2014 Volume 83 Issue 4 Pages 282-289
2014 Volume 83 Issue 4 Pages 282-289
To understand the factors affecting the incidence of blossom-end rot (BER), the effect of the Ca/K ratio (4/12–12/4, in me·L–1) in nutrient solutions and Ca concentration in fractions in the distal part of young tomato fruits immediately before BER symptoms appear were investigated for three seasons. The rate of BER incidence increased with a decrease in the Ca/K ratio in the supplied solutions in the summer and spring, but little difference was observed in the winter. Ca concentration was highest in winter and lowest in summer, and the concentration in fractions decreased with a decrease in the Ca/K ratio of the solutions. When the results of all three experiments were pooled, among the fractions, water-soluble Ca concentration was found to have the highest significance in the relationship to BER incidence. The risk of BER incidence in rapidly growing tomato increased to a critical level when water-soluble Ca in the distal part of the fresh fruit decreased to less than 0.20 μmol·g–1 FW. Multiple-regression analysis revealed that the concentration of water-soluble Ca, which is predominantly recovering apoplastic or cytoplasmic Ca2+, and total Ca, which has been translocated during fruit development, are significantly affected by solar radiation and Ca concentration in the supplied solution rather than air temperature.
In tomato production, blossom-end rot (BER) may cause severe economic losses because of the deterioration of fruit quality and market acceptability. This is especially true for susceptible cultivars as no practical intervention is 100% effective in controlling the development of BER (Ho and White, 2005; Saure, 2001).
BER is believed to result from a lack of Ca2+ in the fruits or parts of fruits, because of poor Ca uptake by the roots, deficient Ca translocation in vascular vessels and/or inadequate Ca partitioning in fruits. Previous studies have suggested that Ca deficiency can be triggered by complex mechanisms that result in the reduction of Ca uptake in either the roots or fruits and inadequate Ca loading into the fruit, consequently leading to abnormal cellular Ca2+ partitioning within the fruit (Adams and Ho, 1992; Ho and White, 2005; Saure, 2001, 2005). BER is generally characterized by a deterioration of cell membranes with subsequent loss of turgor and leakage of cell fluids. Recent studies suggest that BER in fruits may not be caused by a single factor, but most likely by a complex combination of many factors, including both internal causes and external factors (Ho and White, 2005; Saure, 2001). Many environmental factors, such as drought, salinity, light, and temperature, are involved in BER incidence. It has been shown that these factors affect Ca2+ uptake in the entire plant, Ca2+ loading along functional vessels and distribution to fruit (Adams and Ho, 1992; Johkan et al., 2014; Saure, 2001, 2005; Starck et al., 1994). Other limiting factors, such as low Ca content and availability in the soil, inadequate root Ca2+ uptake, Ca2+ competition with other nutrients in the root, as well as leaf and fruit competition for Ca2+ available in the xylem sap, may aggravate BER occurrence (Besford, 1978; Saure, 2001, 2005). In addition, growth regulators have been found to control many cellular processes in plants that can affect Ca2+ uptake, translocation and partitioning at the cellular level and fruit susceptibility to Ca2+ deficiency (Saure, 2005; Starck et al., 1994). However, the translocation of Ca within the whole plant and the causes of Ca deficiency in fruit have not been fully understood. So far, there are discrepancies in terms of understanding the causes of BER and other related factors affecting BER (Adams and Ho, 1992; Saure, 2001, 2005).
It is well known that severe water stress induces BER and simultaneously stimulates sugar accumulation in tomato fruit (Ishigami et al., 1994). In an earlier study, we found that root zone volume and the supplied amount of a nutrient solution significantly affected BER incidence when the amount of supplied solution was controlled in proportion to solar radiation, solar-mediated fertigation control (Yoshida et al., 2007). In a subsequent experiment (Yoshida et al., 2012), we sequentially extracted Ca as (1) water-, (2) 1 N NaCl-, and (3) 0.6 N HCl-soluble fractions of fresh fruit tissue recovering (1) apoplastic or cytoplasmic Ca2+ and loosely wall-bound Ca, (2) Ca pectate in the cell wall, and (3) insoluble Ca phosphate or Ca oxalate, respectively (Ohta et al., 1970). As a result, we found a significant decrease in water-soluble Ca in fruits and an increase in BER incidence with a decrease in Ca concentration in the supplied solutions, and the decrease in water-soluble, but not total, Ca available for physiological processes was supposed to be a major cause or risk indicative parameter of BER incidence in tomato. Except for salinity and form of ionic nitrogen (Minamide and Ho, 1993; Terabayashi et al., 1988), however, the effects of BER inductive factors on fruit Ca concentration in fractions have not been reported.
