2016 Volume 63 Issue 7 Pages 484-490
2016 Volume 63 Issue 7 Pages 484-490
FeB-Ni hard materials were consolidated by both electroless plating and spark sintering processes for the development of ubiquitous hard materials. Uniform nickel layers were formed quantitatively on as-received FeB powder surfaces. The amorphous Ni layers transformed to poly-crystalline during spark sintering. Sintering curves of FeB-Ni compacts showed the similar behaviors regardless of Ni contents, although their apparent relative densities were increased with the increment of Ni contents. Moreover, the sintering mechanism of FeB-Ni and pure Ni compacts consisting of both the plastic deformation and power law creep deformation occurred during spark sintering. Their maximum points of Ḋ were shown in the D of approximately 0.78. The plastic and power law creep deformation were predominantly consolidation mechanisms before and after maximum Ḋ, respectively. Values in HRA of FeB-Ni compacts were decreased with increment of the Ni contents. The compressive stress of FeB-Ni compacts was decreased with the increment of the Ni contents in contrast to the compressive strain.
The combination of WC and Co was the most widely used hard materials in the fields of cutting materials, wear resistant materials, high temperature and corrosion-resistant materials1–4). However, considering the low reserves of the W and Co, the development of ubiquitous hard materials to substitute WC-Co alloys had attracted considerable researcher’s attentions. The metal-carbides, -nitrides and -borides were commonly used as hard materials. It was reported that that iron borides (FeB and Fe2B) with low cost were expected to substitute the WC5–7). On the other hand, the substitution of the Co by Ni or Fe had been investigated in recent years, since Fe and Ni belonged to the transition metal group as Co, and which had excellent mechanical properties8,9). In addition, the reserves of Fe and Ni were rich which resulted in relatively low price compared with Co10). Hard metals were fabricated by vacuum sintering and hot sintering process. These sintering processes relayed on heat transfer, conduction or radiation to sintering the powders which may resulted in grain coarsening11). The enhanced sintering technique such as spark sintering process was applied to overcome these problems. Spark sintering process had the advantage of the rapid densification rate compare with vacuum and hot sintering process12,13). Moreover, the spark sintering process could save more energy than that of vacuum sintering process7). Therefore, the spark sintering process was selected to consolidate the FeB powders in this study. Presently, the FeB-Fe system alloys mixed by elemental blending process were consolidated by spark sintering process in our laboratory5,6). The research results showed that the porosity of the compacts was very high due to the inhomogeneous distribution of the Fe binder phase. It was considered that electroless plating process was an effective method to overcome the problems. Coating on the surface of hard materials could lead to homogeneous distribution of elements, strength the interface between hard phases and achieve full densification of hard materials. Moreover, electroless plating process was suitable for non-conductive materials and materials with irregular shape.
In this study, electroless Ni plating was conducted on as-received FeB powders to obtain homogeneous distribution of Ni. The spark sintering behaviors of the FeB-Ni powders with different Ni contents and their mechanical properties were also investigated. The purpose of the present work was to study the fabrication, sintering behavior and properties of FeB-Ni hard materials.
As-received FeB powders (Fukuda Metal Foil and Powder Co., Ltd.) with mean powder size of 45 μm consisted of 78.4 % Fe, 19.1 % B, 0.04 % C, 0.48 % Si, 0.02 % P, 0.003 % S and 1.9 % Al (mass %). The constructed phases of FeB powders were FeB, Fe2B, B and Fe. Fig. 1 showed the SEM image of as-received FeB powders. The shape of as-received FeB powders showed irregular. Electroless plating was conducted on as-received FeB powders. The FeB powders were simply pretreated before electroless plating to produce a surface with catalytic activity. The solution for the simply pretreatment was hydrochloric acid (50 mL/L). De-ionized water was utilized to clean the powders and removed the remaining liquid after pretreatment. The Ni-B plating solution which contained less than 1 % B (Okuno Chemical Industries Co., Ltd) was used in this study. Electroless plating was performed in a beaker placed in the water bath. The solution was continuously stirred during the electroless plating. The PH and temperature of electroless plating were 6.5 and 333 K, respectively. The FeB-Ni powders were cleaned with de-ionized water after plating. Then the powders were dried in a vacuum oven at 323 K for 2 h.

A SEM image of the as-received FeB powders.
The FeB-Ni powders were consolidated by spark sintering process. During the spark sintering, two graphite punches with diameter of 10 mm and height of 30 mm and a graphite die with an inner diameter of 10 mm, an outer diameter of 40 mm and height of 60 mm were used. Spark sintering process was carried out under a vacuum condition (< 10-2 Pa). The voltage wave forms of spark sintering process had two modes, pulse discharge sintering mode and resistance heating mode10). In pulse discharge sintering mode, the pulse current and voltage were 100 A and 50 V for 900 s under a pressure of 15 MPa. In continuous discharge sintering mode, the sintering process adopted included following steps: firstly, the heating rate with 50 K/min was used to achieve the temperature that 90 K lower than the maximum temperature; secondly, the heating rate with 5 K/min was used to achieve the target temperature and holding for 30 min; finally, the compact was cooling down with the apparatus. A uniaxial pressure of 50 MPa was applied for this mode. The sintering temperature was measured using a thermocouple (R-type) inserted in the cylindrical of the die. The tip of the thermocouple was about 2 mm away to the compact. The voltage, current, temperature and height of the compact were monitored from a software (Naviwave System, SSAlloy Co., Ltd.) of the spark sintering apparatus.
