2020 Volume 52 Pages 1-6
2020 Volume 52 Pages 1-6
Paramecium, a ciliated protozoan, moves using thousands of cilia that cover the entire cell body. When Paramecium comes into contact with harmful substances such as ethanol, its cilia spontaneously detach and motility is substantially reduced. In the present study, Paramecium cells were shown, for the first time, to acquire tolerance to toxic ethanol concentrations after pretreatment with lower, non-toxic concentrations. The effect of pretreatment was characterized by counting numbers of non-deciliated cells. Key findings were: (1) pretreated cells retained the acquired tolerance to ethanol for at least 24 h and lost tolerance within 48 h; (2) the effect of pretreatment was attenuated by protein synthesis inhibitors; (3) the effect of pretreatment depended on duration and ethanol concentration; (4) pretreatment conferred cells the tolerance to not only a toxic concentrations of ethanol but also to lethal concentrations; and (5) cells acquired ethanol tolerance during starvation. Ethanol-induced tolerance in Paramecium may be a primitive model for cellular memory function.
The ciliated protozoan, Paramecium caudatum moves using thousands of cilia that cover the entire cell body. The cilia can be quickly dislodged from the cell surface by shaking in a medium containing ethanol (Machemer & Ogura, 1979; Ogura, 1981). Deciliated cells show reduced motility and sink to the bottom of culture vessels. The ethanol-induced deciliation has been performed for studies in biological research. Although it has been considered that an increase in cytosolic calcium may trigger ciliary detachment, the precise mechanism underlying the ethanol-induced deciliation remains poorly understood. In the present study, cells acquired tolerance to ethanol concentrations that cause deciliation after pretreatment with lower concentrations that do not produce this effect. Characterizing ethanol tolerance acquisition in Paramecium cells might provide clues to the mechanism of the ethanol-induced deciliation.
Paramecium caudatum was cultured in an infusion solution of rice straws in conventional glass test tubes at 25°C. Klebsiella pneumonia cultured separately in a polypeptone-based medium was added to the Paramecium culture as a food source. Paramecium cells were filtered through a rough mesh to remove culture debris, washed three times with a buffered solution consisting of 100 μM CaCl2, 100 μM KCl and 100 μM Tris-HCl (pH 7.2), adjusted at the concentration of 200 cells/ml in a plastic test tube, and kept for 30 min at room temperature.
Deciliation and pretreatmentCells adapted to the buffered solution were deciliated by addition of 100% ethanol to a final concentration of 4% followed by vigorous vortexing for 25 sec. Cells were then washed 3 times by repeated centrifugation at 1,100×g for 90 sec, resuspended in fresh buffered solution, and kept for 30 min at room temperature. Prior to deciliation, some groups of cells were pretreated with lower concentrations of ethanol. Unless otherwise specified, pretreatment was performed by the addition of 100% ethanol to a final concentration of 0.4% followed by gentle agitation and incubation for 2 h. Calcium ion shock was also used for deciliation. Cells adapted to buffered solution were treated with 30 mM CaCl2 on ice and gently mixed for 20 min. Cells were then precipitated by centrifugation at 1,100×g for 90 sec, resuspended in fresh buffered solution and kept for 30 min at room temperature. Some groups of cells were treated with protein synthesis inhibitors, puromycin (10 µM) or cycloheximide (10 µM), for at least 60 min before pretreatment with 0.4% ethanol or treatment with 4% ethanol for deciliation.
Measurement of cell numbersIn all experiments, total volumes of samples and cell suspensions were adjusted to 10 ml and 200 cells/ml respectively in plastic test tubes. After deciliation, cells were kept for 30 min at room temperature. During this period, deciliated cells sedimented spontaneously to the bottoms of the test tubes. Thereafter, supernatant (approx. 9 ml) in which retained non-deciliated cells was transferred to a new test tube to avoid contamination by deciliated and sedimented cells and NiCl2 was added at a concentration of 1 µg/ml to halt movement of non-deciliated cells. Numbers of cells in the collected supernatant were counted under a conventional stereomicroscope and expressed as cells per ml or percent of control.
Statistical analysisAll experiments were conducted in triplicates and results expressed as means±standard errors of replicates. Nonparametric comparisons between two groups were performed with a Mann–Whitney U test and P value <0.05 was used to indicate statistically significant differences.
Deciliation was induced by temporal treatment with 4% ethanol. Almost all cilia on cell surfaces could be detached by this treatment (Fig. 1). Since deciliated cells sedimented to the bottoms of the test tubes, such cells were not present above the bottom of tubes for at least several hours. Compared with a control group (control, 227±34 cells/ml), numbers of motile cells following ethanol-deciliation without pretreatment were obviously low (27±3 cells/ml) (Fig. 2). In contrast, numbers of motile cells following pretreatment were significantly higher (80±8 cells/ml, Fig. 2), indicating that cells acquired tolerance to toxic concentrations of ethanol after pretreatment with a lower non-toxic concentration.
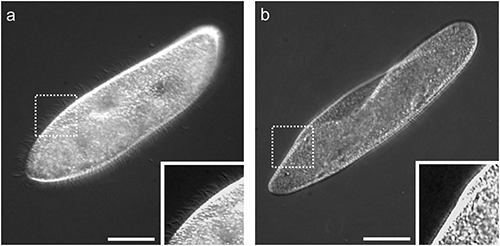
(a) An untreated cell and (b) a deciliated cell. Insets showed magnified images of dotted squares. Almost all cilia covering the cell body surface in the untreated cell (inset in a) were detached by ethanol treatment (inset in b). Bars=50 µm.

