2025 Volume 19 Issue 1 Article ID: oa.2025-0024
2025 Volume 19 Issue 1 Article ID: oa.2025-0024
Objective: 3D DSA performed under carotid artery occlusion tests (Matas and Alcock test) while the operator manually compresses the patient’s carotid artery may be performed as a preoperative evaluation. However, few known studies have quantified the operator’s radiation exposure dose during 3D DSA under carotid artery occlusion tests. In this study, we measured the changes in the operator’s radiation exposure dose during such imaging under different protective measures and assessed alternative protective measures for hand exposure apart from protective gloves and the operator’s head and neck orientation, proposing a new protection method.
Methods: We measured changes in the operator’s radiation exposure dose under different protective measures. Specifically, we measured changes in lens dose on the operator’s head and neck orientation and the use of protective equipment. Furthermore, we evaluated alternative protective measures for hand exposure aside from protective gloves.
Results: In all measurement points, the lower measured dose was recorded when protective measures were implemented. The measured doses to the left and right lenses varied depending on the usage of protective equipment and the orientation of the operator’s head and neck. The lowest measured dose to both lenses was recorded when the protective equipment and ceiling-suspended shield were used, and the operator’s head and neck were turned toward the subject. The hand dose was the lowest when protective gloves were used (316.9 μGy), representing a 72% reduction compared with unprotected conditions. When the neck guard or lead plate was inserted underneath the measurement points, the hand dose decreased by approximately 29% (884.3 μGy) and 43% (657.6 μGy), respectively, compared with unprotected conditions.
Conclusion: Our findings confirmed that operator radiation exposure dose can be reduced through protective measures. The lens exposure dose was minimized when protective equipment and the ceiling-suspended shield were used, and the operator’s head and neck were turned toward the subject. While the protective effect of the lead plates was lower than that of protective gloves—which can be challenging to use during manual compression—the method of inserting a lead plate beneath the patient table and bending it along the shoulder was identified as another useful alternative.
Endovascular treatment is widely utilized for cerebral aneurysms because it is less invasive than surgical procedures.1) However, its associated high radiation exposure of patients and medical staff remains a significant concern.2–4) In 2011, the International Commission on Radiological Protection (ICRP) recommended that the dose limit to the lens for radiation medical workers should be an average of 20 mSv/year over 5 years and should not exceed 50 mSv in any single year.5) In response to this recommendation, the Ministry of Health, Labour and Welfare in Japan revised the Regulations for Prevention of Ionizing Radiation Hazards, which were applied starting from April 1, 2021.6) Given this background, it is evident that there is increasing concern about radiation exposure among radiation medical workers.
In treating cerebral aneurysms, temporary occlusion of the main cerebral arteries is sometimes necessary, highlighting the need to assess the presence of cross-flow through the anterior and posterior communicating arteries before the procedure.7,8) The Matas and Alock tests, which evaluate cross-flow from the anterior and posterior communicating arteries, respectively, are critical preoperative assessments.9) 3D DSA performed under carotid artery occlusion tests may be a preoperative evaluation for cerebral aneurysms located near the anterior and posterior communicating arteries. During this procedure, the operator manually compresses the patient’s carotid artery. However, since not many studies exploring the operator’s radiation exposure dose during 3D DSA performed under carotid occlusion tests have been reported, there is individual variability in the orientation of the operator’s head and neck during imaging. Additionally, since it is hard to manually compress the patient’s carotid artery, protective gloves and similar equipment are often not used near the imaging region. In this study, we measured changes in the operator radiation exposure dose under different protective measures during 3D DSA performed under carotid artery occlusion tests and evaluated alternative protective measures for hand exposure apart from protective gloves and the operator’s head and neck orientation, proposing a new protection method.
An Artis Q biplane system (SIEMENS, Munich, Germany) was used for angiographic imaging. For radiation dose measurement, we utilized the GD-352M fluorescent glass dosimeter with low-energy compensation filters (AGC Techno Glass Co., Ltd., Shizuoka, Japan) and the Dose Ace FGD-1000 series glass dosimeter reader (AGC Techno Glass). Since scattered radiation from the patient is considered the primary source of radiation exposure for medical staff,10,11) which this study also aimed to measure scattered radiation from the subject, no correction was made regarding energy characteristics.
