2014 Volume 83 Issue 2 Pages 122-132
2014 Volume 83 Issue 2 Pages 122-132
A recently popular Japanese yellow-green-skin table grape, ‘Shine Muscat’ (Vitis labruscana Bailey × V. vinifera L.), has the problem of berry skin browning, which occurs at the maturation stage just before harvest. Tiny reddish-brown blotches appear on the surface of berries and considerably decrease the grape’s market value. Although the mechanisms and factors for browning are unknown, we hypothesized the involvement of polyphenol compounds and their oxidation reactions. In this study, the gene expressions of polyphenol oxidase (PPO), stilbene synthase (STS), and chalcone synthase (CHS), which are key enzymatic genes related to the metabolic pathway for polyphenols, were analyzed during berry maturation to examine the molecular basis for browning. Skin browning occurred on several berries in a bunch of ‘Shine Muscat’ from 80 days after full bloom (DAFB), after which the number of berries with skin browning increased, and the browned area spread on the berry surfaces with maturation. Increases in the expression of VvPPO2, VvSTS type B, and VvCHS1 were associated with skin browning, and the trans-resveratrol content also increased in the browning skin, suggesting that biosynthesis and metabolic pathways for phenolic compounds were activated at the time of browning. In terms of VvPPO genes, specific up-regulation of VvPPO2 expression was observed compared with the VvPPO1 gene. The promoter sequence of VvPPO2 contains more Myb binding motifs and W-box motifs than does VvPPO1. The specific up-regulation of VvPPO2 gene expression will play a crucial role in understanding and managing the skin-browning mechanism in the grape berries of ‘Shine Muscat’.
‘Shine Muscat’ (Vitis labruscana Bailey × V. vinifera L.) is becoming a popular table grape cultivar in Japan. ‘Shine Muscat’ was developed in breeding programs conducted by the NARO Institute of Fruit Tree Sciences in Japan. It was originally produced by crossing “Akitsu-21” [‘Steuben’ (V. labruscana) × ‘Muscat of Alexandria’ (V. vinifera)] with ‘Hakunan’ (V. vinifera) in the breeding program, and it acquired the characteristics required to meet current consumers’ taste and desires, such as seedlessness, edible thin skin (pericarp), high Brix, and large berry size (Yamada et al., 2008). ‘Shine Muscat’ is a diploid grape with large berry size, bright yellow-green pericarp, crispy and juicy flesh, and aromatic flavors derived from ‘Muscat of Alexandria’. Seedless berry production is possible using gibberellic acid (GA3) and streptomycin treatment before the anthesis stage. In recent years, the cultivation area has markedly increased in Japan (Ministry of Agriculture, Forestry and Fisheries, 2007–2010).
In skin browning, tiny reddish-brown blotches appear on the surface of the berry pericarp at the maturing stage, and it is becoming one of the most severe problems in ‘Shine Muscat’ cultivation. Once browned, the grapes have a considerably reduced market value. This browning is thought to be a physiological disorder, and similar problems have been observed in other yellow-green-skin table grapes such as ‘Suihou’ and ‘Seto Giants’ (Ogoro et al., 2007). Although the mechanisms and factors causing browning are currently unclear, several technical approaches to overcome this problem are practiced in the cultivation trials.
Most of such browning reactions in plant tissues are caused by the production of polymerized browning substances inside and/or around the cells (Dehon et al., 2002). Polyphenol oxidase (PPO) is one of major enzymes of browning factors, catalyzing the oxidation of phenolic compounds, monophenols, and diphenols to generate reactive quinones, which can be polymerized themselves or with phenols. The polymerized substances appear as a brown color and react with amino acids and proteins (Guyot et al., 1996). In the broad sense, PPO is thought to oxidize o-diphenol to o-quinone. There are three related oxidases, catechol oxidase (diphenol oxidase, o-diphenolase, catecholase, EC1.10.3.1), lac-case (urushiol oxidase, EC1.10.3.2), and tyrosinase (monophenoloxidase, cresolase, EC1.14.18.1), which can be distinguished by differences in the specificity of substrates (Yoruk and Marshall, 2003). Because lac-case does not oxidize monophenols, catechol oxidase and tyrosinase are together referred to as PPO in many cases (Mayer, 2006). Intracellular localization of PPO occurs in the chloroplasts of photosynthetic tissues and the plastids of non-photosynthetic tissues (Vaughn and Duke, 1984). On the other hand, polyphenols, which act as substrates for PPO, are accumulated in vacuoles. The browning reaction by PPO hardly occurs under normal physiological conditions in the cell; however, once the cells are broken by physical injury from the outside and spatial partitioning in the cell is lost, the substrate polyphenols come into contact with the PPO (Vaughn and Duke, 1984; Yoruk and Marshall, 2003). The purpose of PPO in plant cells is actually unknown; however, its role as a biological defense mechanism has long been suggested (Constabel et al., 1995).