We were able to repeatedly control BER incidence by growing tomato plants with a combination of restricted root zone volume and solar-mediated fertigation control. Such a condition was expected to be a useful tool for better understanding the main causes of BER incidence. In this study, we examined the effect of Ca concentration in nutrient solution in three seasons by modifying the Ca/K ratio in solutions to avoid the effect of total concentration or form of ionic nitrogen. Here we report the significant relationship between water-soluble Ca concentration in the distal part of young tomato fruits and the incidence of BER and discuss the experimental and environmental factors affecting fruit Ca concentration in fractions.
Experiments were conducted in a plastic house (10 m × 20 m) covered with 2 layers of plastic films in winter and in another house (6 m × 19 m) in spring and summer at Okayama University. As shown in Table 1, tomato (Solanum lycopersicum L. ‘House Momotaro’) seeds were sown on vermiculite in three seasons and transplanted into 12 cm plastic pots containing ca. 600 mL of 2 peat moss: 1 rock wool granules: 1 perlite mixture, 14 days after sowing. Plants were supplied with a liquid fertilizer containing the following: N, 11.8 mmol·L–1; P, 1.2 mmol·L–1; K, 4.1 mmol·L–1; Ca, 1.2 mmol·L–1; Mg, 0.6 mmol·L–1; and other microelements (OK-F-1; Otsuka AgriTechno, Tokyo, Japan) every 2 days before and 1 day after transplanting. When the third true leaf had fully expanded, six uniformly growing potted plants were selected for each treatment and arranged into 2 rows, 30 cm apart within rows and 150 (winter) or 170 (spring and summer) cm apart between rows. When the 3rd inflorescence flowered, the plants were pinched, leaving 2 leaves above the inflorescence.

Growing conditions and flowering of ‘House Momotaro’ tomato in three experiments (November 2004 to September 2005).
A minimum temperature of 10°C was maintained by heating the plastic houses and adequate ventilation was provided with a fan or through windows when temperature exceeded 28°C. Nutrient solutions were supplied using pot drippers (Netafim Japan, Tokyo, Japan). The amount of the supplied solution was automatically controlled in proportion to the solar radiation to retain 10–20% of the discharged liquid (drainage/supplied solution) as described by Yoshida et al. (2007). In the previous study, the Ca concentration was modified by replacing Ca(NO3)2 with NH4NO3 to maintain total N concentration (Yoshida et al., 2012). In this study, the Ca/K ratio of 8/8 (me·L–1) in Enshi solution was modified to 4/12–12/4 by replacing Ca(NO3)2 and KNO3 alternately (Table 2) in order to maintain total ion concentration and avoid BER inductive effects of NH4-N. Tap water used for preparation of nutrient solutions containing 0.2 mM Ca and 0.1 mM Mg with EC value of 8–10 mS·m–1. The discharged liquid was collected in a bucket every week and the amount and concentration of nutrients were measured as described earlier (Yoshida et al., 2007). Apparent nutrient absorption was calculated weekly as the difference between the supplied and discharged amount of each nutrient.

Concentration of nutrient salts in standard (Ca/K = 8/8) and modified Enshi solutions (mmol·L–1). Microelements were added as in Hoagland and Arnon (1950), and tap water used for preparation contained 0.2 mM Ca and 0.1 mM Mg with an EC value of 9 mS·m–1.