2.3 CharacterizationThe characteristic X-ray images of FeB-Ni powders and the microstructure of the compacts were measured by the electron probe micro analyzer (EPMA, JXA-8900; Japan). Apparent relative density was calculated by the height of the compact dividing the ideal height of 10 mm. Porosity of compacts was measured by image analysis methods14). 5 pictures measured by scanning electron microscope (SEM, TOPCON SM-520; Japan) were used to calculate the average porosity. Phases in the compacts were characterized by X-ray powder diffraction method (XRD, D/max-2500/PC; Japan) using Cu Kα radiation (λ = 1.5406 Å) at 40 kV and 100 mA. Hardness of the compacts was measured by Rockwell hardness tester (A scale). Compressive strength of the compacts was measured at room temperature by using a mechanical testing machine (Autograph DCS-R-5000, Japan) with a constant crosshead speed of 0.05 mm/min.
Fig. 2 showed the relation between electroless plating time and Ni contents plated on surfaces of as-received FeB powders (here after called FeB-0Ni). The Ni contents were increased with the increment of the electroless plating time and temperature. According to this figure, FeB-Ni powders with 10 vol%, 25 vol% and 30 vol% Ni contents (here after called FeB-10Ni, FeB-25Ni and FeB-30Ni) were obtained by controlling of conditions such as electroless plating time and temperature in this study. The compositional image and EPMA mapping analysis of FeB-10Ni powder were shown in Fig. 3 (a) and (b). The compositional image of FeB-10Ni powder and the characteristic X-ray image of Ni showed that uniform Ni layer was formed quantitatively on as-received FeB powder surface. The FeB powders plated with Ni as binder phase avoided the direct contact between hard particles. It was expected to improve the sinterability of FeB hard materials and achieve high apparent relative density by electroless plating. In addition, the Ni and Fe concentration profiles from EPMA line analysis were shown in Fig. 3 (c) and (d). It was found that the thickness of Ni layer formed on FeB powder was about 2 μm. The XRD patterns of FeBNi powders with different Ni contents were presented in Fig. 4. The XRD patterns of FeB and pure Ni were shown as reference. The results revealed that the mainly phases of FeB-Ni powders were Ni, FeB and Fe2B phases. Furthermore, the peaks of Ni became broader compared with the peaks of pure Ni. It was considered that the structure of Ni layers plated on FeB powder surfaces was amorphous.
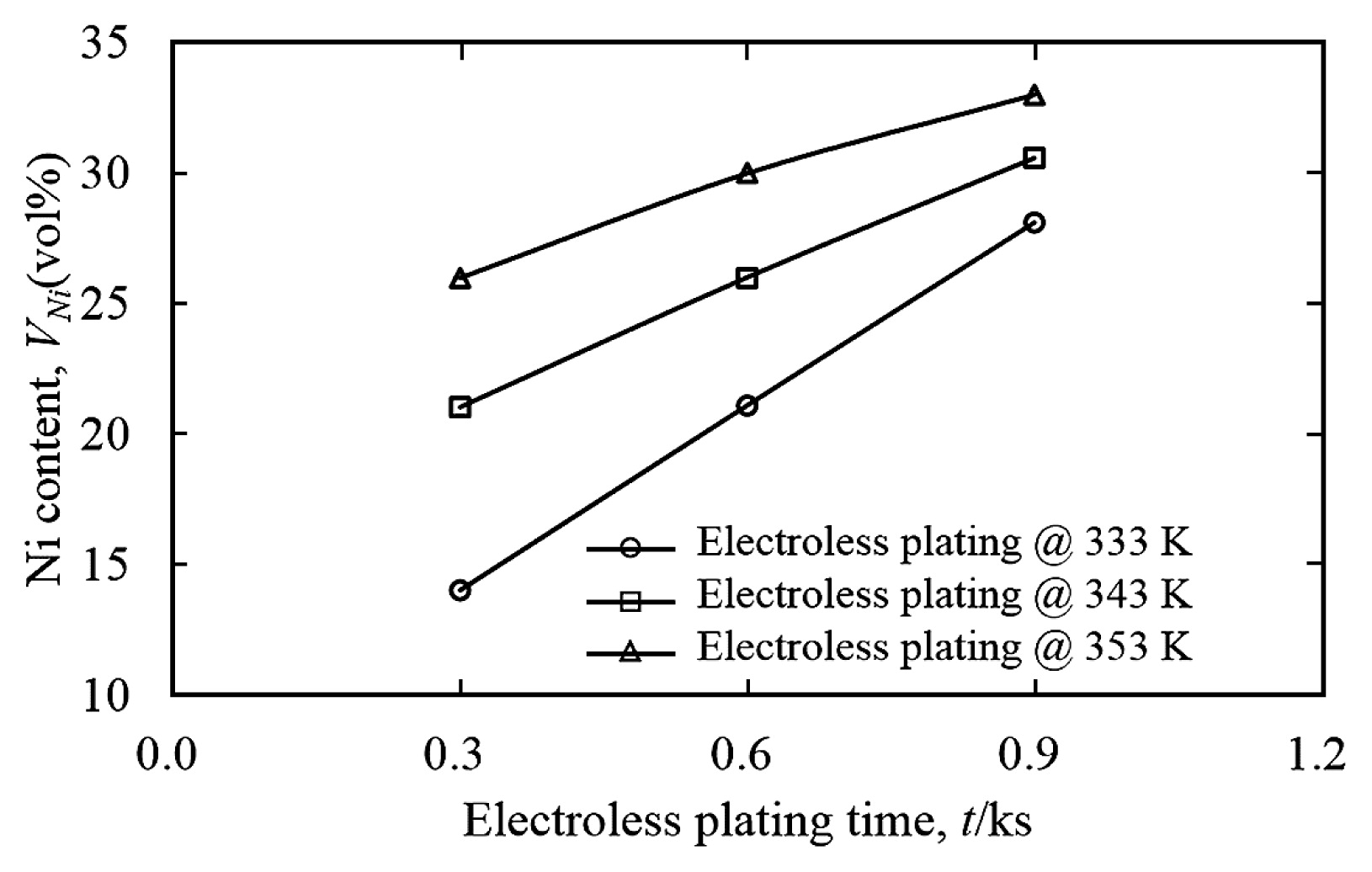
Relation between electroless plating time and Ni contents plated on surfaces of the as-received FeB powders.