Phenylpropanoid biosynthesis is one of the more well-known and well-studied of the metabolic pathways in plant cells. It has been suggested that the phenylpropanoid metabolic pathway is induced by stress (Dixon and Palva, 1995). Stilbene synthase (STS) and chalcone synthase (CHS) are enzymes that catalyze a reaction using p-coumaroyl-CoA and malonyl-CoA as the substrates (Schröder et al., 1988; Schröder and Schröder, 1990). The pathway passing though the STS-catalyzing reaction is called the stilbenoid biosynthetic pathway, and the pathway through the CHS-catalyzing reaction is the flavonoid biosynthetic pathway. The substances synthesized through these metabolic pathways are secondary metabolites used to increase plant adaptability to environmental conditions (Tavares et al., 2013). Biotic and abiotic stresses have been demonstrated to be responsible for polyphenol accumulation, such as anthocyanin accumulation (Winkel-Shirley, 2002). The expression levels of genes encoding enzymes involved in these biosynthetic pathways are correlated with the stress suffered (Tillett et al., 2011). Stilbenoids, including resveratrol and its derivatives, are classified as phytoalexins, which can inhibit the growth of fungi infecting the plant body. On the other hand, in Botrytis cinerea, the conidia treated with resveratrol showed intracellular brown coloration because the resveratrol changed to brown granular material by oxidases in the pathogen (Adrian et al., 1998). This observation implies that resveratrol could be a possible substrate for browning.
In grapevine, the stilbenoid synthetic pathway was found to be activated by abiotic stress factors where STS was localized in the cell walls of berry skin (Fornara et al., 2008). This STS activity has been detected in the primary and secondary cell walls after UV treatment, results that are consistent with a defense function (Pan et al., 2009). In grapevine, CHS is encoded by a small gene family consisting of three homologous genes (Parage et al., 2012; Vannozzi et al., 2012), whereas STS is encoded by a large gene family with 48 homologous genes in the grape genome (Parage et al., 2012; Vannozzi et al., 2012; Velasco et al., 2007).
In this study, we targeted PPO, STS, and CHS genes to analyze their hypothesized involvement in the skin browning of berries in ‘Shine Muscat’. In many cases, physiological disorders of fruits and berries are caused by a complex interaction among factors. This study reveals one aspect of the physiological mechanisms responsible for skin browning in grape berries of ‘Shine Muscat’ using molecular-level analysis.
Three plants of 6-year-old ‘Shine Muscat’ (Vitis labruscana Bailey × V. vinifera L.) were used in this study under forcing culture at Shimane Experimental Station. Forcing cultivation was able to initiate an earlier harvest time by approximately 1 month ahead of rain-cut (normal) cultivation. The experiment was repeated over two growing seasons in 2011 and 2012. Flower clusters were treated with 200 ppm streptomycin (Meiji Seika Pharma, Tokyo, Japan), sprayed 10 days before full bloom, and then immersed in 25 ppm gibberellic acid (GA3; Kyowa Hakko Bio, Tokyo, Japan) solution adding 3 ppm of 1-(2-chloro-4-pyridyl)-3-phenylurea (CPPU; Kyowa Hakko Bio) at full bloom for seedless berry production. The developing bunches were treated again by immersing them in 25 ppm GA3 solution 10 to 15 days after full bloom. Three bunches per plant, nine bunches in total, were used for the analyses. The final bunch size was on average 707.1 and 704.5 g in 2011 and 2012, respectively. The final number of berries was adjusted to 40–45 per bunch each year. The yield of grapes per plant was on average 2,310 and 2,378 kg/10a in 2011 and 2012, respectively.
Two berries from each bunch, or six berries per plant, were periodically harvested 50, 60, and 70 days after full bloom (DAFB). Skin browning (Kasuri-sho in Japanese) was observed in several berries per bunch starting at 80 DAFB. Six normal berries and three to six berries with skin browning were harvested from each plant at 80 and 90 DAFB (Fig. 1). At 100 DAFB, all berries in bunches used for analysis began to show skin browning. Six berries with skin browning from each plant were harvested at 100, 110, and 120 DAFB. Because skin browning occurred in all berries from 100 DAFB onward, we analyzed only browning berries at 100, 110, and 120 DAFB. After harvest, berry skins were collected, immediately frozen in liquid nitrogen, and then stored at −80°C until use.

Skin browning on grape berries of ‘Shine Muscat’. (A) Whole picture of a bunch with skin browning berries 90 days after full bloom (DAFB); (B) Whole picture of a bunch with skin-browning berries at 120 DAFB; (C) apical part of the berry with skin browning at 90 DAFB; (D) apical part of the berry with skin browning at 120 DAFB; (E) surface of the berry skin with browning; (F) longitudinal section of the berry skin with browning and flesh; (G) brown stain around skin cells (close up of panel F).
Total RNA samples of berry skin were prepared by a hot borate extraction method (Wan and Wilkins, 1994). The total RNA sample was quantified using a spectrophotometer and adjusted to 200 ng·L−1. cDNA samples were synthesized from 1 μg total RNA after DNase I (TaKaRa Bio, Shiga, Japan) treatment. Reverse transcription was performed in a 20 μL reaction volume using ReverTra Ace (Toyobo, Osaka, Japan), mixed primers of oligo (dT) 20 primers, and random primers (9 mer), following the manufacturer’s protocol. After the reaction, 20 μL water was added to each reacted sample. Thus, 1 μL cDNA sample was equivalent to 25 ng total RNA. Three replicate samples were prepared for each sampling point.