The date of anthesis was recorded for each flower on four plants in each plot, excluding two of the six plants placed at the end of each treatment. To promote fruit set, 15 mg·L–1 of 2-methyl-4-chlorophenoxyacetic acid (4-CPA) solution was sprayed at the anthesis of the 3rd flower of each inflorescence and the rest of the flower buds were removed. The second fruit of each inflorescence was taken for Ca extraction as described below and the date of visible BER symptoms was recorded for the other fruits. When BER symptoms appeared before sampling, no extraction was performed and only the date of incidence was recorded.
Fruit sampling and sequential calcium extractionFruit samples were taken 20, 15, and 12 days after anthesis (DAA) in experiment I (winter), II (spring), and III (summer), respectively, when the fresh weight (FW) of the fruit reached ca. 15 g and the incidence of BER was first observed. Fruits were weighed and cut along the equatorial plane and the distal part was cut radially into segments less than 5 mm in thickness. Tissue weighing 3–5 g of cut fruit was combined with 10 mL of 99% ethanol in a plastic vial. Vials were shaken 3 times with a vortex mixer for 10 s and stored overnight or longer at 4°C. The supernatant was collected in a glass beaker and subjected to 5 min of ultrasonic extraction by 5 min of reciprocal shaking with 10 mL H2O. This was repeated three times. A combined extract of the water-soluble fraction was dried at 80°C and washed into a volumetric flask with 0.6 N HCl. Then 5 mL of 10 g·L–1 La solution was added before filling up to 50 mL. Extraction using an aliquot of 1 N NaCl was similarly repeated four times with H2O. Similarly, a combined extract of NaCl-soluble fraction was dried and filled up to 