2016 Volume 63 Issue 7 Pages 609-612
2016 Volume 63 Issue 7 Pages 609-612
The cubic perovskite structure was stabilized in solid solution of SrFeO3 and SrNiO3. The compounds were obtained by high pressure synthesis and contained high valence state of Fe4+ and Ni4+ ions in the corner-sharing oxygen octahedra. SrFe1−xNixO3 with x = 0.4 and 0.5 showed ferromagnetic-like behaviors at room temperature, in contrast to the end member perovskite SrFeO3 (x = 0) with helical antiferromagnetism (TN = 134 K). Ni substituton for Fe in the cubic antiferromagnetic SrFeO3 induced the ferromagnetism.
Unusual high oxidation states of some 3d transition-metal cations can be stabilized in oxides. In addition to unusual oxidation states of 2+ and 3+ for Fe typically seen in FeO and Fe2O3, respectively, an unusual high state like 4+ can be stabilized in oxides1–6). The simple cubic perovskite SrFeO3 (see the crystal structure in Fig. 1a) with Fe4+, for instance, is prepared by synthesis under a high-pressure and high-temperature condition. Fe4+ in SrFeO3 is pretty stable at low temperatures because the liner Fe4+-O-Fe4+ bonds stabilize broad conduction bands. The nominal d4 electron configuration of Fe4+ gives an S = 2 high-spin magnetic moment, and the compound shows the antiferromagnetism with the helical magnetic structure below the magnetic transition temperature at 134 K7,8). BaFe4+O3 also crystalizes with the perovskite structure. Interestingly, a weak external field induces ferromagnetism in BaFeO36).
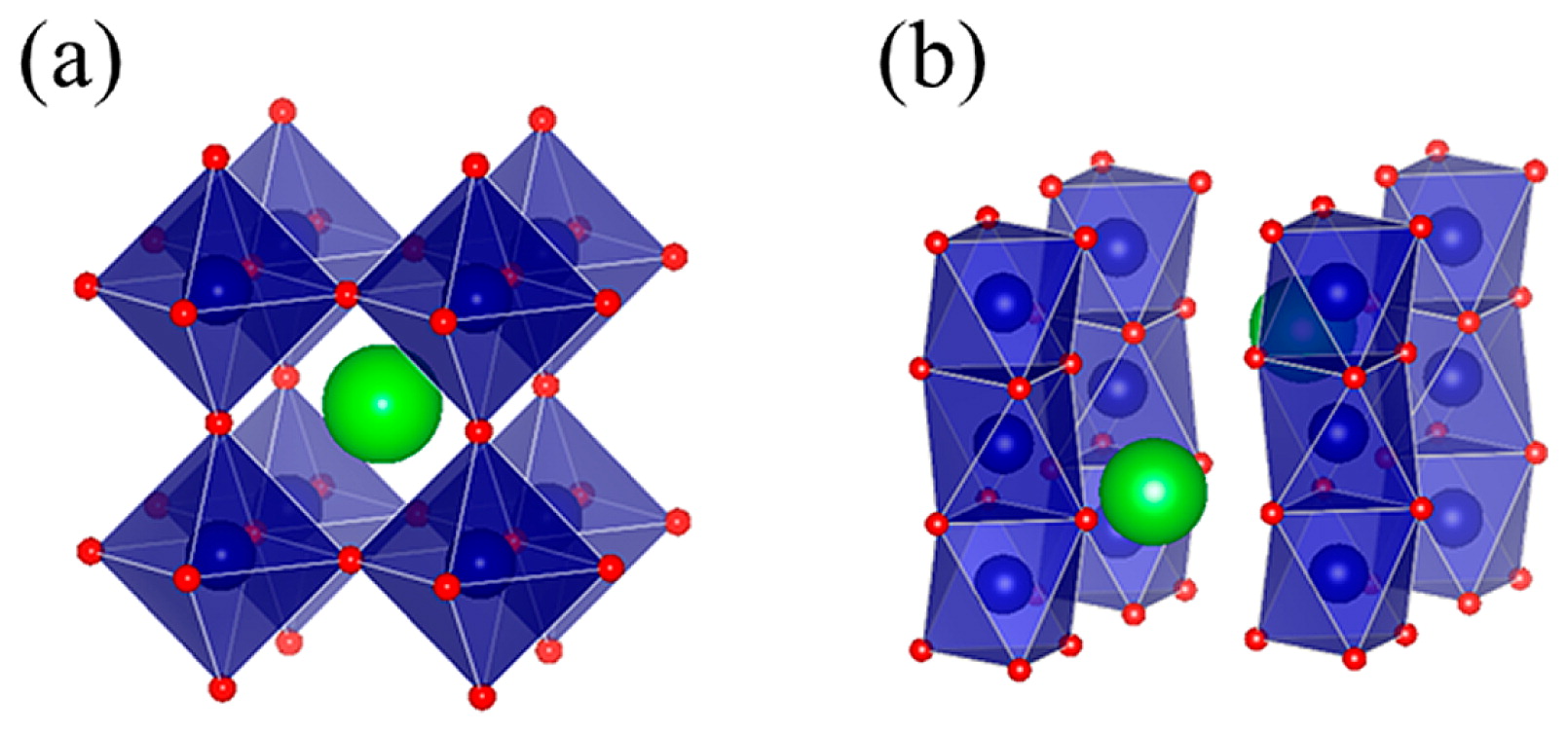
Crystal structures of (a) cubic perovskite and (b) hexagonal perovskite.
On the other hand, a typical oxidation state for Ni is 2+ as seen in NiO, but a very high oxidation state like Ni4+ is stabilized in a few oxides obtained by means of high-pressure synthesis. SrNiO3 and BaNiO3 with Ni4+ crystallize hexagonal perovskite structures, which consist of face-sharing NiO6 octahedra (see the crystal structure in Fig. 1b)9–11). Ni4+ with the nominal d6 electron configuration in the crystal field by six coordinated oxygen ions is considered to have an S = 0 low-spin state. Actually, BaNiO3 is reported to show diamagnetism, suggesting the nonmagnetic Ni4+11,12).