Information of the gene sequences and primer designing for PPO, STS, and CHSInformation on gene sequences for polyphenol oxidase (PPO, catechol oxidase; EC1.10.3.1 and tyrosinase; EC 1.14.18.1), stilbene synthase (STS; EC 2.3.1.95), and chalcone synthase (CHS; EC 2.3.1.74) were obtained from the genome database (Grape Genome Browser), expressed-sequence tag (EST) data (Genbank/DDBJ/EMBL) for grapevines (Vitis vinifera L.) and from recent reports.
For polyphenol oxidase (PPO) genes, the sequence data were collected using the BLAST search program and then assembled and aligned using GENETYX software (Software Development, Tokyo, Japan). At least 4 predicted genes encoding PPO (Accession: XM_002275945, XM_002276076, XM_002275806, XM_002276622) were found in the grape genome stored in the NCBI database. Furthermore, 205 ESTs for the PPO gene were found using a BLAST search in the NCBI database. Due to the alignment and assembly of these sequences, they were consolidated into two types of homologous genes, which we named VvPPO1 and VvPPO2 in this study. They were respectively located on scaffold NW_003724172 and NW_003724396 in the grape genome data of NCBI. These genes were confirmed in the Ensembl Plants (EMBL-EBI) database. The VvPPO1 gene was located on chromosome 10 (Scaffold: FN595540.1, Transcript ID: VIT_10s00116g00560), whereas the location of the VvPPO2 gene was uncertain (Scaffold: Un:30100001...30200000, Transcript ID: VIT_00s0480g00040) in the grape genome. The homology between VvPPO1 and VvPPO2 was 73% in the nucleotide sequences and 63% in the deduced amino acid sequence (Table 1). VvPPO1 was previously studied as GPO1 by Dry and Robinson (1994). Several sequence data for GPO1 (VvPPO1) were stored in the NCBI database (Accession: Z27411, U83274). In contrast, VvPPO2 has not been reported in any grape research so far.

Homology (%) in PPO, STS, and CHS homologous genes of grapevine (Vitis vinifera L.) in the nucleotide coding sequences (CDSs) (left) and deduced amino acid sequences (right).
For stilbene synthase (STS) and chalcone synthase (CHS) genes, the detailed explanations are in recent reports (Goto-Yamamoto et al., 2002; Gutha et al., 2010; Parage et al., 2012; Vannozzi et al., 2012). The 48 predicted STS genes in the grape genome have been divided into three groups based on phylogenic analysis (VvSTS types A, B, and C in this study) (Parage et al., 2012; Vannozzi et al., 2012). According to Vannozzi et al. (2012), five of the predicted genes are type A, 22 are type B, and 13 are type C. The remaining eight genes do not have complete ORFs. Although the homologies in the deduced amino acid sequences among types A, B, and C were low, the nucleotide sequences showed higher homology (Table 1). CHS genes in the grape genome were reported by Goto-Yamamoto et al. (2002) and Gutha et al. (2010). Three genes encoding CHS, VvCHS1 (AB015872), VvCHS2 (AB066275), and VvCHS3 (AB066274) are found in the grape genome. According to Goto-Yamamoto et al. (2002), their homologies were considerably high in deduced amino acid sequences (Table 1).
Gene-specific primers of each gene for PCR analysis were designed using the online software Primer3 (Rozen and Skaletsky, 1999) and OligoAnalyzer 3.1 (http://www.idtdna.com/analyze-2013Integrated DNA Technologies, http://www.idtdna.com/analyzer/Applications/OligoAnalyzer/, December 3, 2013). Several gene-specific primers used in this study were designed in other research (Table 2).

Nucleotide sequences and Tm (°C) of primers used in this study.
The expression levels of polyphenol oxidase genes (VvPPOs, VvPPO1, and VvPPO2), stilbene synthase genes (VvSTSs), and chalcone synthase (VvCHSs) were quantified by qRT-PCR using real-time PCR (TaKaRa Thermal Cycler Dice TP8000; TaKaRa Bio) following the manufacturer’s instructions. All reactions were performed in 20 μL volumes: 1.6 μL cDNA sample, 10 μL SYBR Premix Ex Taq II (TaKaRa Bio), 0.8 μL forward and reverse primers (10 μM each), and 6.8 μL water. The PCR was carried out under the following conditions; 95°C for 30 sec, then 50 cycles of 95°C for 5 sec, annealing temperature for 10 sec, and 72°C for 20 sec. Dissociation analysis was performed to confirm the specific amplification. The Elongation Factor 1 (EF1) gene (Hanana et al., 2007) and the Ubiquitin (Ubq) gene (Czemmel et al., 2009) were used as the internal control (reference) genes (Table 2). The reactions were performed at least twice for each sample. The threshold cycle (Ct) values were measured using the software provided (version 3.1; TaKaRa Bio), and expression levels were calculated after normalizing with two internal control genes.
