2014 Volume 83 Issue 4 Pages 317-321
2014 Volume 83 Issue 4 Pages 317-321
Mature pollen grains of Lilium cultivar, with their germ pores folded in upon themselves, were observed under a scanning electron microscope (SEM). The conventional pretreatment process requires aldehyde fixation, dehydration, drying and metal sputtering for SEM observation. These complicated and laborious procedures can considerably alter the morphology of pollen grains. In order to omit this conventional pretreatment process, we established a novel technique utilizing an ionic liquid (IL) that is composed solely of ions, namely, a liquid salt that can remain in a molten state even at room temperature. IL-treated pollen grains could be observed under vacuum conditions without artifacts, and furthermore, a satisfactory SEM image could visualize pollen grains in a wet state. The possible direction of future studies on ionic liquids in the SEM field is also discussed.
Scanning electron microscopy (SEM) can provide important information on various surface morphologies by scanning them with an electron beam (Goldstein et al., 2003). When the pollen grain of flowering plants is observed by SEM, each pollen grain possesses a species-specific pattern on its surface. Because these species-specific patterns show wide variation, they can be used to identify and classify the plant species. The sample chamber of a conventional SEM must be kept dry and under high-vacuum conditions; in such an environment, pollen grains can be observed in the state in which germ pores are folded in upon themselves. A pretreatment process consisting of aldehyde fixation, dehydration, drying, and metal sputtering is required for the observation of pollen grains using a typical SEM system because the grains contain water and have no conductivity. However, this conventional pretreatment process often results in considerable morphological alteration of the surface structure of pollen grains; that is, these complicated and laborious procedures create artifacts.
An ionic liquid (IL) composed solely of ions is a liquid salt that can remain in a molten state even at room temperature (for review, see Torimoto et al., 2010). ILs can be utilized for studies under vacuum conditions because of their peculiar physicochemical properties, which include negligible vapor pressure and high ionic conductivity (Armand et al., 2009; Rogers, 2007; Rogers and Seddon, 2003; Seddon, 2003; Tsuda et al., 2011a). Recently, Kuwabata et al. (2006) showed that ILs could be observed by SEM without the electron-charge accumulation from the so-called charge-up effect, although ILs possess ionic conductivity. It was subsequently demonstrated that IL-treated specimens swollen with water could be observed by SEM without the laborious pretreatment processes (Arimoto et al., 2008c; Dwiranti et al., 2012; Ishigaki et al., 2011a, b, c; Takahashi et al., 2013; Tsuda et al., 2011b). These results led us to observe wet pollen grains treated with an IL under vacuum conditions. In this study, we present the first satisfactory SEM images of wet pollen grains, that is, without a folded germ pore, by using IL treatment.
The procedure for IL treatment of pollen grains from the Oriental hybrid lily ‘Siberia’ is shown in Figure 1. Mature anthers were rinsed in 10% (v/v) sucrose solution to remove viscosity. The released pollen grains were then suspended in 1% (v/v) IL in absolute ethanol for 10 s. The IL used in this study was choline lactate {[Ch][CH3C(OH)CO2], a clear and fluid liquid at room temperature} that was highly purified (Vijayaraghavan et al., 2010) to make it usable in SEM analysis. Pollen grains were pipetted well and then transferred onto conductive carbon tape. In order to volatilize the ethanol, the specimen was blown with compressed air for 10 s.