The protective equipment used included a 0.25 mmPb lead-equivalent protective apron (Maeda Co., Ltd., Tokyo, Japan), a 0.25 mmPb lead-equivalent neck guard (Maeda Co., Ltd., Tokyo, Japan), 0.07 mmPb lead-equivalent protective glasses (Toray Industries, Inc., Tokyo, Japan), 0.04 mmPb lead-equivalent protective gloves (Flare Co., Ltd., Tokyo, Japan), a 0.5 mmPb lead-equivalent ceiling-suspended protective shield (MAVIG, Munich, Germany), and a 1 mm-thick lead plate.
We used EZR X11 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) for all statistical analysis. The Wilcoxon signed-rank test was applied for comparisons between 2 groups, while the Friedman test was used for multiple-group comparisons. The threshold for statistical significance was set at p <0.05. In cases where the Friedman test indicated significance, Holm’s multiple comparison test was conducted for the post hoc analysis.
This study was conducted under the assumption of a rotational imaging setting, in which the operator typically cannot perform manual compression using their dominant hand from the head side of the patient. Therefore, it was assumed that the operator would stand on the manual compression side next to the bed and use the hand closest to the compression site.
Comparison of the radiation dose under different protective measuresTo simulate the operator’s standing position during 3D DSA performed under manual carotid artery occlusion tests, a Rando phantom (Kyoto Kagaku Co., Ltd., Kyoto, Japan) was stacked to a height of approximately 168 cm (which corresponds to the height of an average adult male).12) The phantom was positioned 60 cm below the patient’s neck and 50 cm away from the center (Fig. 1). Fluorescent glass dosimeters were placed at positions simulating the operator’s left and right lenses, left and right thyroid glands, and gonads (Fig. 2). Three protective setups were used:
Type 1: No protective equipment.
Type 2: Protective apron, neck guard, and protective glasses.
Type 3: Protective apron, neck guard, protective glasses, and ceiling-suspended shield.


A 16 cm CTDI phantom (Kyoto Kagaku) was placed to simulate the patient’s head, and imaging was performed using the protocol (tube voltage: 70 kV, tube current: 300 mA, magnification: 42 cm, collection angle: 200°). The radiation dose to the operator was measured. Measurements were conducted by varying the phantom’s standing position (right or left side of the patient table) and the protective setup (Type 1, Type 2, or Type 3) (Fig. 3). The upper edge of the ceiling-suspended protective shield was aligned with the phantom’s head height and installed vertically relative to the table. Ten measurements were taken for each imaging condition.

Using the geometric configuration and imaging conditions outlined in the previous paragraph, the lens dose was measured under 3 head and neck orientations: facing forward, turned 45° toward the subject, and turned 45° to the opposite side of the subject (Fig. 4). Additionally, measurements were conducted by varying the phantom’s standing position (right or left side of the patient table) and the protective setup (Type 1, Type 2, or Type 3). Ten measurements were taken for each imaging condition.

A 16 cm CTDI phantom and a Rando phantom were placed on the patient table to simulate the patient’s head and body below the neck, respectively. A glass rod with a diameter of 1.5 cm, corresponding to the thickness of a finger, was positioned at the location simulating manual carotid artery compression by the operator (Fig. 5A). A fluorescent glass dosimeter was attached to the tip of the glass rod, and imaging was performed under the same conditions to measure the hand dose. Using the same geometric configuration, imaging was performed under the following protective conditions: wearing protective gloves, covering the site from above with a neck guard, inserting the neck guard between the bed and headrest, and inserting the lead plate between the bed and headrest and bending it along the shoulder (Fig. 5B–5E). The hand dose was measured 10 times for each condition.

The research within our submission has been approved by the ethics institutional review board of Dokkyo Medical University, Saitama Medical Center.
The changes in radiation dose due to the different protective measures are shown in Fig. 6. In all measurement points, lower measured doses were recorded with the implementation of protective measures. For the lenses and thyroid glands on both sides, higher measured doses were recorded at the measurement points on the C-arm side when protective equipment such as protective aprons and glasses were not used (standing on the left side: right lens 40.4 μGy, left lens 20.8 μGy (p = 0.0054), right thyroid 48.0 μGy, left thyroid 33.0 μGy (p = 0.0057), standing on the right side: right lens 19.1 μGy, left lens 35.7 μGy (p = 0.0058), right thyroid 24.7 μGy, left thyroid 45.2 μGy (p = 0.0057)). Regarding the operator’s standing position, similar results were obtained regardless of whether the phantom stood on the right or left side of the patient table.