To determine the expression of each homologous gene in the STS and CHS gene families, three types of stilbene synthase homologous genes (VvSTS types A, B, and C) and three chalcone synthase homologous genes (VvCHS1, 2, and 3), were analyzed using RT-PCR because the amplification of primer dimmers hindered the individual analysis of each homologous gene in the real-time PCR method. The RT-PCR was performed in 20 μL reaction volume including 1 μL cDNA, 1 × PCR buffer, 0.2 mM dNTP mixture, 0.4 μM each forward and reverse primer, and 0.5 U Ex-Taq (TaKaRa Bio) using the following program cycle: initially 94°C for 5 min, then 27 or 30 cycles of 94°C for 30 sec, followed by annealing temperature for 30 sec, and 72°C for 30 sec. The PCR products were run on a 1% agarose gel using electrophoresis and detected under UV radiation after ethidium bromide staining.
Quantification of resveratrol content in berry skinPolyphenols including resveratrol were extracted according to the method described by Romero-Pérez et al. (2001). In brief, frozen berry skin samples (1 g) were homogenized with 25 mL ethanol/water (8:2, v/v) and incubated at 60°C for 30 min. After centrifugation (8,000 rpm, 15 min), the supernatant was transferred to a flask, and the solvent was completely evaporated using a rotary evaporator (Tokyo Rikakikai, Tokyo, Japan). The solid residue was dissolved in 2 mL of 50% ethanol and transferred to a centrifugal microtube. After centrifugation (12,000 rpm, 15 min), the supernatant was transferred to a new microtube and stored at −80°C until analysis.
The resveratrol content was determined using the Ultra Fast Liquid Chromatography (UFLC) system (Prominence UFLC LC-20AD; Shimadzu, Kyoto, Japan) equipped with a degasser (DGC-20 A3), auto sampler (SIL-20AC HT), column oven (CTO-20A), diode array detector (SPD-M20A), and a 50 × 3.0-mm Inertsil ODS-3 column (GL Science, Tokyo, Japan). The stored samples were filtered through a 0.2-μm membrane and injected using an auto sampler. The column temperature was 40°C, and the elution profiles of solvents A and B were as follows: 0 min (16.5/83.5 = A/B), 3 min (18/82), 7 min (23/77), 10 min (25/75), 15 min (31.5/68.5), 17 min (100/0), 20 min (16.5/83.5), where solvent A was acetonitrile (CH3CN) and solvent B was ultrapure water (H2O). The injection volume was 20 μL and the flow rate was 1.0 mL min−1. A standard sample of trans-resveratrol (3,4′,5-Trihydroxy-trans-stilbene; Sigma Chemical, St. Louis, MO, USA) was used for quantification using the calibration curve method. The system control and data analysis were conducted using the LC-Solution software workstation (Shimadzu) following the manufacturer’s instructions.
Quantification of flavonoid content in berry skinFrozen berry skin samples (1 g) were crushed in liquid nitrogen and homogenized with 25 mL ethanol. The homogenized samples were sonicated in an ultrasonic bath at 60°C for 30 min and centrifuged (10,000 rpm, 15 min). The following procedure was the same as for the resveratrol analysis above.
Total flavonoid content was determined by a simple spectrophotometric method described by Mammen and Daniel (2012). In brief, 0.25 mL 10% AlCl3 (w/v), 0.1 mL of 1 M potassium acetate and 0.25 mL distilled water were added to 0.5 mL sample solution and incubated at room temperature for 1 h. The absorbance was measured at 415 nm with a Shimadzu UV-VIS scanning spectrophotometer (UV-mini-1240; Shimadzu). Quercetin (Sigma Chemical, St. Louis, MO, USA) was used for the standard curve, and total flavonoid content in the sample was calculated as quercetin equivalent amount (μg·g−1 FW). The trans-resveratrol was un-detectable by this aluminium ion complexation method using a spectrophotometer.
Sequence analysis of PPO genes in ‘Shine Muscat’The genomic DNA of ‘Shine Muscat’ was extracted from young leaves using the cetyltrimethyl ammonium bromide (CTAB) method (Kim et al., 1997). Synthetic oligo primers for gene isolation and sequencing were designed based on sequences from the grape genome database (Table 2). In the first step, DNA fragments covering the promoter and open reading frame (ORF) regions of PPO genes were amplified from 100 ng ‘Shine Muscat’ genomic DNA by PCR using Phusion High-Fidelity DNA polymerase (New England Biolabs, Ipswich, MA, USA) with VvPPO1-6F and VvPPO-5R primers (for VvPPO1 promoter), VvPPO2-14F and VvPPO-5R primers (for VvPPO2 promoter), VvPPO1-10F and VvPPO1-7R primers (for VvPPO1 ORF), or VvPPO2-11F and VvPPO2-9R primers (for VvPPO2 ORF). The PCR products were cloned into the pGEM-T Easy vector (Promega, Madison, WI, USA) and sequenced using ABI PRISM 3100 (Applied Biosystems, Foster city, CA, USA). At least three independent clones were fully sequenced using internal primers for PPO genes to cover promoter and ORF sequences. The sequence data were assembled and aligned using GENETYX software. The sequence motifs in the promoter regions were examined by the plant cis-acting regulatory DNA elements (PLACE) online program (Higo et al., 1999; Prestridge, 1991).