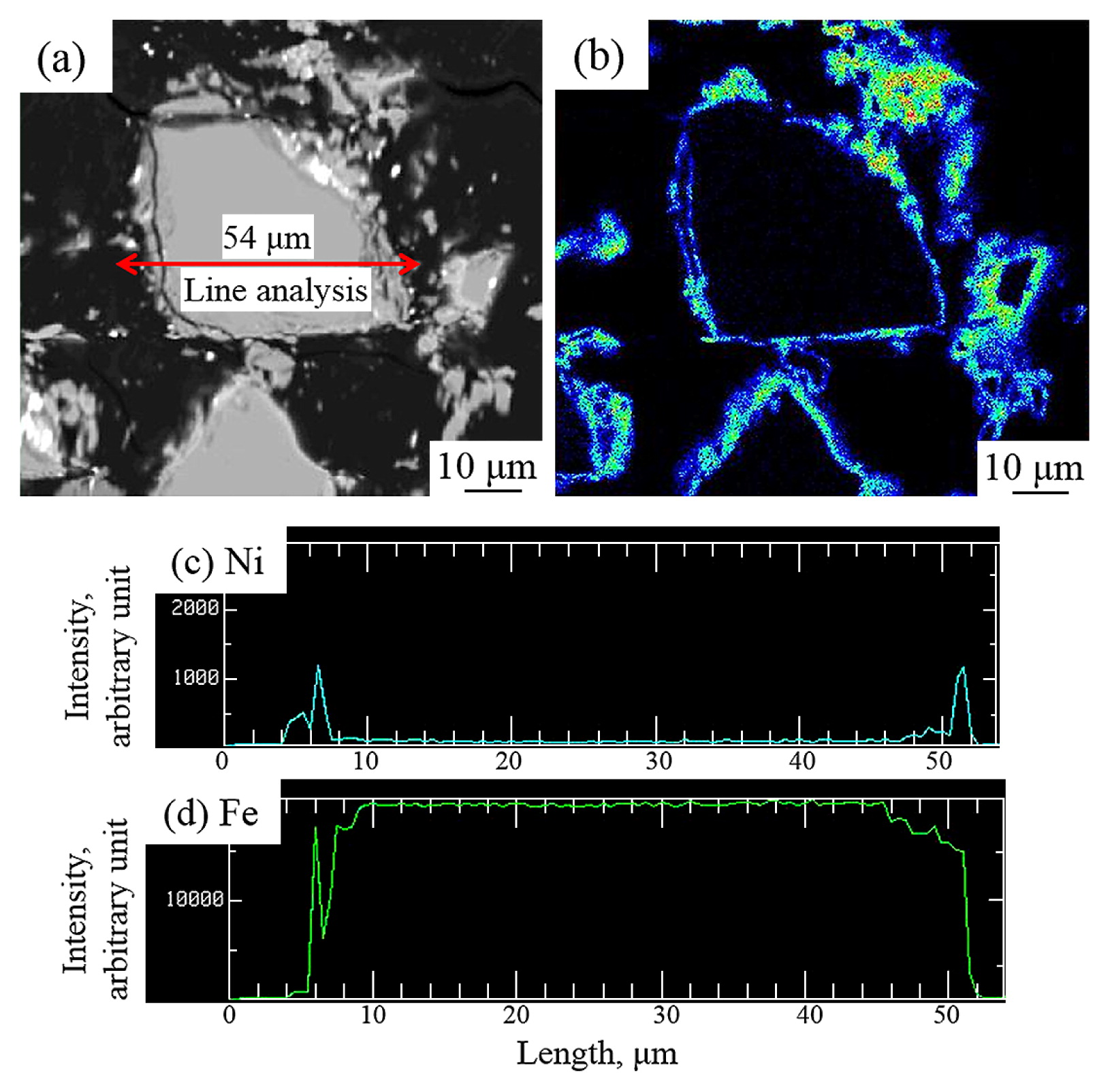
A compositional image (a) and the EPMA mapping of FeB-10Ni powder (b). Ni (c) and Fe (d) concentration profiles from EPMA line analysis. The line analysis track shown with red line was indicated in (a).

XRD patterns of the (a) FeB-10Ni, (b) FeB-25Ni and (c) FeB-30Ni powders used in experiments. XRD patterns of the (d) FeB-0Ni and the (e) pure Ni powders were as reference.