50 mL. Extraction with an aliquot of 0.6 N HCl was similarly repeated four times and a combined extract of HCl-soluble fraction was filled up to 50 mL with La solution and 0.6 N HCl. Ca concentration was determined by atomic absorption spectrometry (Z-6000; Hitachi, Tokyo, Japan) and described as μmol·g–1 FW.
Statistical analysisMicrosoft Excel spreadsheets were used for statistical analysis of the data.
As shown in Table 1, mean temperature during 30 days after the beginning of flowering was lowest in winter and about 10°C higher in spring and summer. Day length was longest in spring and shortest in winter. However, solar radiation was highest in summer and lowest in winter, and was intermediate in spring because of the early onset of the rainy season at the beginning of June.
In spring and summer, the absorbed amount of Ca decreased and that of K increased with a decrease in the Ca/K ratio in the supplied solution (Table 3). In winter, however, the absorbed amount of Ca decreased with a decrease in the Ca/K ratio (P = 0.06), while little difference was observed in K absorption. No significant effect of the Ca/K ratio was observed for other nutrients throughout the three experiments.
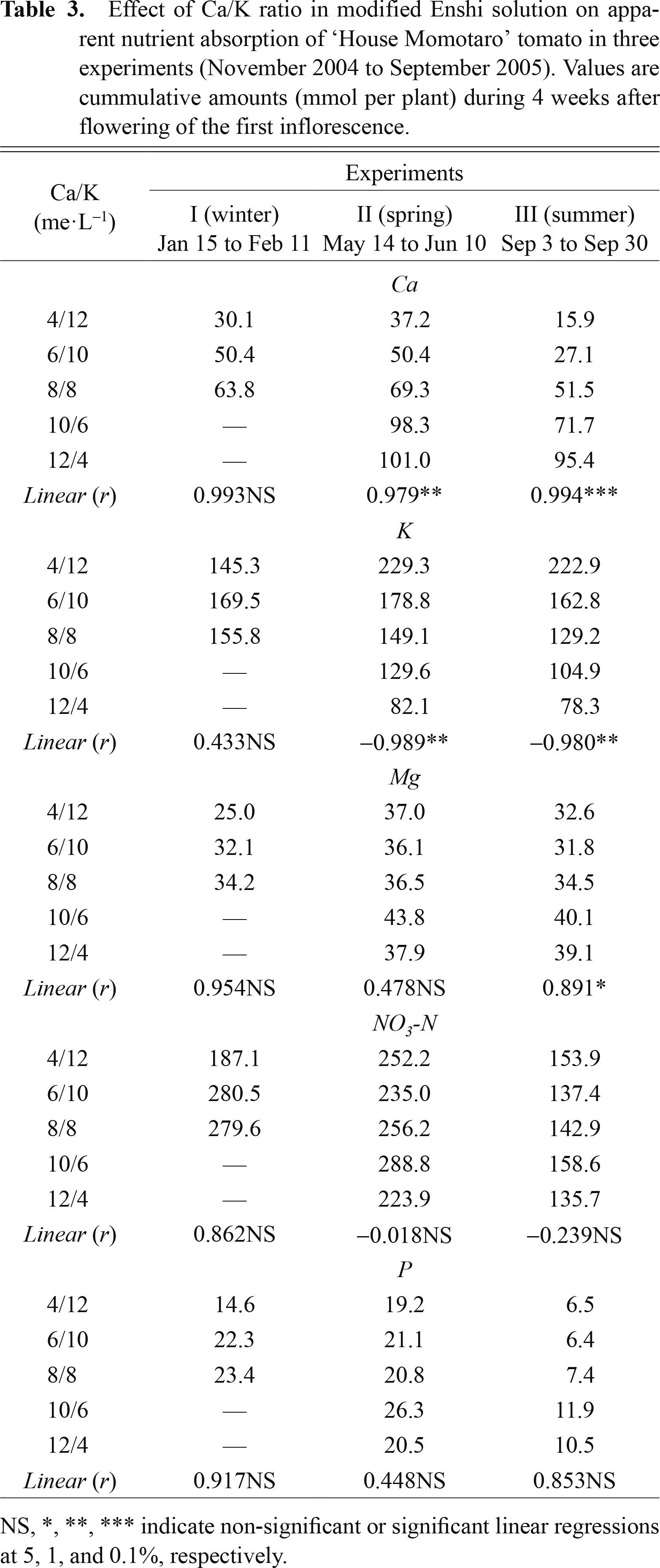
Effect of Ca/K ratio in modified Enshi solution on apparent nutrient absorption of ‘House Momotaro’ tomato in three experiments (November 2004 to September 2005). Values are cummulative amounts (mmol per plant) during 4 weeks after flowering of the first inflorescence.
Vegetative growth of tomato plants supplied with the lowest concentration of Ca solution (4/12) was less vigorous compared to the other plants. Leaves were slightly thin and yellowish (data not shown), but no difference was observed in flowering or the appearance of flowers (Table 1).