Skin browning was observed from 80 days after full bloom (DAFB). At first, only a few berries in each bunch displayed browning, and the degree of browning and the number of browned berries increased over time. Once a few skin-browned berries appeared in a bunch, browning symptoms would spread to all berries in the bunch by 100 DAFB. Skin browning was observed mainly in the apical part (distal to peduncle) of berries (Fig. 1B, D). Skin-browned berries were located randomly in a bunch and there was no trend in their occurrence. By 80 DAFB, the berries were in their third growth stage (stage III) following veraison (Mullins et al., 1992). The area of skin browning increased with the progression of maturation. Brown substances were detected only in the peel (peri-carp), particularly in intercellular spaces and cell walls, by microscopic observation. We considered the browning substances to be oxidized phenolic compounds existing originally in intercellular spaces or weeping from neighboring cells (Fig. 1E, G). The browning substances were lodged in the berry’s skin, and the grape flesh was never affected by this staining (Fig. 1F). The quality of taste of the skin-browned berries was the same as that of normally matured berries (Kanazawa and Takahashi, 2011; Mochida et al., 2013), and the skin-browning pattern observed in ‘Shine Muscat’ was similar to the berry-skin browning observed in other yellow-green table grapes, including ‘Suihou’ and ‘Seto Giants’ (Ogoro et al., 2007).
Expression of PPO, STS, and CHS genes in the berry skin of ‘Shine Muscat’Expression of two PPO genes, three types of STS genes, and three CHS genes were analyzed periodically in berry skin after the veraison stage (around 50 DAFB). First, the bulk expressions of PPO genes (VvPPOs; including VvPPO1 and VvPPO2), STS genes (VvSTSs; including 48 STS genes), and CHS genes (VvCHSs; including VvCHS1, VvCHS2, and VvCHS3) were determined by quantitative RT-PCR (real-time PCR) using primer sets designed in conserved sequences. Following bulk analysis, the expression levels of each type of gene were determined by qRT-PCR for VvPPO1 and VvPPO2 and by RT-PCR for VvSTS types A, B, and C, VvCHS1, VvCHS2, and VvCHS3 using type-specific primers for each gene (Table 2).
The bulk expression of PPO genes (VvPPOs) in berry skin decreased with maturation from 50 to 80 DAFB; however, this decrease was slightly smaller in skin-browning berries than in normal berries. The expression level after 80 DAFB remained almost unchanged even with progressive browning (Fig. 2A). VvPPO1 expression showed a similar pattern to VvPPOs expression, and the expression level significantly decreased with time from 50 to 80 DAFB. Although the expression level was temporarily increased at 90 DAFB in both normal and browning berries, the expression decreased again and stayed low in skin-browning berries after 100 DAFB (Fig. 2B). VvPPO2 expression showed a contrasting pattern to that of VvPPO1. The expression level of VvPPO2 was low at 50 DAFB, increased slightly at 60 DAFB, and then decreased at 70 and 80 DAFB in normal berries. In contrast, a significant increase in the expression level of VvPPO2 was observed after 80 DAFB in skin-browning berries. At 100 and 110 DAFB, the VvPPO2 expression level in browning berries reached about five-fold that of normal berries at 50 DAFB (Fig. 2C). Similar expression patterns were observed in the two experimental years, 2011 (Fig. 2) and 2012 (data not shown); this expression increment corresponded to the occurrence of skin browning. The regulation of VvPPO2 gene expression is considered to play major roles in the polyphenol oxidase enzyme-related browning reaction in berry skin.

Expression of PPO genes in the berry skin of ‘Shine Muscat’. (A) VvPPOs (including VvPPO1 and VvPPO2), (B) VvPPO1, and (C) VvPPO2 expression analysis by qRT-PCR. All data were normalized by VvEF1 and VvUbiquitin and are shown as values relative to the maximum point, set at 1.0. Values are the means ± SE (n = 6).
The bulk expression of STS genes (VvSTSs) showed a similar pattern to that of VvPPO2. The expression level was very low at 50 DAFB and increased with berry maturation (Fig. 3A). A similar expression pattern was observed in both experimental years (data not shown). A significant increase in the expression level was observed in the skin-browning berries, as with VvPPO2. In the results of RT-PCR using type-specific primers, changes in the expression level in VvSTS type B genes showed a similar pattern to VvSTSs expression changes; therefore, VvSTS type B genes were considered the main contributors to VvSTSs expression. The VvSTS type C genes were consistently expressed from 60 DAFB through to the end of the experimental period. The expression of VvSTS type A genes was hardly detectable under this experimental condition (Fig. 3B). Vannozzi et al. (2012) reported that the expression patterns of VvSTS genes changed in response to several stresses such as UV-C radiation, downy mildew infection, and wounding. More specifically, UV-C radiation induced VvSTS type B, VvSTS type C, and VvSTS type A (mentioned as “group B”, “group C”, and “group A”, respectively, in the original paper) gene expressions in this order, and VvSTS type B (“group B”) expression under stress conditions appeared to contribute to the increase in biosynthetic capability of the stilbene pathway (Vannozzi et al., 2012). Our type-specific RT-PCR data were consistent with these previous results. Thus, UV radiation may be one of the factors for skin browning in ‘Shine Muscat’.