Fig. 5 showed the relation between sintering temperature and apparent relative density of FeB-10Ni, FeB-25Ni, FeB-30Ni compacts sintered at 1273 K, FeB-0Ni compacts sintered at 1382 K and pure Ni sintered at 1418 K. The sintering behaviors of FeB-Ni compacts with different Ni contents were shown between those of FeB-0Ni compact and pure Ni compact. Sintering curves of FeB-Ni compacts with different Ni contents showed the similar sintering behaviors regardless of Ni contents, although their apparent relative densities were increased with the increment of Ni contents. The apparent relative densities of FeB-Ni compacts with different Ni contents were increased monotonously in the temperature range below 1200 K. And then the apparent relative density became almost constant above this temperature. Moreover, the slope of sintering curves below about 1200 K for FeB-Ni compacts with different Ni contents was higher than that of FeB-0Ni compacts during spark sintering due to the deformation of plated Ni layers on the FeB powder surfaces. The slope in the sintering curves meant the consolidation rate in the temperature range below 1200 K. On the other hand, the deformation of FeB hard phases could not be expected below 1200 K because of the slightly increment of the apparent relative density according to the sintering curve of FeB-0Ni compact5). It was found that the increment of the apparent relative density of FeB-Ni compacts was mainly due to the addition of Ni. As shown in Fig. 5, the sintering curve of FeB-0Ni compact with low apparent relative density suggested poor sinterability. In contrast, the sintering curve of the pure Ni compact showed good sinterability.
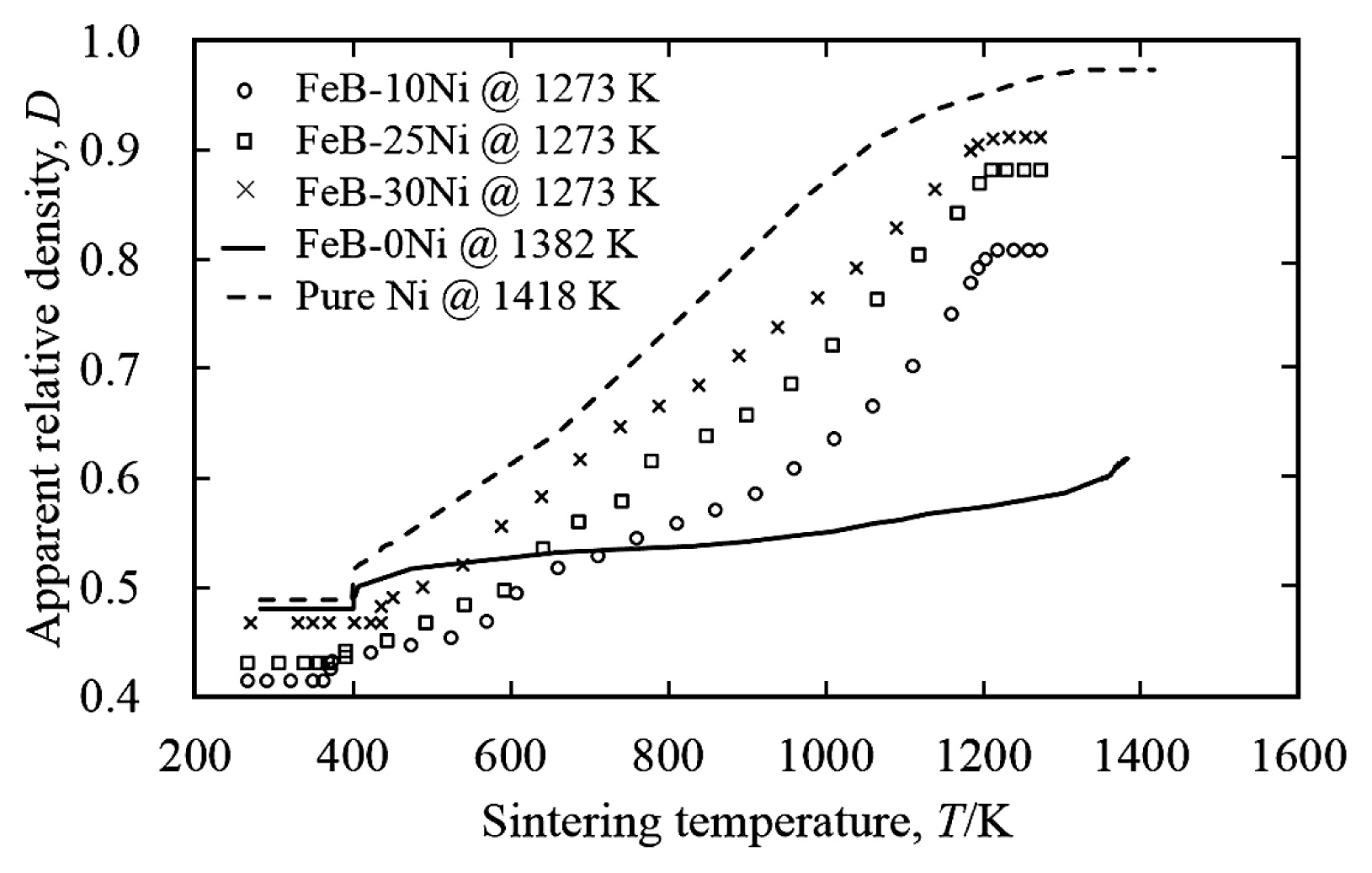
Relation between sintering temperature and apparent relative density of the FeB-10Ni, FeB-25Ni, FeB-30Ni compacts sintered at 1273 K, the FeB-0Ni compact sintered at 1382 K and the pure Ni compact sintered at 1418 K.
In order to investigate the mechanism of densification in spark sintering process, the densification rate of FeB-Ni with different Ni contents, FeB-0Ni and pure Ni compacts were obtained experimentally as shown in Fig. 6. The densification rate, Ḋ, was obtained by dividing the apparent relative density increment, dD, by time increment, dt.