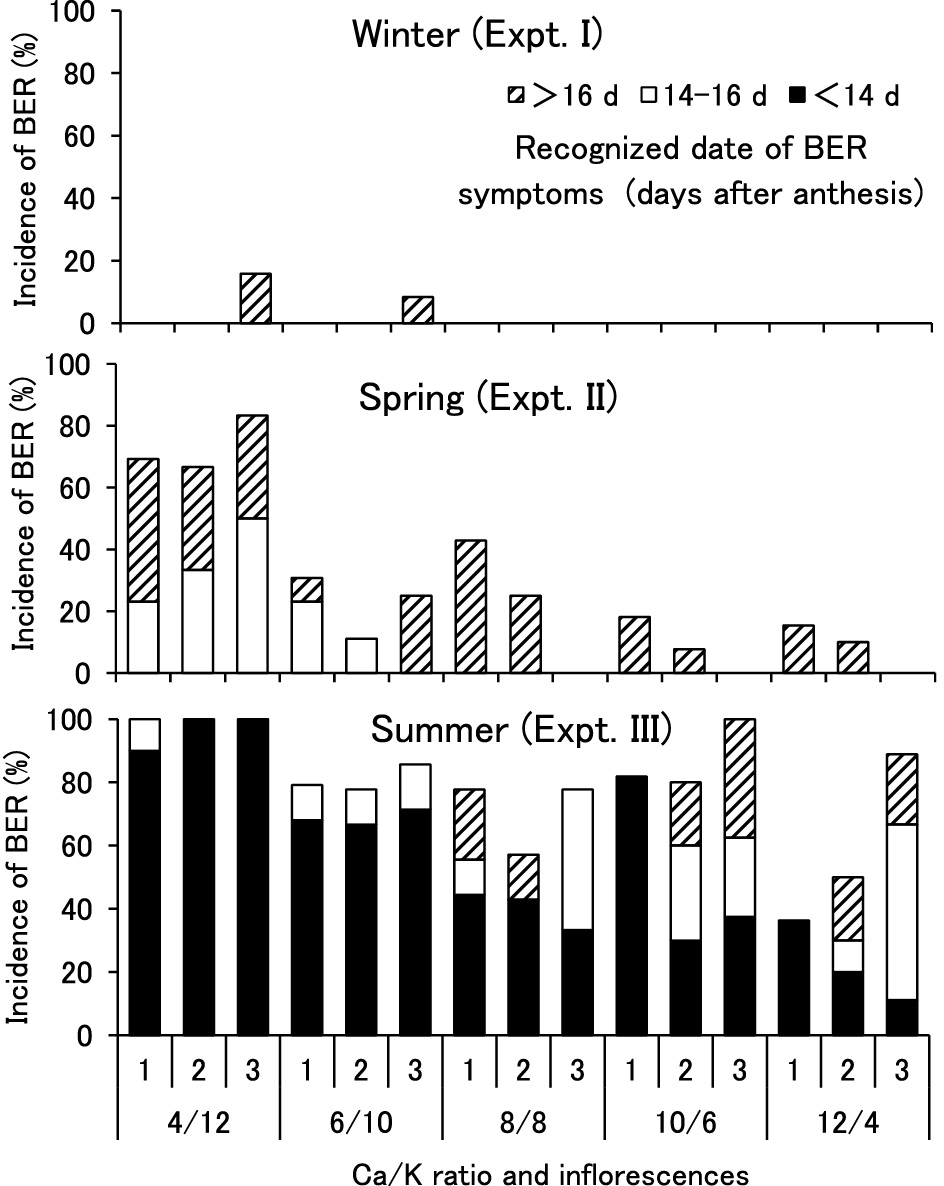
The rate of BER incidence was much higher in the plants supplied with a low concentration of Ca/K solution and it decreased with an increase in the Ca/K ratio in the supplied solution (Fig. 1). In winter, BER developed only in a small number of fruits on the 3rd inflorescence. In summer, BER developed most quickly and severely among the three experiments and the development was intermediate in spring. Symptoms of BER could be seen later than 20 DAA in winter, but appeared before 14 DAA in 80% of BER-infected fruits in summer. The rate of BER incidence was lower in the 3rd inflorescence than in the lower inflorescences in spring, whereas it was a slightly higher in the 3rd inflorescence than in the others in summer or winter.
Seasonally, total Ca concentration in the distal part of the tomato fruit was highest in winter and lowest in summer (Fig. 2). Mean values of total Ca in fruits supplied with standard solution (8/8) were 0.72, 0.53, and 0.35 μmol·g–1 FW in winter, spring, and summer, respectively. Except in winter, the concentration significantly decreased with a decrease in the Ca concentration in the supplied nutrient solution and an increase in the order of inflorescence (Table 4). Total Ca concentration was 0.28 and 0.26 μmol·g–1 FW in the 3rd inflorescence of plants supplied with 4/12 solution to 0.80 and 0.54 μmol·g–1 FW in the 1st inflorescence of plants supplied with 12/4 solution in spring and summer, respectively. In winter, however, the concentration of 0.56 μmol·g–1 FW in the 1st inflorescence of plants supplied with 4/12 solution was the lowest and 0.93 μmol·g–1 FW in the 3rd inflorescence of plants supplied with 8/8 solution was the highest.