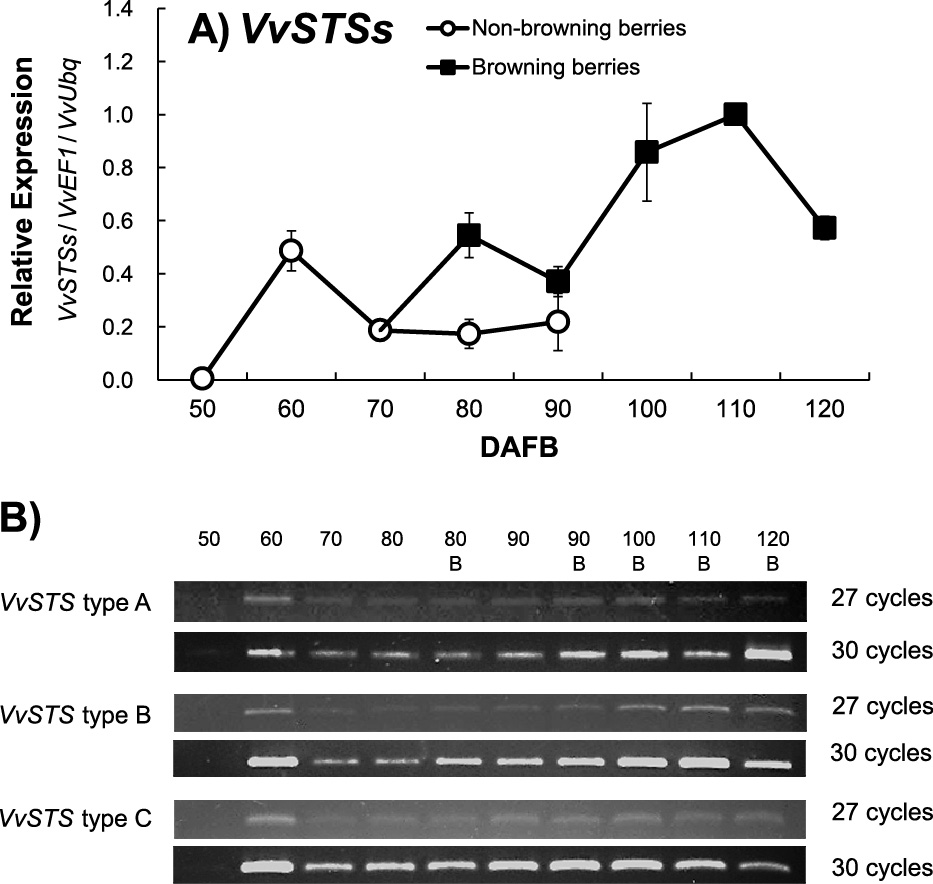
Expression of STS genes in berry skin of ‘Shine Muscat’. (A) VvSTSs (including all 48 homologous genes) expression analysis by qRT-PCR. Each data point was normalized by VvEF1 and VvUbiquitin and is a value relative to the maximum point, set as 1.0. Values are the means ± SE (n = 6); (B) type-specific expression analysis by RT-PCR using 27 or 30 cycles. VvSTS type A, B, and C genes were separately amplified by RT-PCR using type-specific primers (Table 2), and reference expressions of internal control genes, VvEF1, and VvUbiquitin for each sample are shown in Figure 4B.
The bulk expression of CHS genes (VvCHSs) also showed a similar pattern to VvPPO2 and VvSTS expression. The expression level increased from 50 DAFB to 80 DAFB, and a further increase was observed after skin browning occurred (Fig. 4A). A similar expression pattern was observed in the two experimental years (data not shown). Type-specific RT-PCR showed that the periodical expression pattern of VvCHS1 corresponded to the VvCHS qRT-PCR result (Fig. 4B). Therefore, VvCHS1 would contribute to the total VvCHS expression in all three types. VvCHS2 was consistently expressed throughout berry maturation after veraison, whereas VvCHS3 was hardly detected under this experimental condition. CHS is an important enzyme in the flavonoid synthesis pathway and shares substrates (p-coumaroyl CoA and malonyl-CoA) with STS. The expression of VvCHSs in berry skin is known to correlate well with the total amount of extracted anthocyanins from grapes of various skin colors (Boss et al., 1996a); however, in grapes, UDP glucose, flavonoid-3-O-glucosyltransferase (UFGT), has been found to be a key enzyme for anthocyanin biosynthesis rather than CHS (Boss et al., 1996a, b). Moreover, R2R3 Myb transcription factors including the MybA gene have also been identified as key regulatory genes for anthocyanin biosynthesis in the flavonoid pathway (Azuma et al., 2008; Czemmel el al., 2012; Kobayashi et al., 2002). In this study, up-regulation of the gene expressions of VvSTSs and VvCHSs, and specifically VvSTS type B and VvCHS1, was observed in skin browning. The stilbenoid and flavonoid biosynthetic pathways are suggested to be activated and functioning during skin browning, even in yellow-green-skin grapes.