Relation between densification rate and apparent relative density of the FeB-10Ni, FeB-25Ni, FeB-30Ni, FeB-0Ni and pure Ni.
| (1) |
where D and t were apparent relative density and the corresponding sintering time, respectively. It was reported that plastic deformation of the compacts occurred before reaching maximum point of the Ḋ. After that power law creep deformation of compacts occurred using pure copper, pure Ti and Al2O3 added copper powders15–19). The peaks of Ḋ of FeB-10Ni, FeB-25Ni and FeB-30Ni compacts occurred at 0.76, 0.79 and 0.78, respectively which were similarity. On the basis of the previous results, it was considered that the plastic deformation of Ni layer in FeB-Ni compacts occurred before reaching maximum point of the Ḋ. After that power law creep deformation of Ni layer occurred in compacts. Pure Ni compact showed high densification rate, and its peak value was 0.74 at D which was close to FeB-10~30Ni compacts. In contrast, the value in Ḋ peak of FeB-0Ni compact was much lower than those of FeB-Ni compacts. It was proved that the FeB phase showed little deformation in this temperature range below 1200 K. Therefore, the plastic deformation of Ni layer caused by Joule’s heat generation among particles played the dominant role in increasing the apparent relative density for FeB-Ni compacts. It meant that increment of apparent relative density from about 0.6 for FeB-0Ni compact to 0.8 for FeB-10Ni caused by the electroless plating 10 vol% Ni on FeB powder surfaces. Moreover, the apparent relative density could be further improved by more Ni addition.
3.3 Microstructure and mechanical propertiesFig. 7 showed compositional images of the (a) FeB-10Ni, (b) FeB-25Ni, (c) FeB-30Ni compacts sintered at 1273 K; (d) FeB-0Ni compact sintered at 1382 K. It was noted that, uniform Ni layers formed on FeB powder surfaces were still maintained after consolidation. The FeB-Ni compacts with different Ni contents achieved less porosity due to less FeB-FeB contacts during sintering. Moreover, the Ni binder phase filled the existing voids between FeB particles. The average porosities in FeB-10Ni, FeB-25Ni and FeB-30Ni compacts sintered at 1273 K were 13.5 %, 12.8 % and 5.3 %, respectively. As reference, the porosity of FeB-0Ni compact was 28.1 %. It was considered that the porosity was decreased with increment of the Ni contents. Fig. 8 showed XRD patterns of the (a) FeB-10Ni, (b) FeB-25Ni, (c) FeB-30Ni compacts sintered at 1273 K, (d) FeB-0Ni compact sintered at 1382 K and (e) pure Ni compact sintered at 1418 K. XRD patterns of FeB-Ni compacts with different Ni contents revealed the presence of FeB, Fe2B and Ni peaks. There were main constituent FeB phase of 92 %, 82 %, 69 % and 63 % in FeB-0, 10, 25 and 30 Ni compacts. As shown in contrast to Figs 4 and 8, Ni phases with crystalline structure were detected in the XRD patterns indicating that the structure of the Ni layers was transformed from amorphous to crystalline.
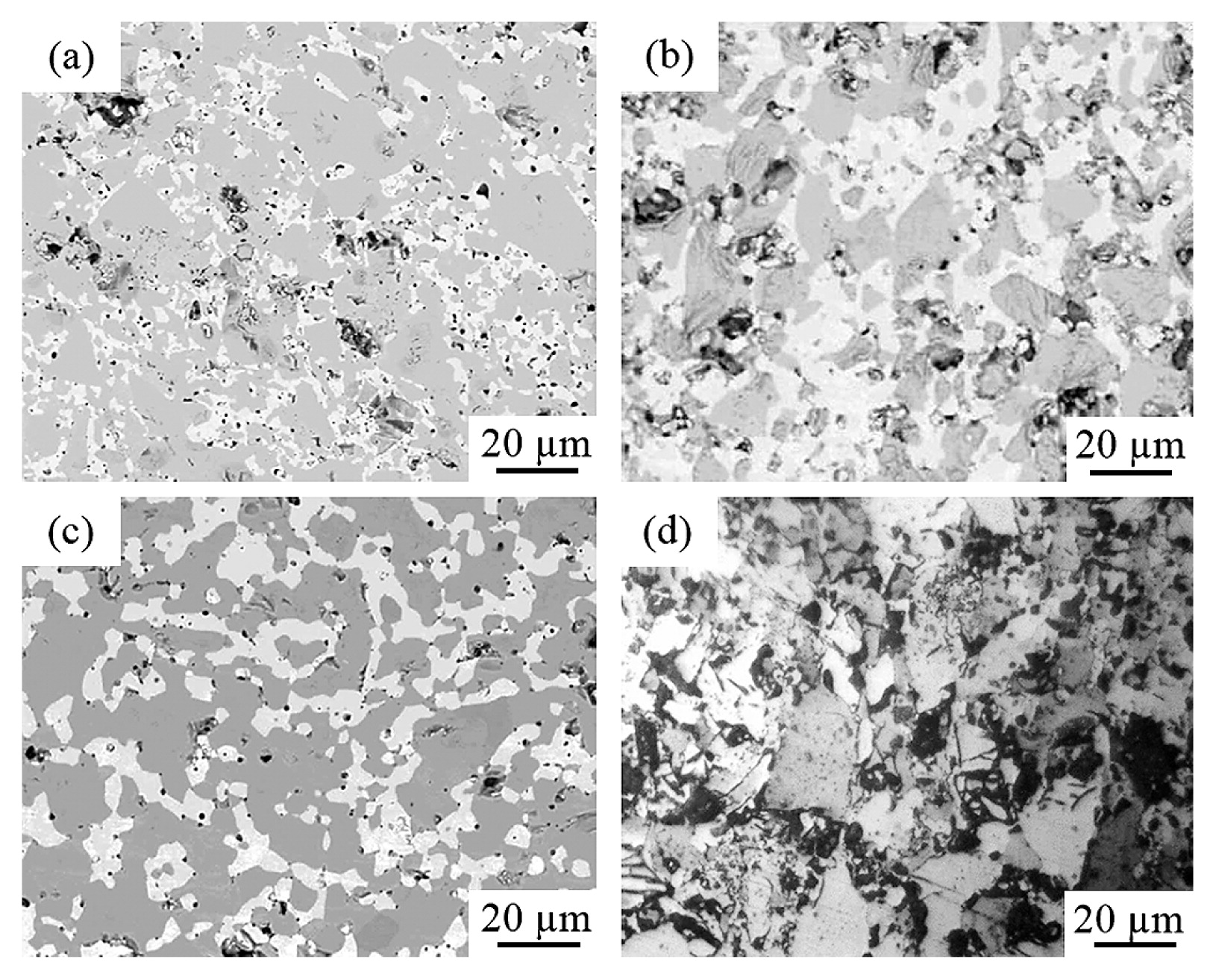
Compositional images of the (a) FeB-10Ni, (b) FeB-25Ni, (c) FeB-30Ni compacts sintered at 1273 K; (d) FeB-0Ni compact sintered at 1382 K.