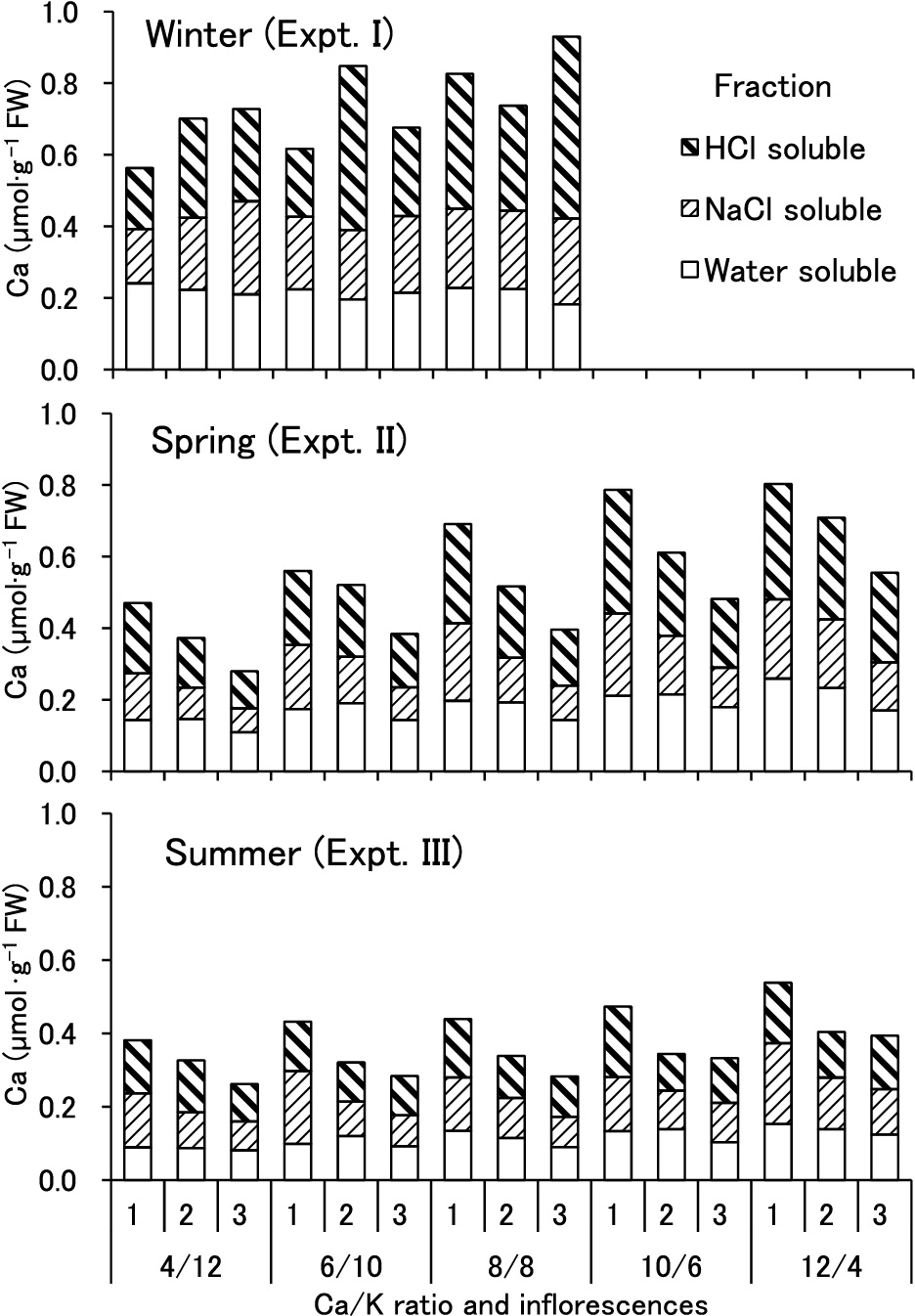

Similarly, Ca concentration in the three fractions significantly decreased with a decrease in the Ca/K ratio in the supplied nutrient solution and an increase in the order of inflorescence in spring and summer, but not in winter (Fig. 2 and Table 4). In particular, water-soluble Ca concentration in the distal half of the tomato fruit increased linearly with an increase in the Ca/K ratio in the supplied solution in spring and summer, but not in winter (Fig. 3). When the results of the three experiments were pooled, although significant multiple regressions were represented statistically, except for the HCl-soluble Ca fraction, the coefficient of determination (adjusted R2) revealed that only less than 10% of variation in the Ca concentration of fractions in young tomato fruits could be explained by the two explanatory variables.