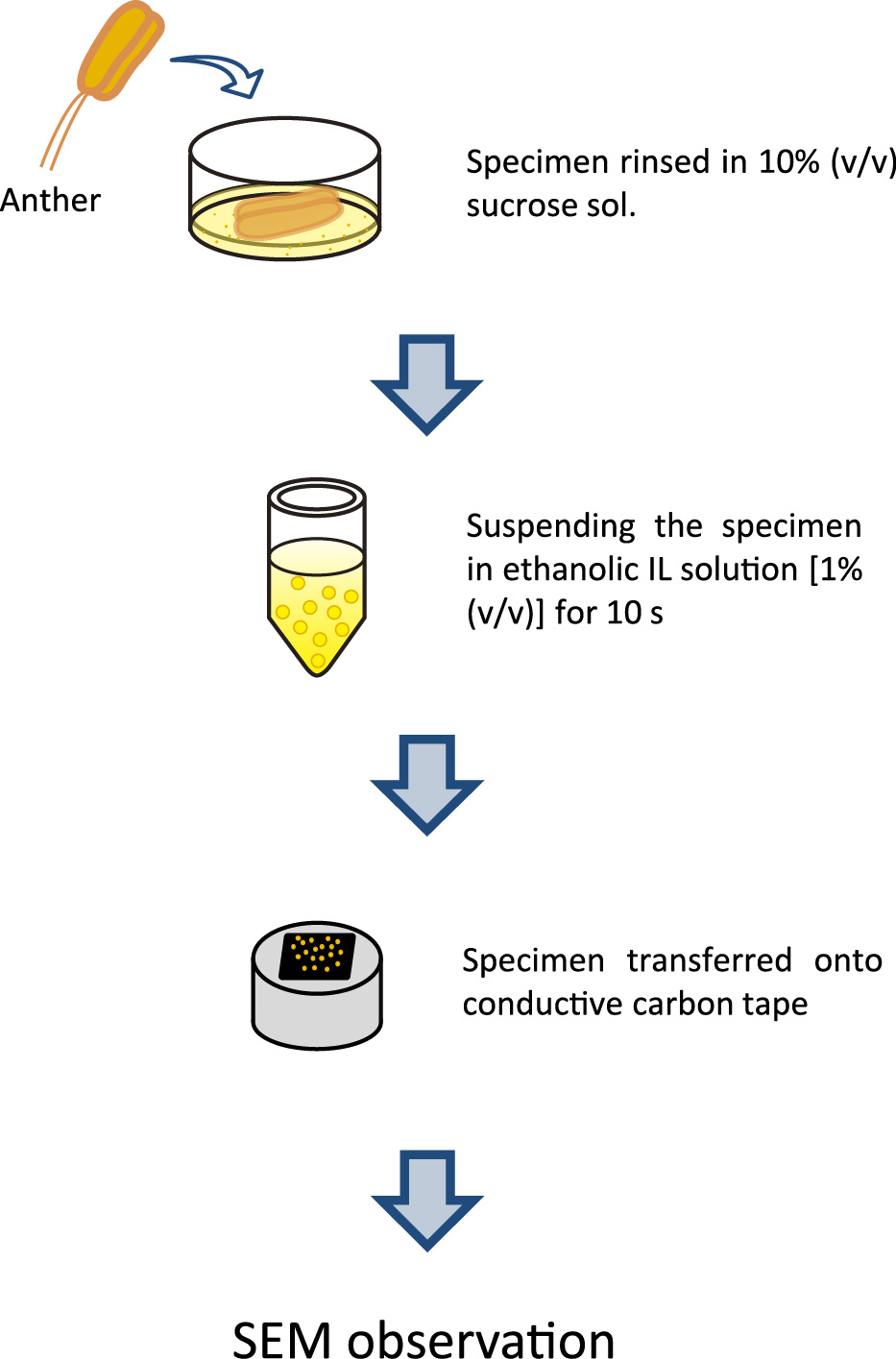
Novel protocol for the pretreatment of lily wet pollen grains from Lilium for SEM observation.
A Hitachi S3000N SEM (Hitachi High-Technologies, Tokyo, Japan) was used for the observations. The SEM was used in “high”-vacuum mode (< 1 Pa). The accelerating voltage employed was 5 kV, and the working distance used was ca. 10 mm. For each sample observed by SEM, microphotographs at different magnifications were taken.
Preparation of protoplastsPretreated pollen grains were directly suspended in the enzyme solution, which contained 1% (w/v) Macerozyme R-10 and 1% (w/v) Cellulase Onozuka R-10 (both from Yakult Honsha, Tokyo, Japan) dissolved in washing solution [0.6 M sorbitol, 10 mM CaCl2·2H2O and 5 mM 2-morpholino-ethanesulfonic acid (MES) with the pH adjusted to 5.6] (Tanaka et al., 1987). We used 2 mL of the enzyme solution per anther. The enzyme treatment was carried out at 30°C with reciprocal shaking (stroke 25 mm) at 100 rev./min under dark conditions. After the enzyme treatment, protoplasts were washed three times with the above washing solution by centrifugation at 1000 rpm (180 × g) for 3 min. The final suspension was observed under an inverted microscope (Olympus IX71; Olympus, Tokyo, Japan).