XRD patterns of the (a) FeB-10Ni, (b) FeB-25Ni, (c) FeB-30Ni compacts sintered at 1273 K, (d) FeB-0Ni compact sintered at 1382 K and (e) pure Ni compact sintered at 1418 K.
Fig. 9 showed the relation between HRA and porosity of the FeB-10Ni, FeB-25Ni, FeB-30Ni compacts sintered at 1273 K, and FeB-0Ni compact sintered at 1382 K. The value of HRA of FeB-10Ni compact sintered at 1273 K was higher than that of FeB-25Ni and FeB-30Ni compacts sintered at the same temperature. It was considered that the increment of the soft Ni contents resulted in the decreased of the hardness. In addition, the value of HRA of FeB-30Ni compact was close to that of FeB-25Ni compact due to the lower porosity. Moreover, the value of HRA of FeB-10Ni compact sintered at 1273 K was higher than that of FeB-0Ni compact sintered at 1382 K, although the Ni with low hardness was added. It was mainly because the porosity of FeB-10Ni compact was lower than that of FeB-0Ni compact.
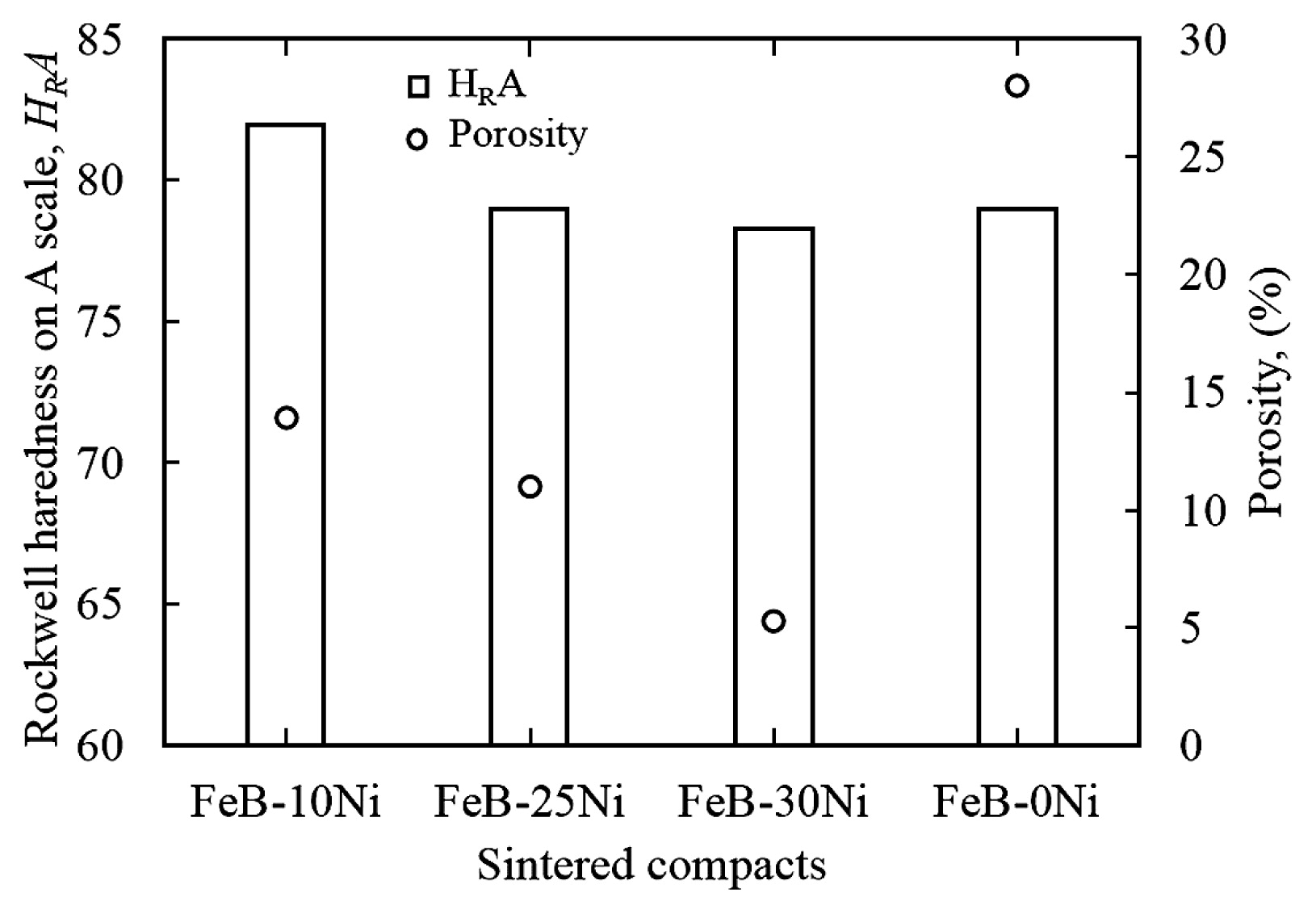
Relation between HRA and porosity of the FeB-10Ni, FeB-25Ni, FeB-30Ni compacts sintered at 1273 K, and FeB-0Ni compact sintered at 1382 K.