Our primary concern in this study is which crystal structure is stabilized in a solid solution of simple perovskite SrFeO3 with Fe4+ and hexagonal perovskite SrNiO3 with Ni4+. Magnetic properties of SrFe1−xNixO3 (SFNO) are also investigated.
The solid solution samples were prepared by means of high pressure synthesis. Stoichiometric amounts of Fe2O3 and NiO were first mixed, ground in a ball mill, and calcined at 1273 K for 24 hours in air. The obtained powders were then mixed with an appropriate amounts of SrO2 and 25 wt% KClO4 oxidizing agent, sealed in platinum capsules, and treated at high-temperature and high-pressure conditions. For the synthesis below 10 GPa a DIA-type cubic anvil press, where six tungsten-carbide (WC) superhard alloy anvils synchronously compress the sample through a pyrophyllite medium, was used. For the synthesis from 10 up to 15 GPa a 6–8 Kawai-type multi-anvil high-pressure apparatus was used. The octahedral sample cell assembly is compressed by second-stage eight truncated WC anvils (Fig. 2) loaded on the six first-stage anvils. After the reaction, the sample was taken out and the residual KClO4 and KCl in the sample were removed by washing with distilled water and acetone in turn.

Octahedral sample cell assembly in second-stage eight truncated WC anvils.
Phase identification was made by X-ray diffraction (XRD) with a Bruker D8 ADVANCE diffractometer using a Cu-Kα radiation source. The detailed crystal structure of the identified phase was analyzed with synchrotron XRD data. The room temperature pattern produced at a wavelength of 0.77425 Å was collected with a large Debye-Scherrer camera with an image plate at beamline BL02B2 in SPring-8. The obtained XRD data were analyzed by the Rietveld method using the program RIETAN-FP13). Oxidation state of Fe in the sample was estimated by the isomer shift (IS) value in 57Fe Mössbauer spectrum by using a 57Co/Rh radiation source. The velocity scale and the IS were determined by using pure iron metal.