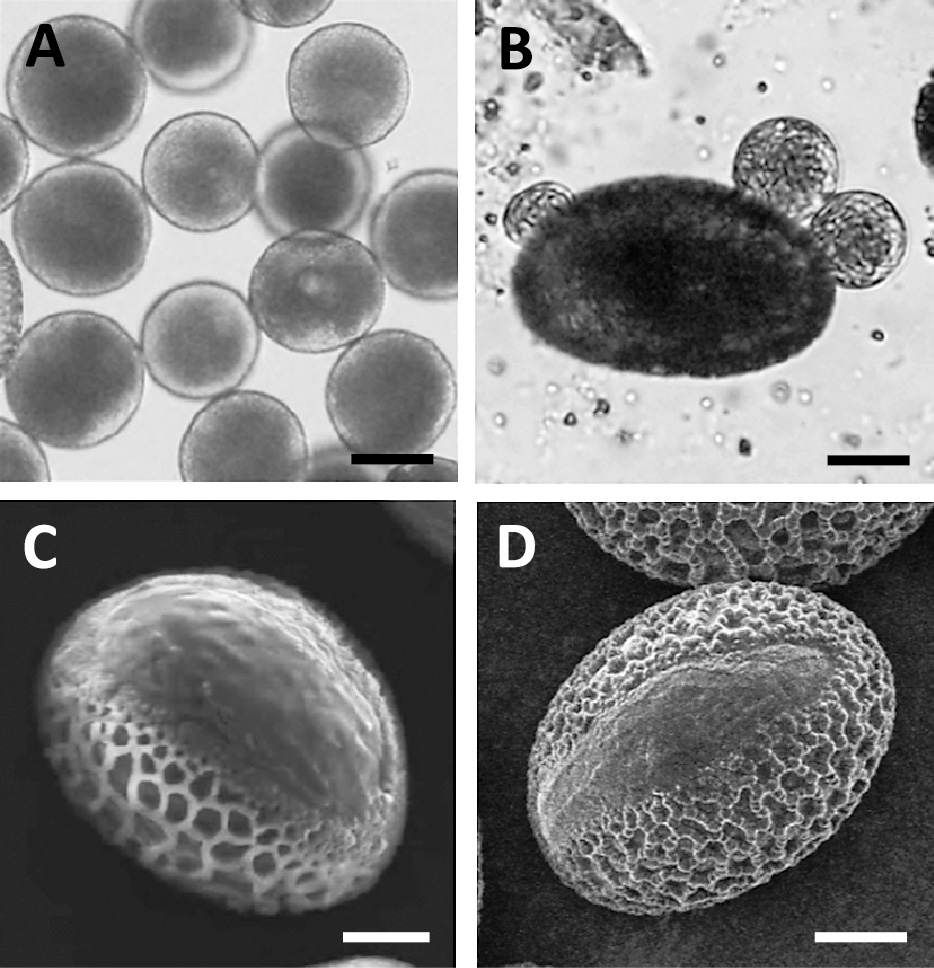
Upon maturation, lily pollen grains dry to a low moisture content, and the germ pores close for protection against the dry environment. When dry pollen grains arrive at the stigma of the pistil, they immediately swell under the wet conditions present there, and these wet pollen grains then germinate and elongate the pollen tube. Mature pollen grains pretreated with sucrose solution to remove their viscosity changed into wet pollen grains (Fig. 2A), and then the wet pollen grains reverted back to their dry state after a few minutes under an optical microscope (Fig. 2B). Under SEM observation, wet pollen grains that had not been treated with fixation, dehydration and ultra-thin coating with metal appeared to be dry pollen grains; that is, they appeared as crushed and fossil-like pollen grains because vaporizing water existed in the pollen grains under vacuum conditions (Fig. 2C). Moreover, we could not obtain SEM images even of the dry pollen grains due to their charge-up effect (Fig. 2D).