The changes in lens dose according to the phantom’s head and neck orientation are shown in Fig. 7. In Type 1, when the phantom’s head and neck were either facing forward or turned to the opposite side of the subject, a higher measured dose was recorded at the C-arm side measurement points (standing on the left side, facing forward: right lens 40.4 μGy, left lens 20.8 μGy (p = 0.0054), standing on the right side, facing forward: right lens 19.1 μGy, left lens 35.7 μGy (p = 0.0058), standing on the left side, opposite side of the subject: right lens 40.4 μGy, left lens 20.8 μGy (p = 0.0054), standing on the right side, opposite side of the subject: right lens 19.1 μGy, left lens 35.7 μGy (p = 0.0058)). In contrast, when the phantom’s head and neck were turned toward the subject, the measured doses to the left and right lenses were relatively similar.

In Type 2, when the phantom’s head and neck were either facing forward or turned toward the subject, the measured doses to the left and right lenses were relatively similar. However, when the head and neck were turned to the opposite side of the subject, a higher measured dose was recorded at the C-arm side (standing on the left side, opposite side of the subject: right lens 19.2 μGy, left lens 2.8 μGy (p = 0.0058), standing on the right side, opposite side of the subject: right lens 5.6 μGy, left lens 18.0 μGy (p = 0.0058)). These findings indicate that head and neck orientation causes lens dose variations between the left and right sides.
In Type 3, a comparison of the integrated dose of the left and right lenses recorded the lowest dose when the head and neck were turned toward the subject (standing on the left side: facing forward 12.6 μGy, turned toward the subject 5.4 μGy, opposite side of the subject 12.3 μGy. The Friedman test revealed a statistically significant difference (p < 0.001), and the Holm test revealed statistically significant differences between all pairs (p = 0.017) except for the combination of “facing forward” and “opposite side of the subject” (p = 0.832), standing on the right side: front of head and neck 9.4 μGy, turned toward the subject 3.7 μGy, opposite side of the subject 10.5 μGy. The Friedman test revealed a statistically significant difference (p <0.001), and the Holm test revealed statistically significant differences between all pairs (p = 0.017) except for the combination of “facing forward” and “opposite side of the subject” (p = 0.09). Regarding the operator’s standing position, similar results were obtained regardless of whether the phantom stood on the right or left side of the patient table.
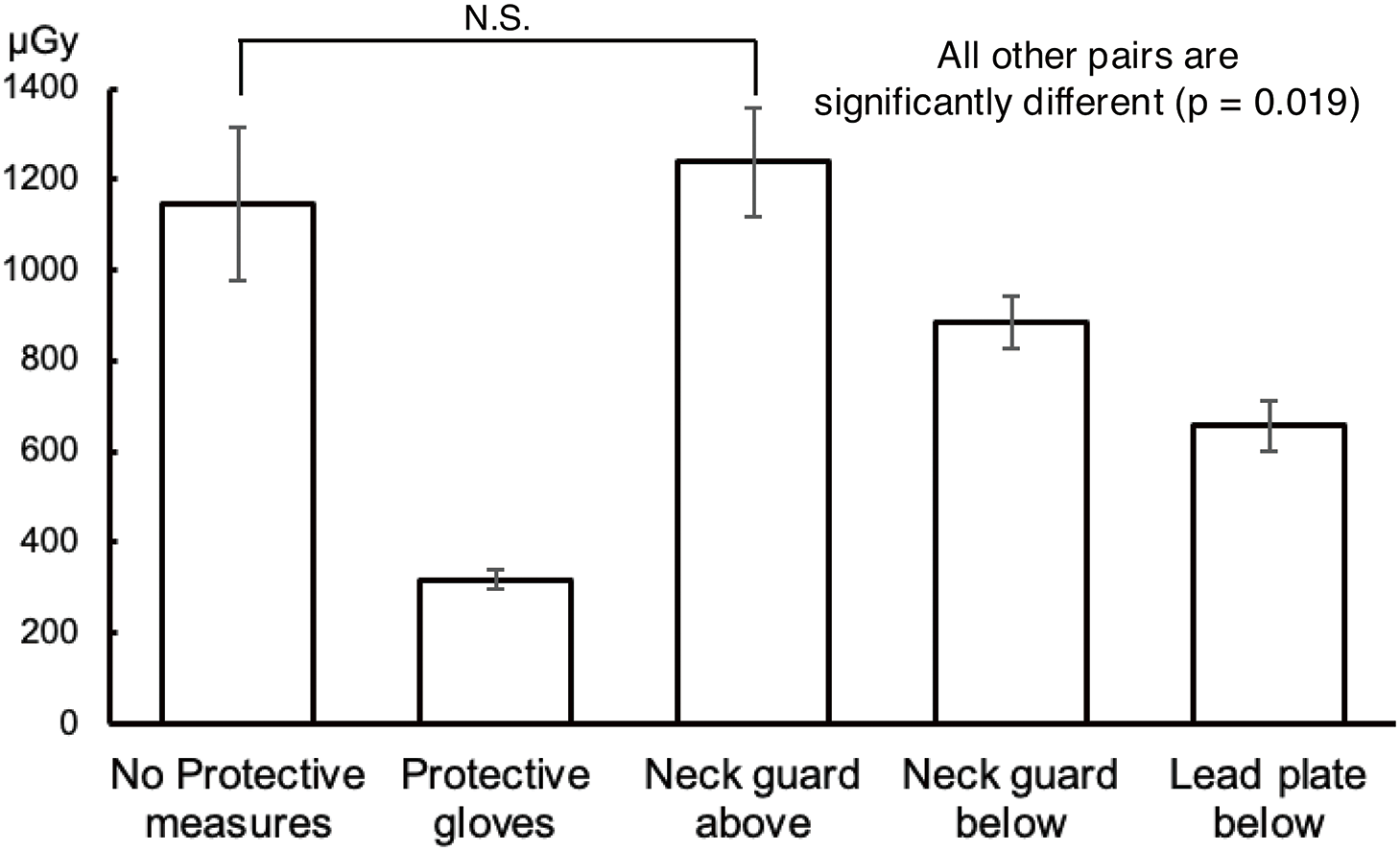