Fig. 10 showed compressive stress-strain curves of the FeB-10Ni, FeB-25Ni, FeB-30Ni compacts sintered at 1273 K, and FeB-0Ni compact sintered at 1382 K. FeB-Ni compacts showed the high compressive strain more than 40 %, although that of approximately 20 % was shown in the FeB-0Ni compact. The compressive strain of FeB-Ni compacts was increased with the increment of the Ni contents. Although the compressive stress of FeB-Ni compacts was decreased with the increment of the Ni contents, the compressive stress of FeB-Ni compacts was still lower than that of FeB-0Ni compact. It was considered that the Ni phase surrounding FeB hard phase improved the interfacial strength between FeB phases. The typical fracture surface of FeB-25Ni compact was shown in Fig. 11 (a). The fracture mode of FeB-25Ni was the mixture of intragranular fracture and intergranular fracture of Ni phases. As shown in Fig. 11 (b), the interfacial strength between FeB and poly-crystalline Ni phase was improved through circumference of all FeB phases by their reactions. In contrast, the fracture mode of FeB-0Ni compact was intergranular fracture among every particle. In addition, grain boundaries between FeB phases with many voids were clearly observed in the FeB-0Ni compacts indicating low interfacial strength compared with the FeB-Ni compacts. Low interfacial strength resulted in low compressive stress which corresponded to that of compressive tests as shown in Fig. 10.
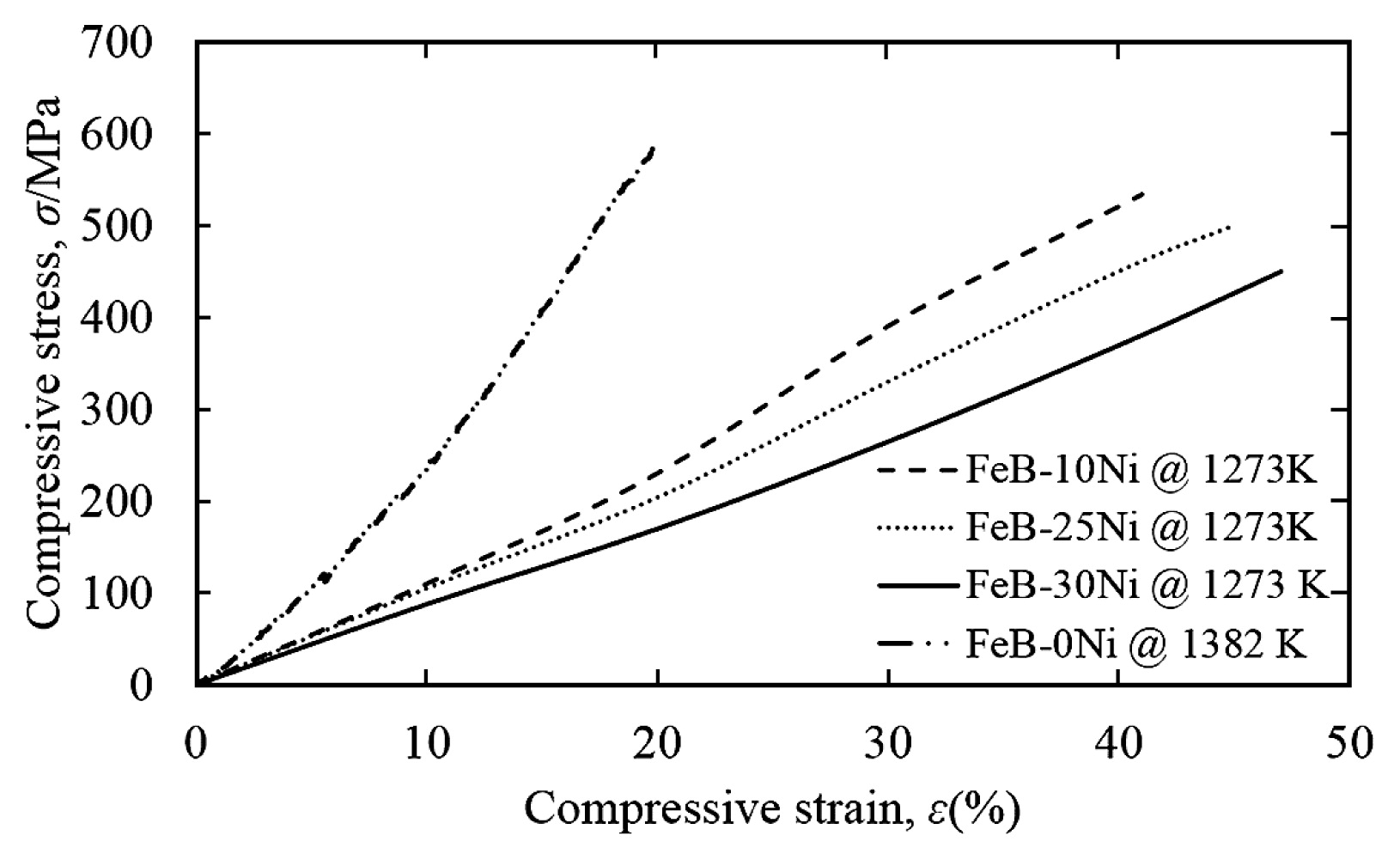
Compressive stress-strain curves of the FeB-10Ni, FeB-25Ni, FeB-30Ni compacts sintered at 1273 K, and FeB-0Ni compact sintered at 1382 K.