Mature lily pollen grains whose viscosity was eliminated show the characteristic appearance of wet pollen grains (A). Dry pollen grains originated from the pollen grains shown in (A) after several minutes of observation under an optical microscope (B). Wet pollen grains without conventional pretreatment, such as fixation, dehydration, and metal sputtering, changed into dry pollen grains under SEM observation (C), and electron beam irradiation accumulated on their surfaces, an effect known as the charge-up effect (D). Bars = 50 μm.
An IL is a type of salt that stays in a molten state at room temperature (Torimoto et al., 2010). ILs do not vaporize under a vacuum, so they can be used as samples in research requiring vacuum conditions. When wet pollen grains pretreated with IL were observed by SEM, many drops of IL containing pollen grains were confirmed under vacuum conditions. This indicates that the IL remains on the surfaces of pollen grains under a vacuum field, and there is no problem with regard to charging, although the pollen grains themselves were not observed (Fig. 3A). Kuwabata et al. (2006) reported the direct observation of IL by SEM. Direct SEM observation of pollen grains pretreated with a 33% (v/v) ethanolic IL solution showed some degree of reticulate structure on the surface of the grains (Fig. 3B). However, the utilization of a 5% (v/v) ethanolic IL solution clarified the morphological view of the germ pores of grains in spite of the presence of excess IL (Fig. 3C). Pollen grains under SEM observation could avoid the charging behavior irrespective of the low concentration of IL in the ethanolic solution and these results indicated that ILs possesses high ionic conductivity. In addition, the pollen grains treated with the 100% EtOH used for IL dilution were observed under dry conditions with the accumulation of electron charges (Fig. 3D). When we employed 1% (v/v) ethanolic IL solution, the SEM images of germ pores showed that the pollen grains were wet (Fig. 3E–H). The pretreatment method using IL allowed us to obtain SEM images of wet pollen grains successfully without engaging in complicated and labor-intensive pretreatment procedures. In addition, the IL protocol presented here is superior to the conventional protocol, which often alters the morphology of biological samples, and allows the observation of the original features of pollen grains.