Expression of CHS genes in the berry skin of ‘Shine Muscat’. (A) VvCHSs (VvCHS1, VvCHS2, and VvCHS3) expression analysis by qRT-PCR. Data were normalized by VvEF1 and VvUbiquitin and are shown as values relative to the maximum point, set as 1.0. Values are the means ± SE (n = 6); (B) type-specific expression analysis by RT-PCR using 27 cycles. VvCHS1, 2, and 3 genes were separately amplified by RT-PCR using type-specific primers (Table 2), and VvEF1 and VvUbiquitin were amplified as internal control references for each sample.
The skin browning in berries of ‘Shine Muscat’ was found to be associated with the activation of the stilbenoid and flavonoid biosynthetic pathways and a polyphenol oxidization reaction by VvPPO2 in the berry skin.
Sequence analysis of PPO genes in ‘Shine Muscat’In this study, VvPPO1 (Accession: AB871370) and VvPPO2 (AB871371) genes were isolated and sequenced in ‘Shine Muscat’. The deduced amino acid sequences of these genes in ‘Shine Muscat’ were identical to the predicted gene from the grape genome data of ‘Pinot Noir’. The homology between VvPPO1 and VvPPO2 was 63% in the deduced amino acid sequence (Table 1). It is generally believed that amino acid sequences having more than 45% similarity (homology) have the same function as proteins (Mount, 2004). Therefore, 63% homology implies their similar structure and function as enzymatic proteins for VvPPO1 and VvPPO2.
Expression analysis of VvPPOs revealed that VvPPO2 expression was specifically up-regulated in skin-browning berries. To elucidate the regulatory elements for the gene expression, approximately 840 bp promoter sequences for VvPPO1 and VvPPO2 in ‘Shine Muscat’ (AB871370 and AB871371) and ‘Pinot Noir’ genome data (NW_003724172 and NW_003724396) were scanned using the PLACE program (Fig. 5). A W-Box motif, a binding domain of the WRKY protein known as a stress responsive element (Ülker and Somssich, 2004), binding motifs of Myc and Myb genes, which are transcription factors involved in the flavonoid biosynthesis pathway (Czemmel et al., 2012), and phytohormone responsive motifs, including abscisic acid (ABA)-related, ethylene-related, and gibberellin acid (GA)-related motifs, were plotted on the promoter sequences (Table 3; Fig. 5). The promoter sequences of VvPPO1 and VvPPO2 are highly conserved between ‘Pinot noir’ and ‘Shine Muscat’ respectively. In a comparison of VvPPO1 and VvPPO2 promoter sequences, without considering the motif directions, VvPPO1 contains more ABA-and ethylene-related motifs, whereas VvPPO2 contains more Myb binding and W-box motifs. ABA is generally considered an important phytohormone in grape berry maturation (Coombe and Hale, 1973) and recently, Myb transcription factor genes were also considered to be key regulators of polyphenol biosynthesis in grapevine (Czemmel et al., 2012; Höll et al., 2013). The specific increase in VvPPO2 gene expression during skin browning may be regulated and mediated by Myb transcription factors and W-box motifs responding to several stresses at the berry maturation stage.

Location of cis-acting motifs in the promoter sequences of VvPPO1 and VvPPO2 in ‘Pinot Noir’ (NW_003724172 and NW_003724396) and ‘Shine Muscat’ (AB871370 and AB871371). Putative cis-acting motifs of ABA-related (A), ethylene-related (E), GA-related (G), W-Box (W), Myc binding (Mc), and Myb binding (Mb) detected by the PLACE program are plotted on the schematic illustration of the promoter regions (−840 to 0 bp) of VvPPO1 and VvPPO2. The types of each motif are represented as the number listed in Table 3. Multiple numbers at the same location mean that multiple motifs are adjacent or overlap.

Cis-acting motifs in the promoter sequences of VvPPO1 and VvPPO2 detected by the PLACE program.
The amount of trans-resveratrol contained in the berry skin of ‘Shine Muscat’ increased with maturation. Although there were no significant differences in the amount of trans-resveratrol between skin-browning and normal berries at 80 and 90 DAFB, we observed a trend toward greater trans-resveratrol accumulation in the browned skins (Fig. 6).

Trans-resveratrol content in the berry skin of ‘Shine Muscat’ from 50 days after full bloom (DAFB) to 120 DAFB. Different letters indicate that the values were significantly different according to Tukey’s multiple range test at P < 0.05. ND: not detected at 50 DAFB. Values are the means ± SE (n = 6).