Relationship between the mean value of water-soluble Ca concentration in the distal half of tomato fruit and Ca concentration in the supplied nutrient solution in each experiment. NS, **, *** indicate non-significance or significance at 1 and 0.1%, respectively.
With respect to K concentration in fruits, no significant effect of the Ca/K ratio in the supplied solution, order of inflorescence, or cropping season was observed. Mean value of total K was 20.8 μmol·g–1 FW as a whole, ranging from 15.6 μmol·g–1 FW in the 3rd inflorescence of plants supplied with 12/4 solution in summer to 24.9 μmol·g–1 FW in the 1st inflorescence of plants supplied with 6/10 solution in summer. The lowest K concentration of 4 me·L–1 might still not be deficient for a developing young tomato fruit.
Relationships between BER incidence and fruit Ca concentration in fractionsCorrelation coefficients between the rate of BER incidence in each inflorescence and mean Ca concentration in fractions are shown in Table 5. Only the water-soluble fraction significantly correlated with BER incidence in spring, and the fraction represented the largest coefficient value of –0.707 among the fractions in summer. When all data were pooled, there were significant negative relationships between the rate of BER incidence and Ca concentration in all fractions with the water-soluble fraction representing the largest value. In a rapidly growing tomato fruit, which is the most sensitive to BER (Ho and White, 2005), water-soluble Ca may be the most important factor directly relating to the physiological process of BER development.

Correlation coefficients between the rate of blossom-end rot (BER) incidence in each inflorescence and Ca concentration in the distal half of tomato fruit in fractions in each experiment.
The rate of BER incidence was plotted against the water-soluble Ca concentration, as shown in Figure 4. In spring, the relationship was less significant than in summer. This may be due to the absence of BER fruit in the 3rd inflorescence of plants grown with solutions containing a high rate of Ca, where water-soluble Ca concentrations of 0.14 to 0.18 μmol·g–1 FW were lower than that of 0.19 to 0.26 μmol·g–1 FW in 1st or 2nd inflorescences (Figs. 1 and 2). Flowers of the 3rd inflorescence opened in late May and early June in the spring experiment (Table 1) and the rainy season started at the beginning of June in 2005. The daily mean solar radiation of 8.4 MJ·m–2·day–1 in late May decreased to 4.5 and 4.1 MJ·m–2·day–1 in early and mid June, respectively. As several authors reported earlier, decreased solar radiation (Sonneveld and Voogt, 1991) and also increased humidity (Bradfield and Guttridge, 1984) may have affected the decrease in BER incidence. In summer, the water-soluble Ca concentration of 0.18 μmol·g–1 FW was the highest in the 3rd inflorescence of plants supplied with 12/4 solution where BER symptoms appeared in 36% of the fruits and the concentration was less than 0.14 μmol·g–1 FW in the other inflorescences, where more than 50% of fruits developed BER.

Relationships between the rate of blossom-end rot (BER) incidence in each inflorescence and the mean value of water-soluble Ca concentration in a distal half of tomato fruit. *, *** indicate significance at 5 and 0.1%, respectively.
From these results, it was concluded that the risk for BER increases when the water-soluble Ca concentration in the distal half of young tomato fruits drops below 0.20 μmol·g–1 FW and BER develops when environmental and plant conditions are favorable for rapid fruit growth. As Saure (2001) summarized in his review, various cultural practices and environmental factors, such as fruit thinning (de Kock et al., 1982), increased daily radiation (Sonneveld and Voogt, 1991), increased temperature or a combination of warm weather and high irradiance (Ho et al., 1993; Wui and Takano, 1995), have been reported to increase the incidence of BER by promoting the rate of fruit growth. Accelerated fruit growth increases Ca demand in the fruit for cell expansion and Ca transport to the fruit. However, the supply is often restricted due to competition with vegetative organs because such conditions are also favorable for plant growth and evaporation (Ho and White, 2005). Rapid fruit growth may cause a further decrease in water-soluble Ca concentration and finally increase the risk of BER in tomato to a critical level. Consequently, severe and early development of the symptoms appeared in fruits in inflorescences containing less than 0.15 μmol·g–1 FW of water-soluble Ca.
