2014 Volume 83 Issue 4 Pages 322-326
2014 Volume 83 Issue 4 Pages 322-326
Premature softening during low-temperature storage is a major issue in the red kiwifruit (Actinidia chinensis Planch.) cultivar ‘Rainbow Red’. The objective of this study was to investigate the effect of low temperature on ethylene sensitivity in this cultivar. We demonstrate how ethylene preconditioning at 4°C and 25°C interacted with more rapidly ripening at the lower temperature in ‘Rainbow Red’ kiwifruit. The expression of ripening-related genes ACS1, ACO3, EIL4, ERF14, and PGB was at the basal level during ethylene preconditioning at 4°C and 25°C, and rapidly increased with ethylene treatment following ripening. These results suggest that low-temperature storage enhances ethylene sensitivity in ‘Rainbow Red’.
Fruits can be classified as climacteric or nonclimacteric, depending on the presence or absence of marked ethylene production during ripening and on their response to exogenous ethylene. Kiwifruit (Actinidia chinensis Planch.) is classified as a climacteric fruit because of its high sensitivity to ethylene (Pratt and Reid, 1974).
The efficacy of the interaction between low temperature and ethylene has been studied in pear (Leliévre et al., 1997) and apple (Jobling et al., 1991; Leliévre et al., 1995). Ritenour et al. (1999) reported different responses of kiwifruit to ethylene treatment in relation to the duration of low-temperature storage. Kim et al. (1999) suggested that the decrease in fruit firmness of kiwifruit during low-temperature storage is associated with ethylene signaling. However, decreases in fruit firmness under low-temperature storage are caused by basal ethylene, exogenous ethylene, high sensitivity of the fruit to ethylene, and/or another unexplained mechanism. Possible reasons for the effects of low temperature include ethylene sensitivity and expression patterns of ripening-related genes.
‘Rainbow Red’ is a commercially important early-season cultivar in Japan. The fruits have a deep red color around the core, and the contrasting red and yellow-green cross-sectional appearance is particularly striking and decorative. The fruit has a sweet taste when ripe, with a mean soluble solid concentration of > 18% and total acid content of approximately 1.5%. These desirable characteristics have led to its popularity, but a premature decrease in fruit firmness during low-temperature storage is a major problem in this variety. The objective of this study was to investigate how low temperatures influence ethylene sensitivity in the cultivar ‘Rainbow Red’.
‘Rainbow Red’ kiwifruits (A. chinensis Planch.) were obtained from an orchard in Shizuoka, Japan. Full bloom was recorded on April 30. After harvesting on September 5, careful selection was conducted to exclude fruits with physical injury, disease, or pest damage. The kiwifruits were stored at either 4°C or 25°C for eight days prior to ethylene treatment (Fig. 1). In kiwifruits, the symptoms of chilling injury were previously reported to be significantly lower in those stored above 1°C (Lallu, 1997). To avoid chilling injury, we set the storage temperature to 4°C. At the higher temperature, one portion of the fruit was subjected to ethylene treatment (24 h in a desiccator with 50 ppm ethylene), whereas the other was stored at 25°C in ambient air. After both ethylene and no-ethylene treatments, fruits were stored at 25°C. Five fruits per treatment were sampled, at 1, 9, 13, 19, and 23 days after harvest (DAH), and evaluated for ethylene production, core and flesh firmness, soluble solid content (SSC), and titratable acidity (TA). After removal of the peel, the fruit flesh was frozen in liquid nitrogen and stored at −80°C for RNA extraction.
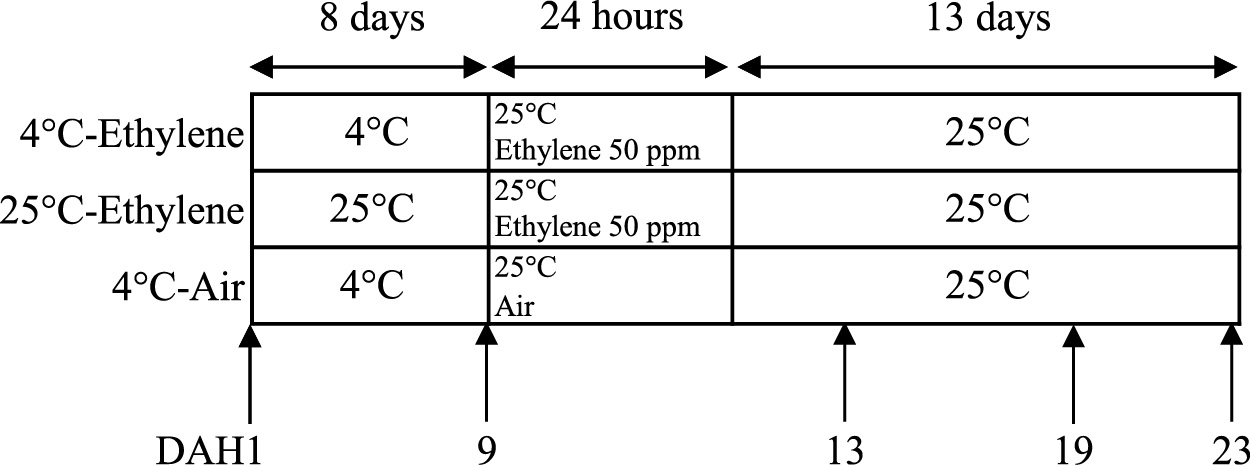
Schematic representation of the experimental design to test the efficacy of low temperature on ‘Rainbow Red’ kiwifruit. Fruits were pretreated at 4°C or 25°C for 8 days. One portion of the fruits was used for ethylene (50 ppm) treatment at 25°C for 24 h, whereas the other was stored at 25°C in ambient air. Fruits were investigated at the time points indicated by arrows.