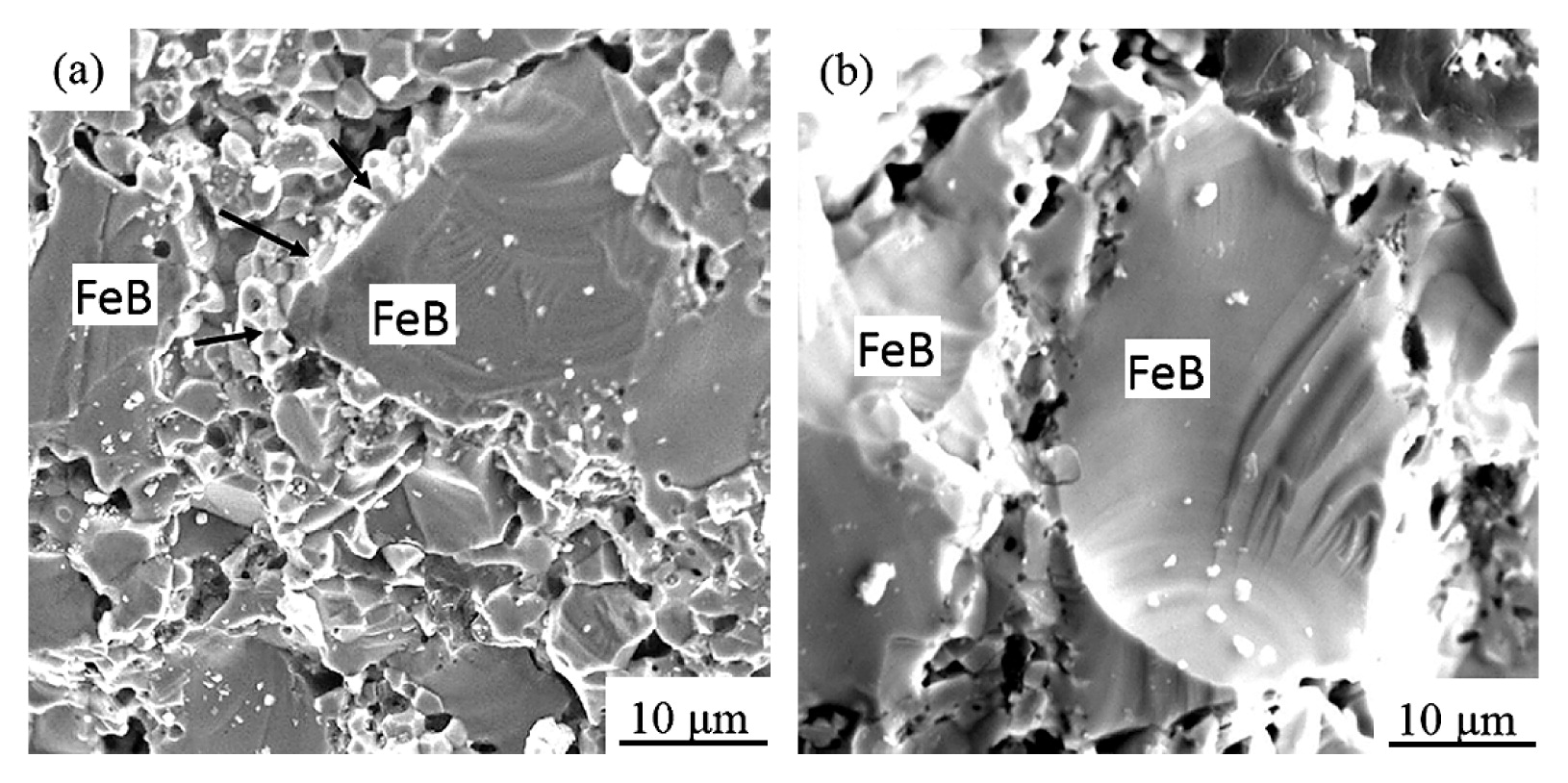
SEM images of fracture surfaces of the (a) FeB-25Ni compact sintered at 1273 K and the (b) FeB-0Ni compact sintered at 1382 K. The Ni phases surrounding the FeB phase were indicated by the arrows.
In this study, the intention was that the preliminary investigation of the development of hard materials which refer to the substitution of WC for ubiquitous FeB and the substitution of Co for Fe/Ni. The spark sintering process with energy conservation was utilized to consolidate FeB-Ni materials. Electroless Ni plating process was conducted on the FeB powder surfaces to obtain the homogeneous distribution of Ni. The sintering mechanism was investigated to determine the feasibility for the FeB-Ni materials as hard materials. In addition, the basic performances were also studied by the Rockwell hardness and compressive strength tests. It was conclude that FeB-Ni materials had the possibility to substitute the WC-Co hard materials. In the future, the mechanical properties refer to the practical application such as the fracture toughness, wear resistance and bending strength would be measured to obtain the high performance hard materials.
The FeB-Ni hard compacts were fabricated by both eletroless plating and spark sintering processes. Their sintering behaviors and characteristics were investigated in this study.
This work was supported by JSPS KAKENHI Grant Number 26340101.