Factors affecting water-soluble calcium concentration in fruitIn spring and summer, water-soluble Ca concentration in the distal half of the tomato fruit decreased with a decrease in the Ca/K ratio in the supplied solution, but not in winter (Fig. 3). The concentration that negatively correlated with BER incidence was apparently highest in winter and lowest in summer. Along with Ca supply, several factors, such as the order of inflorescences (El-Gizawy and Adams, 1986; Nukaya et al., 1995), solar radiation (Sonneveld and Voogt, 1991), and temperature (Ho et al., 1993), have been reported to affect BER incidence. Except for salinity (Minamide and Ho, 1993), however, the effects of these factors on fruit Ca concentration in fractions have not been reported. Therefore, multiple regression analysis was conducted to clarify the contribution of experimental and environmental factors to BER incidence through Ca nutrition (Table 6). The coefficient of determination thus obtained (adjusted R2) revealed that 55% of the variation in water-soluble and total Ca concentration in the distal part of young tomato fruit could be explained using the explanatory variables. Adjusted R2 was smallest in the NaCl-soluble fraction followed by the HCl-soluble fraction. When Ca was extracted before BER development from fruits that appeared healthy, there was a possibility that the cell wall of the normally developing fruits contained a sufficient amount of NaCl-soluble Ca for cross-linking of pectic substances. Consequently, the smallest value of R2 may have been obtained in the NaCl-soluble fraction.

Parameters of multiple regression in Ca concentration in the distal half of tomato fruit in fractions (μmol·g–1 FW; individual values in Figure 2) against Ca concentration (me·L–1) in nutrient solution, order of inflorescences (1st to 3rd), and daily mean of cumulative solar radiation (MJ·m–2·day–1) and air temperature (°C) during the early period of fruit development (n = 141). See Tables 1 and 2.
Among the four variables, Ca in the supplied solutions and solar radiation had the largest effect on the water-soluble fraction, which correlated with the incidence of BER most significantly and also with total Ca. Many authors have reported that low Ca supply induces BER development (Adams and El-Gizawy, 1988; de Kreij, 1996; Maynard et al., 1957; Raleigh and Chucka, 1944; Ward, 1973). Ward (1973) and Nonami et al. (1995), however, had some doubts regarding Ca2+ deficiency as the only cause of BER, because of discrepancies in the published values for Ca2+ in fruits with and without BER. As Ho and White (2005) have shown, Ca has been measured as total Ca (mainly Ca oxalate and Ca pectate) rather than as the fraction of Ca relevant to a particular cell function (Minamide and Ho, 1993), and this may be one of the reasons why it is difficult to find a universal critical Ca level for the induction of BER in fruit. In sequential fractionation in our experiments, the (1) water-, (2) NaCl-, and (3) HCl-soluble fractions of fresh fruit tissue were predominantly involved in recovering apoplastic or cytoplasmic Ca2+ and loosely wall-bound Ca, Ca pectate in the cell wall, and insoluble Ca phosphate or Ca oxalate, respectively (Minamide and Ho, 1993; Minamide et al., 1987; Ohta et al., 1970). Among them, the water-soluble fraction correlated most significantly with BER incidence (Table 5) and reflected the changes in experimental and environmental conditions that have been reported as BER-inducing factors (Table 6).
In conclusion, Ca deficiency in the distal part of young tomato fruit is the major cause of BER and 0.15 to 0.20 μmol·g–1 FW of water-soluble Ca concentration may be critical for the development of BER symptoms in rapidly growing fruit. Ho and White (2005) also concluded that BER is a symptom of Ca deficiency in distal fruit tissue during rapid cell expansion and that, in practice, spraying Ca onto young tomato fruit is the most effective measure to minimize BER incidence. To confirm the importance of water-soluble Ca, further analysis of the effects of other experimental and environmental factors, such as spraying, humidity, water stress, and cultivar differences, on water-soluble Ca are needed.