Magnetic properties of the samples were measured using a SQUID magnetometer (MPMS, Quantum Design). Temperature dependence of magnetic susceptibility was measured in a 10 kOe magnetic field at temperatures from 5 to 300 K. Field dependence of the magnetization was measured at several temperatures under fields ranging from −24 to 24 kOe.
Fig. 3 shows XRD patterns of SFNO with x = 0, 0.5, 0.75 and 1.0 recorded using Cu-Kα as the radiation source. The observed pattern for the sample with x = 0.5 is well reproduced with the cubic Pm3̅m perovskite structure, which is the same as that for SrFeO3 (x = 0.0), although a very small amount of NiO is seen. As shown in Fig. 4, the lattice parameters for the cubic perovskites (0.0 ≤ x ≤ 0.5) decrease linearly with increasing the amount of Ni, also confirming the successful substitution of Ni for Fe in SrFeO3. Note that even with the exceptional peak shape resolution of the SXRD patterns there was no obvious splitting of the stronger Bragg reflections indicative of cubic symmetry. On the other hand, the XRD pattern for the sample with x = 0.75 consists of mixed phases. Although the sample includes some mounts of cubic perovskite phase, the obtained lattice parameter for the cubic phase is the same as that for SrFe0.5Ni0.5O3 (Fig. 4). Attempts to prepare a single phase sample for x = 1.0 were unsuccessful. Although SrNiO3 was reported to crystalize with the hexagonal perovskite, the observed XRD pattern did not show that the sample include such a phase. We thus conclude that the solid solubility limit of x in the cubic perovskite SFNO is about 0.5 by the synthesis under present high pressure conditions.

XRD patterns for SFNO (x = 0, 0.5, 0.75, and 1).

Composition dependence of the cubic lattice parameter of SFNO. The blue line is a linear fit for the change, following the Vegard’s law.
A typical Rietveld refinement result for the cubic Pm3̅m perovskite SFNO (x = 0.4) is shown in Fig. 5 and Table 1. The refined occupancy for the oxygen site, 1.005(7), confirms that the oxygen sites are fully occupied. From the refinement result, the Fe(Ni)-O bond distance, 1.91772(1) Å are obtained, and the valence states of Fe and Ni are estimated to 3.92 and 4.06, respectively, from the bond distance with a bond valence sum method. The values are very close to 4+, suggesting unusual high oxidation states for both Fe and Ni in the compound. The obtained bond valence sum values are also quite consistent with the nominal composition of SFNO, which gives an average oxidation state for the B-site cation 4+.

Synchrotron X-ray diffraction pattern for the x = 0.4 sample and the result of the Rietveld refinement. The red dots and the black solid line represent the observed data and fitted pattern, respectively. The purple and orange vertical marks show the Bragg peak positions of SFNO (x = 0.4) and NiO, respectively. The bottom blue solid line represents the difference between the observed and calculated intensities.
| Atom | g | x | y | z | 100 × Uiso/Å2 |
|---|---|---|---|---|---|
| Sr | 1 | 0.5 | 0.5 | 0.5 | 0.53(2) |
| Fe | 0.6 | 0 | 0 | 0 | 0.38(3) |
| Ni | 0.4 | 0 | 0 | 0 | 0.38(3) |
| O | 1.005(7) | 0 | 0 | 0.5 | 0.77(6) |
Space Group Pm3̅m ; a = 3.83545(1) Å
Rwp = 8.86 %
The valence state of Fe is also examined by the Mössbauer spectroscopy. Fig. 6 shows the Fe Mössbauer spectroscopy result for SrFe0.5Ni0.5O3 obtained at 5 K. The spectrum consists of a single component with IS of 0.09 mm/s relative to α-Fe and a hyperfine field (HF) of 293 kOe. These values are very close to those for SrFeO32). The results, especially the small IS value, lead to a conclusion that the oxidation state of Fe in the cubic perovskite SFNO is kept at 4+ in the range of 0 ≤ x ≤ 0.5. Importantly, the results also give the unusually high oxidation state of 4+ for the substituting Ni in the compounds.