The total flavonoid content in the skin peaked at 50 DAFB and then it decreased toward 80 DAFB, although the difference was not significant. There was no difference in the flavonoid content between skin-browning and normal berries at 80 DAFB, while the flavonoid content appeared to increase in the browned skins with maturation after 90 DAFB, although the difference was not significant (Fig. 7).

Flavonoid content in the berry skin of ‘Shine Muscat’ 50 days after full bloom (DAFB) to 120 DAFB. Total flavonoids were detected by the aluminium ion complexation method using a spectrophotometer at 415 nm. All data are shown as the quercetin equivalent amount. The flavonoid content was relatively high in veraison stage grape at 50 DAFB and in skin-browning berry at 90–120 DAFB, although none of the differences were significant (P > 0.05; Tukey’s multiple range test). Values are the means ± SE (n = 6).
Correlations between tissue browning and polyphenol content have been studied well in apple and lettuce (Holderbaum et al., 2010; Tanaka et al., 2011). In apple, fruit having higher polyphenol content has higher potential for browning. The major polyphenol in the fruit is chlorogenic acid or epicatechin, although it differs among apple cultivars (Holderbaum et al., 2010). In lettuce, browning by cutting was inhibited by cinnamalde-hyde treatment or mild heat treatment, which can reduce phenylalanine ammonia-lyase (PAL) activity and inhibit polyphenol biosynthesis. The lower polyphenol content by those treatments was correlated with the degree of browning (Tanaka et al., 2011). In this study, the increase in trans-resveratrol content, and probably also the increase in flavonoids, was related to the browning of the berry skin. Although the major browning compounds are unclear in ‘Shine Muscat’, their positive correlations indicate that skin browning is probably accelerated by the accumulation of polyphenols reacting with PPO enzymes in the berry skin.
Phenylpropanoid is the backbone of flavonoid and stilbenoid biosynthesis, and the synthetic pathways are usually activated by stresses such as light, drought, and temperature (Dixon and Palva, 1995). Resveratrol is often accumulated under abiotic or biological stress and is considered a “phytoalexin”, intended to protect the plant body from further stresses. On the other hand, accumulated amounts of resveratrol differ greatly by genotype and the growing conditions of grapevines (Gatto et al., 2008; Jeandet et al., 1991). Flavonoid biosynthesis and accumulation are controlled by many endogenous and/or exogenous factors, such as phyto-hormones, water stresses, temperature stresses, and light conditions (Braidot et al., 2008). The accumulation of trans-resveratrol and flavonoids was related to skin-browning symptoms and berry maturation of ‘Shine Muscat’ and the rate of the increase of trans-resveratrol was clearly higher than that of flavonoids. The bio-synthetic pathway of trans-resveratrol is probably more dominant or susceptible to the stress responses and correlates with skin browning. Strictly, it is difficult to conclude that an increase in the trans-resveratrol content of berry skin was caused by browning rather than by simple berry maturation. However, the fact that trans-resveratrol was contained in berry skin at maturation is beneficial information for marketing ‘Shine Muscat’ eaten without peeling.
Perspective on molecular approach for skin-browning problem in ‘Shine Muscat’Molecular and genetic approaches to reveal tissue-browning mechanisms have been conducted in several plant species. In pineapple, two PPO genes are involved in a physiological disorder called “blackheart” browning, caused by chilling and wounding, and these gene expressions were expected to be controlled by GA-related and Myb binding motifs on the promoter (Stewart et al., 2001; Zhou et al., 2003). In tomato, a transgenic plant study showed that activation of the transcription of the PPO gene occurred at the same site as tissue browning by external stresses such as pathogens or wounding (Thipyapong and Stefens, 1997). Research that shows the relationship between tissue browning induced by stresses and PPO gene expression is an essential approach.
In red wine grapes, abiotic stresses from several treatments enhance the gene expressions of STS and CHS and the biosynthesis of polyphenols including trans-resveratrol and flavonoids (Bonghi et al., 2012). In the context of biosynthesis progression, polyphenol oxidase, laccase, and peroxidase play important roles in metabolic pathways such as lignin biosynthesis and phenol compound polymerization (Pezet et al., 2003; Tavares et al., 2013; Zamboni et al., 2010). Recently, molecular bases for the regulation of these biosynthetic pathways have been researched intensively in grapevine (Czemmel et al., 2012; Tillett et al., 2011; Vannozzi et al., 2012). In the berry skin of ‘Shine Muscat’, similar induction and activation of those biosynthetic pathways could have been replaced by skin browning because increases in the expression levels of VvSTS type B and VvCHS1 were observed along with browning. The up-regulation of stilbenoid biosynthesis is obvious because the trans-resveratrol content increased with skin browning. Considering the gene expression data, the flavonoid biosynthesis is also probably up-regulated. Moreover, it is interesting that one of the polyphenol oxidase genes, VvPPO2, showed a specific increase in expression level with skin browning. Although further characterization of VvPPO2 and an enzyme activity assay is necessary, the VvPPO2 gene and the enzyme may lead to understanding the skin-browning process at a molecular level.