved in fruit softening in avocado (Sakurai and Nevins, 1997), tomato (Sakurai and Nevins, 1993), grape (Yakushiji et al., 2001), and kiwifruit (Terasaki et al., 2001). These studies and our results indicate that xyloglucan in particular degraded in ‘Nanko’ fruits. Xyloglucan is strongly held at the surface of cellulose fibers (Beuer et al., 1973; Keegstra et al., 1973) and contributes to strengthening of the cell wall (Scheller and Ulvskov, 2010). In ‘Nanko’, the cellulose fraction decreased during the softening process (Fig. 2). Similar phenomena have been reported in dwarf Cavendish banana (Desai and Deshpande, 1978), apple (Siddiqui et al., 1996), and pear (Yamaki et al., 1979). These results indicate that xyloglucan and cellulose linkages loosen in ‘Nanko’ fruits during the softening process. Therefore, coordinated degradation of pectin, hemicellulose, and cellulose occurs specifically in ‘Nanko’ fruits during the softening process and results in softer fruits than ‘Gojiro’.
We gratefully acknowledge Yohei Arimoto for assistance with this investigation.