Comparison of the hand dose under different protective measuresThe changes in hand dose due to protective measures are shown in Fig. 8. The hand measured dose without any protective measures was 1145.7 μGy. The lowest dose was recorded when protective gloves were used, resulting in a reduction of approximately 72% compared with the unprotected condition. In contrast, when the site was covered from above using a neck guard, no reduction was recorded, and the measured dose increased. When the neck guard or lead plate was inserted beneath the measurement points, the measured dose was reduced by approximately 29% and 43%, respectively, compared with the unprotected condition (no protection: 1145.7 μGy, protective gloves: 316.9 μGy, neck guard above: 1239.8 μGy, neck guard below: 884.3 μGy, lead plate below: 657.6 μGy. The Friedman test revealed a statistically significant difference (p <0.001), and the Holm test revealed statistically significant differences between all pairs (p = 0.019), except for the combination of “no protection” and “neck guard above” (p = 0.375).
In this study, we measured operator’s exposure dose during 3D imaging in carotid occlusion tests under various conditions, a topic that has not been previously reported. Our results showed that alternative protective measures, such as the use of lead plates for hand exposure, protective glasses, and ceiling-suspended shields, as well as turning the operator’s head and neck toward the subject, were the most effective in reducing radiation exposure to both lenses.
Comparison of the radiation dose under different protective measuresMonte Carlo simulations have demonstrated that neck guards containing 0.5 mm-thick lead can reduce the equivalent dose to the thyroid gland to 1/12 of the initial value (91.7%,% reduction), while those containing 0.35 mm-thick lead can reduce it to 1/7 of the initial value (85.7% reduction).13) In this study, the use of a neck guard resulted in a reduction to 1/8 of the initial value (87.6% reduction). However, neck guards can be uncomfortable when worn tightly around the neck, which is why operators tend to wear them more loosely for comfort. If the neck guard slips down to the level of the larynx, the upper portion of the thyroid gland may remain unprotected, thereby reducing the protective effect.14) The measured dose to the gonads was also reduced by 91% with protective equipment, consistent with the reduction rate reported in published guidelines.15)
The primary source of radiation exposure for operators during angiographic procedures is scattered radiation from the subject and the movable collimator.16) Therefore, the higher measured dose recorded at the C-arm side measurement points for the lenses and thyroid gland, when protective equipment such as aprons and protective glasses were not used, can be attributed to exposure from scattered radiation originating from these sources.
Comparison of the lens dose based on head and neck orientationAs previously mentioned, the primary sources of operator radiation exposure during angiographic procedures are scattered radiation from the subject and the movable collimator.16) Therefore, when the operator’s head and neck are either facing forward or turned to the opposite side of the subject, a higher measured dose is recorded at the C-arm side lens. In contrast, when the head and neck are turned toward the subject, the operator faces the scattering source directly, resulting in a more balanced measured dose between the left and right lenses.