Mössbauer spectrum of SFNO (x = 0.5) obtained at 5 K. The solid blue line is the Lorentzian fit. The velocity scale was determined relative to pure iron metal.
The temperature dependence of the magnetic susceptibility of SFNO (0 ≤ x ≤ 0.5) is shown in Fig. 7. As reported previously, SrFeO3 (x = 0) shows an antiferromagnetic-like transition at 134 K. The behaviors of the samples with x = 0.1 and 0.2 are similar to that of the sample with x = 0. On the other hand, large increases in magnetization with decreasing temperature are observed for the samples with x = 0.4 and 0.5. Field dependence of magnetic moment at 5 K confirmed the ferromagnetic-like behaviors for the x = 0.4 and 0.5 samples with saturation magnetizations 2.38 and 1.80 μB/f.u., respectively, as shown in Fig. 8. Even at room temperature the large saturation magnetizations of 1.16 μB/f.u. (x = 0.4) and 0.98 μB/f.u. (x = 0.5) are observed. Thus, the results show that the Ni4+ substitution in the antiferromagnetic SrFeO3 induces ferromagnetism with a transition temperature higher than room temperature.
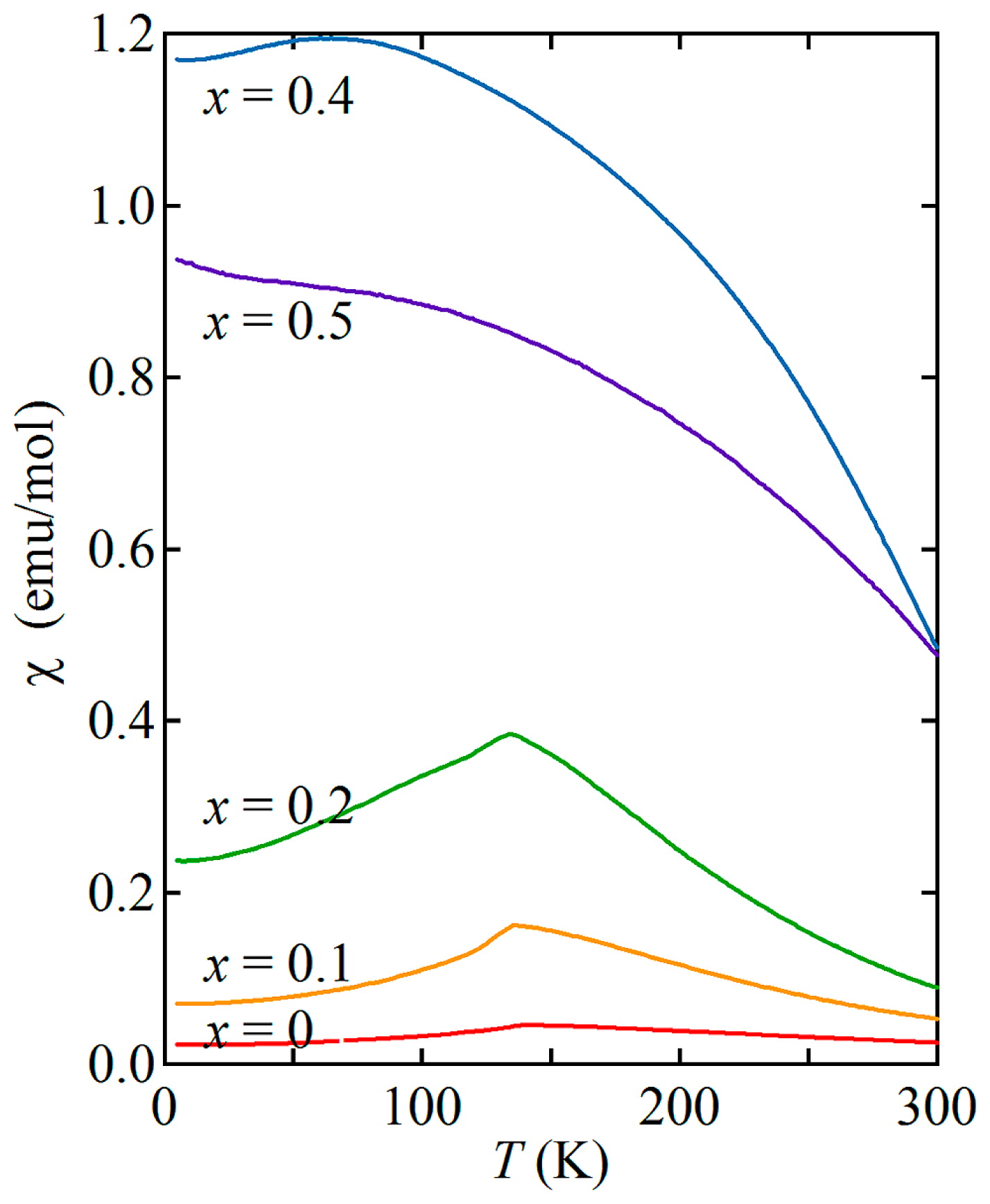
Temperature (T) dependence of magnetic susceptibility (χ) for SFNO measured at 10 kOe on heating after zero-field cooling.

Magnetic field (H) dependence of magnetic moment (M) at 5 K for SFNO.
Ni4+ is believed to have intrinsically an S = 0 low-spin state like that of the Ni4+ in BaNiO3. Considering that Fe4+ spins in SrFeO3 shows helical magnetic structure, the obtained results appear to indicate that the ferromagnetism is induced by introducing nonmagnetic Ni4+ ions into the helical antiferromagnetic Fe4+ spin sublattice. However, it is interesting to see a recent result by Chen, et al.14), where they computed a hypothetical cubic perovskite SrNiO3 by a first-principles method and found that the ground state of SrNiO3 is ferromagnetic. They also claimed that the Ni4+ in SrNiO3 has the magnetic moment of 0.855~1.689 μB depending on the on-site