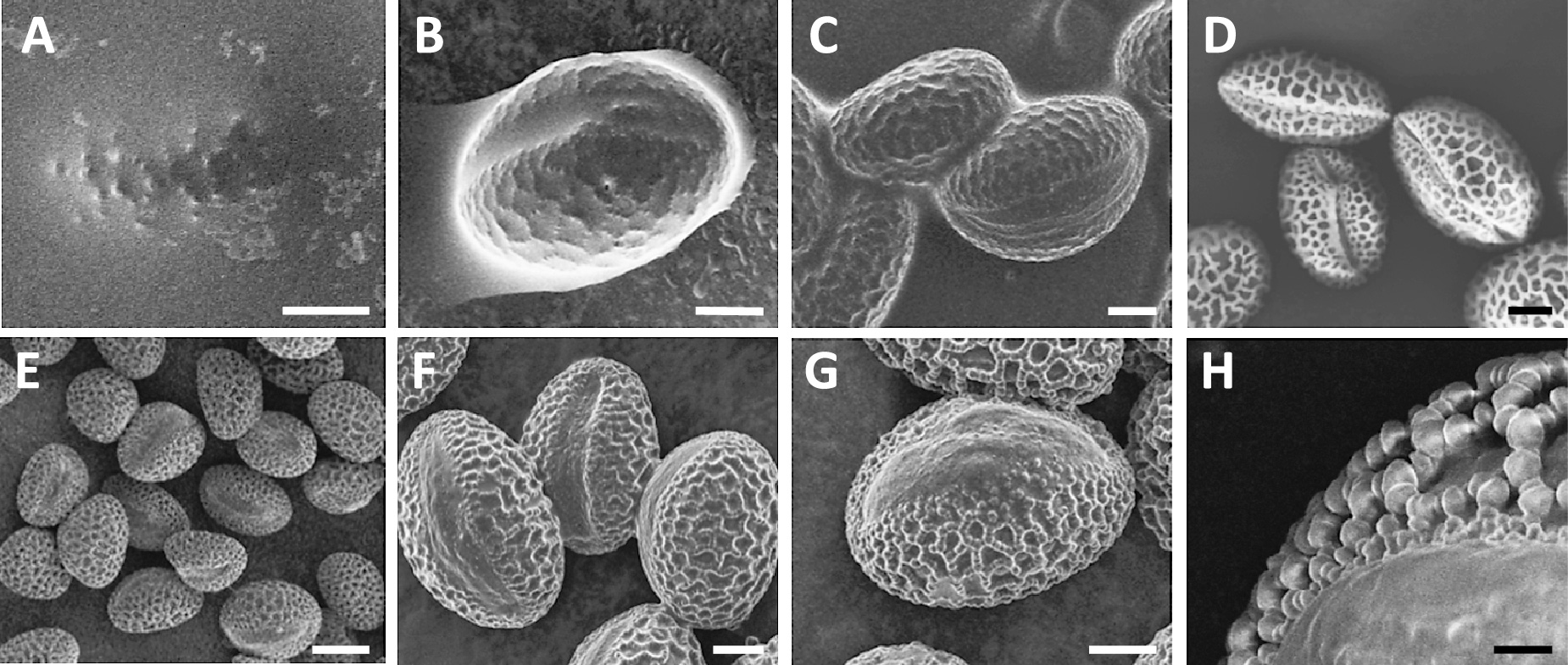
SEM observations of wet pollen grains pretreated with 100% (v/v) IL (A), 33% (v/v) (B), 5% (v/v) (C), and 1% (v/v) ethanolic IL solution (E–H). Wet pollen grains treated with 100% EtOH changed into dry pollen grains with the charge-up effect (D). Increasing the magnification of the SEM enabled us to observe the surfaces of the wet pollen grains without charging behavior (H). Bars = 20 μm (A–D, F, G), 50 μm (E), and 5 μm (H).
Since the conventional metal-coating process is performed using one-directional spraying of the specimens with a metal element, the escape area of sputtering would occur on the round surface of pollen grains. When the IL protocol was used, pollen grains were considered to have been coated uniformly and slightly with IL by means of having been suspended in ethanolic IL solution, so the primary electron beams would be well grounded during the SEM observation. We observed wet pollen grains under high magnification (× 2500) without charging behavior (Fig. 3H). When the pollen grains were treated with a buffer comprised of inorganic salts and sugars prior to ethanolic IL treatment, we successfully obtained SEM images of the wet pollen grains at an extremely high magnification (× 20000) without charging behavior (data not shown). It was considered that an increase in the boiling point may occur in the pollen grains. These results indicate that it will be possible to detect various elements including the germ pores and reticulate structure in grains by this method. Such elucidation of the features of the microstructure in pollen grains by SEM should be very useful for the identification of plant species. Thus, the knowledge obtained from SEM images of wet pollen grains could lead to new discoveries in palynology.