Previous studies have reported that radiation exposure can be reduced with the use of protective glasses and ceiling-suspended shields.17) However, certain areas, such as the lower and central portions of protective glasses, tend to have unavoidable gaps. The orientation of the operator’s head and neck likely influenced the amount of scattered radiation entering through these gaps, causing variations in the protective effect and measured dose. Additionally, it has been reported that the protective effect varies depending on the distance from the ceiling-suspended shield.18)
In this study, the lowest lens-measured dose on both sides was recorded when the protective equipment and the ceiling-suspended shield were used, and the operator’s head and neck were turned toward the subject. This result is likely due to the reduced distance between the operator and the shield, which minimized the scattered radiation entering through gaps. These findings suggest that the use of protective equipment and ceiling-suspended shields, combined with turning the head and neck toward the subject, is an effective way of mitigating radiation exposure to the operator’s lenses.
Comparison of the hand dose under different protective measuresDuring rotational angiographic imaging, the X-ray tube rotates under the patient table (under-table configuration), resulting in a significant amount of scattered radiation from below. In this study, a lower measured dose was recorded when the neck guard or lead plate was positioned beneath the phantom’s neck region, possibly because these measures effectively shielded against scattered radiation from below. In clinical settings, inserting a neck guard between the subject and the bed may be challenging depending on the subject’s physique. Furthermore, the neck guard may not fit properly if the subject’s neck is large. In contrast, a lead plate is thinner than a neck guard, making it easier to place between the subject and the bed. Its size and shape can also be easily adjusted, enabling customization according to the subject’s physique. When the neck guard was placed over the measurement points from above, a higher measured dose was recorded. This may be attributed to the detection of the additional scattered radiation from the neck guard itself.
The lowest measured dose was recorded when protective gloves were used, as they completely covered the hands where the dosimeter was attached, preventing the entry of scattered radiation through any gaps. Unlike other protective methods, the protective gloves provided a higher protective effect because they placed a lead-containing shield directly between the scattering radiation source (the subject) and the dosimeter. However, in some countries, it is reported that an estimated 60%–90% of medical workers engaged in radiation-related diagnosis and treatment use protective aprons for radiation protection, and only 10%–40% use protective gloves and protective glasses.19) Wearing protective gloves can reduce tactile feedback,14) making delicate procedures such as carotid artery palpation more difficult. Additionally, if the gloved hand enters the irradiation field, the system compensates for the attenuation of X-ray caused by the glove, potentially increasing the X-ray dose and inadvertently raising the patient’s exposure dose, annulling the protection of the operator’s hand.20)
From the results of this study, it was found that protective gloves have the highest reduction effect, and their use is desirable. Since the shielding performance and maneuverability differ among types of protective gloves,21) it is essential to select gloves that are well-suited to the operator’s individual requirements. In addition, the use of a lead plate was shown to be a practical alternative when protective gloves are not worn due to discomfort or when the gloved hand enters the irradiation field.
This study has several limitations. This study was conducted in a fixed environment using a phantom. Therefore, it does not consider changes in radiation exposure due to the subject’s physique, the radiation field, the operator’s physique, and the operator’s posture during imaging (such as leaning out from the opposite side of the compression side). Additionally, there are differences in the types, range of motion, and usage of protective equipment between facilities. Furthermore, in a clinical environment, the distribution of scattered radiation may not necessarily be symmetrical due to the arrangement of surrounding equipment. However, this study provides valuable insights into radiation exposure reduction strategies for operators performing 3D imaging under carotid occlusion tests. The proposed protective measures may contribute to improved radiation safety in clinical practice.
Our findings confirmed that operator radiation exposure can be mitigated through protective measures. However, for lens exposure, the protective effect was influenced by the usage of protective equipment and the operator’s head and neck orientation. This study focused on measurements taken during rotational imaging, where the operator must manually compress the patient’s carotid artery as the C-arm rotates, making it hard to position the ceiling-suspended shield optimally. Our findings suggest that, at the shield position employed in this study, the combination of protective equipment and a ceiling-suspended shield, with the operator’s head and neck turned toward the subject, minimizes the bilateral lens radiation exposure. Additionally, recent advancements in protective glasses tailored to individual face shapes can minimize gaps between the lenses and the face,22) further contributing to the lens exposure dose reduction. While the protective effect of the lead plates was lower than that of protective gloves—which can be challenging to use during manual compression—the method of inserting a lead plate beneath the patient table and bending it along the shoulder was found to be another useful alternative.
The authors declare that they have no conflicts of interest.