Ethylene production was determined by incubation of an individual fruit in a plastic 250-mL container for 1 h at 25°C, after which 1 mL of headspace gas was withdrawn and injected into a gas chromatograph (GC-8A; Shimadzu Co., Ltd., Kyoto, Japan). Core and flesh firmness was determined by measuring compression using a Texture Analyzer (TA-XT2; Texture Technologies Corp., Scarsdale, NY, USA) with a 5-mm diameter columnar plunger. Each fruit was subjected to a compression speed of 1 mm·s−1 after contact and penetration to 10 mm. SSC of the fruit juice was measured using a digital refractometer (DBX-55A; Atago Co., Ltd., Tokyo, Japan). TA of the fruit juice was determined by titration using 0.1 N NaOH and expressed as percentage citric acid equivalents.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)Total RNA was extracted from the frozen samples using the method for polysaccharide-rich fruit tissues by Ikoma et al. (1996), and treated with DNase (RNeasy; QIAGEN, Hilden, Germany) to eliminate any DNA contamination. First-strand cDNA was synthesized from total RNA using a reverse transcription kit (TaqMan Reverse Transcription Reagents; Applied Biosystems Japan Ltd., Tokyo, Japan) according to the manufacturer’s instructions, and was used as a template for amplifying cDNA fragments encoding the five ripening-related genes 1-aminocyclopropane-1-carboxylate (ACC) synthase gene ACS1, ACC oxidase gene ACO3, EIN-like gene EIL4, ethylene response gene ERF14, and polygalacturonase gene PGB. Expressed sequence tags of the five ripening-related genes were obtained from GenBank (National Center for Biotechnology Information) using BLAST analysis (http://www.ncbi.nlm.nih.gov/BLAST/). Oligonucleotide primers were designed according to each gene’s region. It was confirmed that there was only one PCR product for each pair of primers. To verify that primers amplified the target gene-coding regions, all PCR products were resequenced. The sequences of all primers used for reverse transcription qRT-PCR are presented in Table 1.

Oligonucleotide primers used for amplification of cDNA by qRT-PCR.
qRT-PCR was performed using a Real Time PCR System 7500 (Applied Biosystems Japan Ltd.) with a Power SYBR Green PCR (Applied Biosystems Japan Ltd.), as described in the manufacturer’s protocol. 18S rRNA was used as internal standard in all experiments. The means of three individual PCR experiments were determined from separate but concurrent reactions.
Ethylene production and fruit quality were evaluated during the investigation period. Ethylene production was low in both 4°C and 25°C pretreatments at 9 DAH (Fig. 2A). At 13 DAH, ethylene production of the fruits that received 4°C pretreatment (4°C-ethylene) increased more rapidly than for those that received 25°C pretreatment (25°C-ethylene), whereas the ethylene untreated fruits at 4°C storage (4°C-air) yielded a low level of ethylene. Core and flesh firmness levels were similar for both the 4°C and 25°C pretreatments at 9 DAH (Fig. 2B, C), whereas at 13 DAH, softening occurred more rapidly in 4°C-ethylene than in 25°C-ethylene. Although the firmness in 4°C-air decreased more slowly than that in 4°C- and 25°C-ethylene, SSC was similar for both the 4°C and 25°C pretreatments at 9 DAH (Fig. 2D). Although TA of 4°C-air gradually decreased compared with those of 4°C- and 25°C-ethylene, it was similar to both at 9 DAH (Fig. 2E). At 13 DAH, TA decreased more rapidly in 4°C-ethylene than in 25°C-ethylene and in 4°C-air more slowly than in 4°C- and 25°C-ethylene.

Ethylene production and quality characteristics of ‘Rainbow Red’ kiwifruit. (A) Ethylene production (nL·g−1·h−1), (B) Core firmness (g), (C) Flesh firmness (g), (D) SSC, and (E) TA. Data are mean ± SD of five individual fruits. Arrows indicate the time point of ethylene treatment (9 DAH).
These results show that fruits under 4°C-ethylene ripened more rapidly than those under 25°C-ethylene after ethylene treatment. In contrast, fruits under 4°C-air poorly ripened throughout the investigation.