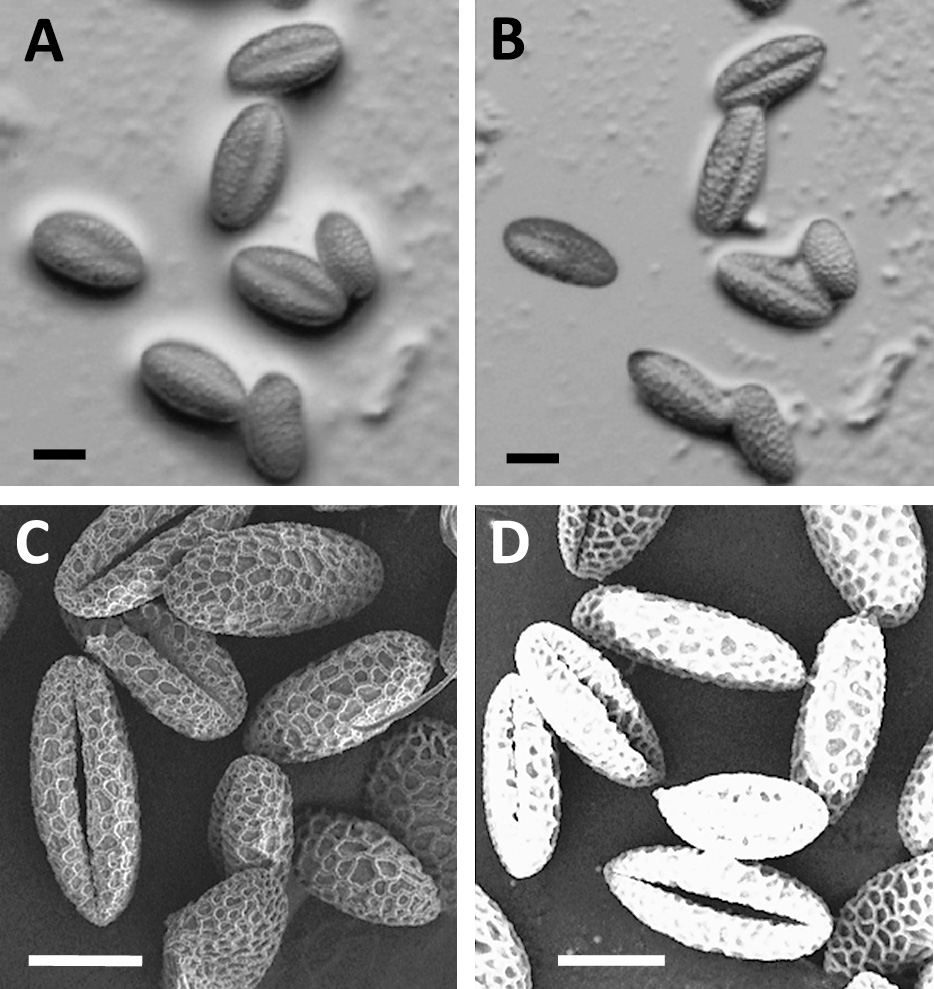
Protoplasts derived from pollen grains of Lilium longiflorum have been frequently isolated (Fig. 4A), and the enzyme compositions have been well studied (Tanaka, 1988; Tanaka et al., 1987). When the best prescription for L. longiflorum was applied to the pollen grains of the Oriental hybrid lily, multiple protoplasts and/or spheroplasts emerged from the germ pores of pollen grains (Fig. 4B). The SEM observation of pollen grains of both varieties pretreated with ethanolic IL revealed that the germ pore size of L. longiflorum was larger than that of the Oriental hybrid lily (Fig. 4C, D). The differences in the isolation frequencies in pollen protoplasts may have been caused by the constituents and sizes of the germ pores in pollen grains. In order to determine the enzyme composition for the digestion of recalcitrant pollen grains in the Oriental hybrid lily, it would be very valuable to detect the elements of the tissues composing the germ pores of grains through SEM observation.
Protoplasts isolated from Lilium longiflorum pollen grains through a Percoll cushion (A). The enzyme composition employed for L. longiflorum led to the emergence of protoplasts and/or spheroplasts from the germ pores of Oriental hybrid lily pollen grains (B). SEM images showing the differences in the germ pore size of ethanolic IL-treated pollen grains from L. longiflorum (C) and Oriental hybrid lily (D). Bars = 50 μm (A), 20 μm (B–D).
In preliminary experiments, we employed 14 types of potentially biocompatible ILs, such as imidazolium-, alkane-, and choline-based ILs, for the pretreatment of pollen grains. The choline lactate showed high biocompatibility with the wet pollen grains, and the SEM images taken after their pretreatment were almost identical to the optical images. It has been reported that choline-based ILs have favorable biocompatibility (Gutiérrez et al., 2010; Vijayaraghavan et al., 2010). We could not show whether the water in the pollen grains was replaced by choline-based IL, but considering that the pretreatment procedure has been completed for tens of seconds, the surface of wet pollen grains would be coated physically with choline salts. On the basis of this concept, the IL-treated pollen grains could include water during SEM observation, so they may be alive under vacuum conditions. Recently, an in situ SEM observation system for metal electrodeposition from ILs in real time was developed (Arimoto et al., 2008a, b). The novel technique presented herein could be used to obtain a moving image of biological events under such an SEM field.