Coulomb interaction (U = 3.0~11.0 eV). Although the cubic SrNiO3 perovskite could not be synthesized in our present experiments, the calculation result suggest that the Ni4+ in the NiO6 octahedron can be magnetic. Given the predicted Ni4+ magnetic moment of 1.17 μB by assuming a typical U value like 5.0 eV, the observed saturation magnetization (2.38 μB/f.u.) for SrFe0.6Ni0.4O3 leads to Fe4+ magnetic moment 3.19 μB. Very recently, we have performed magnetic circular dichroism experiment and obtained the spin moments of 3.70 μB for Fe and 1.26 μB for Ni by using magnetooptical sum rules15), and the values are quite consistent with the present values. Therefore, the Ni4+ ions in SFNO have substantial magnetic moments, contrary to expectation of nonmagnetic low spin state (t2g6, S = 0) in an octahedral crystal field.
Note, as shown in Fig. 6, the unusual high oxidation state of Fe4+ remains down to low temperature and anomalies due to charge disproportionation of Fe are not observed. This also suggests that unusual high oxidation state of Ni4+ is kept intact and that the charge disproportionation of Ni and charge transifer between Fe and Ni do not occur. Because the crystal structures keep the cubic symmetry for the samples with x ≤ 0.5, linear Fe(Ni)-O-Fe(Ni) bonds hold the broad bands in the elecctronic structure and make the metallic state stable.
Solid solution samples of SrFe1−xNixO3 with unusual high valence Fe4+ and Ni4+ were prepared by high-pressure and high-temperature synthesis. The cubic perovskite structures were obtained up to x = 0.5, and single phase samples could not be obtained for 0.5 < x ≤ 1.0. Ni substituton for Fe (x = 0.4 and 0.5) in SrFeO3 induced the ferromagnetism, and the large saturation magnetizations were observed. The ferromagnetic transition temperatures are higher than room temperature. The SPring-8 experiments were approved by JASRI (2015A1016 and 2015A1014). This work was partly supported by KAKENHI and JST-CREST.