Difference in storage temperature influences expression patterns of ripening-related genes after ethylene treatmentExpression patterns of five ripening-related genes were evaluated during the investigation period. Expression levels of the ethylene biosynthesis genes ACS1 and ACO3 were low at both 4°C and 25°C pretreatments at 9 DAH (Fig. 3). At 13 DAH, ethylene treatment following 4°C storage (4°C-ethylene) led to more rapid expression than that following 25°C storage (25°C-ethylene), which was concomitant with increased ethylene production. No-ethylene treatment following 4°C storage (4°C-air) led to a low level of expression. Expression levels of ethylene response genes, EIL4 and ERF14, were low under both 4°C and 25°C pretreatments at 9 DAH. At 13 DAH, 4°C-ethylene led to more rapid expression than 25°C-ethylene. Expression levels of the cell wall degradation-related gene (PGB) were low for both the 4°C and 25°C pretreatments at 9 DAH.

Transcription of ethylene biosynthesis genes (ACS1 and ACO3), ethylene signaling genes (EIL4 and ERF14), and cell-wall degradation-related gene (PGB) in ‘Rainbow Red’. The steady-state levels were normalized to 18S rRNA. Data are mean ± SD of three individual experiments. Arrows indicate the time point of ethylene treatment (9 DAH).
Low-temperature storage is a major technology that is widely used to extend the postharvest life of fresh horticultural produce. Low-temperature storage is considered to inhibit most cell metabolic activities, thereby delaying fruit ripening and plant senescence (Hardenburg et al., 1986; McGlasson et al., 1979). In addition, low temperature is known to influence the expression patterns of ethylene synthesis, ethylene receptors, and ripening-related genes in several fruits. In pear, chilling has been observed to activate the transcription of ACS and ACO genes (Leliévre et al., 1997). Expression levels of ACS and ACO were also increased by chilling in apple (Tian et al., 2002). Ritenour et al. (1999) suggested that the gene expression of ACS and/or ACO of kiwifruit is induced during chilling and that transcripts of ACS are accumulated. In the present study, although ethylene preconditioning at 4°C led to more rapid ripening of kiwifruit than that at 25°C (Fig. 2), the expression of ACS1 and ACO3 was at the basal level at both temperatures (Fig. 3). However, after ethylene treatment, the expression of both genes rapidly increased. These results differ from those for pear and apple and suggest that low-temperature storage enhanced the expression of ACS1 and ACO3 genes after ethylene treatment in ‘Rainbow Red’ kiwifruit.
Yin et al. (2010) suggested that the EIL and ERF ethylene response gene families are involved in the regulation of ripening in kiwifruit. Yin et al. (2009) reported five ethylene receptor genes, two CTR1-like genes, and four EIL genes showing changes in expression during low-temperature storage. EIL4 and ERF14 could potentially enhance ethylene efficiency under low-temperature storage conditions. In the present study, the expression of EIL4 and ERF14 was at the basal level under ethylene preconditioning at both 4°C and 25°C (Fig. 3). However, after ethylene treatment, EIL4 and ERF14 expression rapidly increased, as did that of ACS1 and ACO3. These results suggested that EIL4 and ERF14 are unrelated to the molecular mechanism of enhancement of ethylene efficiency under low temperature. The EIL expression pattern suggested that the level of EIL protein, rather than the level of EIL mRNA, is subject to ethylene-induced regulation (Guo and Ecker, 2004). In the present study, low-temperature storage enhanced ethylene sensitivity after ethylene treatment in ‘Rainbow Red’. However, it remains unclear how the molecular mechanisms of low-temperature storage enhance ethylene efficiency. We infer that a low temperature influences ethylene receptor genes and EIL protein.
In the no-ethylene treatment with 4°C storage (4°C-air), the firmness decrease and SSC increase gradually continued without measurable ethylene production (Fig. 2). Mworia et al. (2012) reported that low temperature modulated the ripening of kiwifruit in an ethylene-independent manner, suggesting that ripening is inducible by low-temperature signals. Given that the phenomenon was reported in ‘Rainbow Red’ (Asiche et al., 2012), we considered the observations in 4°C-air to be equivalent.
In the present study, premature fruit ripening during low-temperature storage was rendered more effective by ethylene preconditioning. Yano and Hasegawa (1993) suggested that kiwifruits do not initiate ethylene production without causing diseases such as ripe rot or exposure to exogenous ethylene. Exogenous ethylene arising from diseased fruit is likely to induce softening and ethylene production in intact neighboring fruit. In fruit distribution and storage, it is difficult to avoid mixing healthy and diseased fruit, and this mixing may be a factor responsible for premature fruit softening during low-temperature storage of ‘Rainbow Red’.
In summary, we have confirmed that ethylene preconditioning at 4°C and 25°C influenced ripening in ‘Rainbow Red’ kiwifruit, with the lower temperature leading to more rapid ripening than the higher one. The expression of the ripening-related genes ACS1, ACO3, EIL4, ERF14, and PGB was at the basal level in ethylene preconditioning at 4°C and 25°C, rapidly increasing with ethylene treatment following ripening. These results confirm that low-temperature storage enhances ethylene sensitivity in ‘Rainbow Red’.