Pretreatment was performed by the addition of ethanol at a final concentration of 0.4% followed by gentle agitation. Deciliation was performed by the addition of ethanol at a final concentration of 4% followed by vigorous vortexing. Compared to a control group (open bar, 227±34 cells/ml), numbers of motile cells after ethanol-deciliation without pretreatment were decreased (filled bar, 27±3 cells/ml). However, numbers were significantly increased with pretreatment (shaded bar, 80±8 cells/ml). An asterisk represents a statistic difference (P<0.05).
Retention of tolerance was investigated by exposing tolerant cells to 4% ethanol 24 h or 48 h after pretreatment. During this time, cell proliferation did not occur since cells were kept in buffered solution containing no nutrients. Numbers of motile cells at 24 h after deciliation were significantly higher in the pretreated group (19±1% of control) than in the group without pretreatment (8±1% of control) (Fig. 3). Thus, ethanol tolerance was retained for at least a day. However, 48 h after pretreatment, no significant difference in numbers of motile cells was observed between pretreated and non-pretreated groups (13±2% and 15±2% of control, respectively, Fig. 3). Pretreated cells retained acquired tolerance for at least 24 h and lost it within 48 h.

Pretreatment was performed by the addition of ethanol at a final concentration of 0.4%. After deciliation, compared to groups without pretreatment (filled bars), numbers of motile cells in groups with pretreatment (shaded bars) were significantly higher after 24 h (8±1% of control without pretreatment and 19±1% of control with pretreatment), but not after 48 h (13±2% of control without pretreatment and 15±2% of control with pretreatment). Asterisks represent a significant difference (P<0.05).
The mechanism underlying acquired tolerance was investigated by inhibiting protein synthesis in Paramecium cells with puromycin (10 μM) or cycloheximide (10 μM). After deciliation, no significant difference was seen in numbers of motile cells between untreated and protein synthesis inhibitor-treated groups (data not shown), indicating that these inhibitors have no effect on the deciliation process. After deciliation, numbers of motile cells after ethanol pretreatment were high (31±3% of control) compared with motile cells without pretreatment (11±2% of control) (Fig. 4). However, when cells were treated with the protein synthesis inhibitors for 60 min before pretreatment, numbers of motile cells after deciliation were comparable with that in non-pretreated cells (14±2% of control in the puromycin-treated group and 14±2% of control in the cycloheximide-treated group, Fig. 4). The fact that Paramecium cells failed to acquire ethanol tolerance when protein synthesis was inhibited, suggests that ethanol tolerance is mediated by synthesis of a certain protein or proteins.

Pretreatment was performed by the addition of ethanol at a final concentration of 0.4%. After deciliation, compared to cells without pretreatment (filled bar, 11±2% of control), numbers of motile cells were significantly higher with pretreatment (extreme left shaded bar, 31±3% of control). However, tolerance was eliminated in cells treated with protein synthesis inhibitors for 60 min before pretreatment (14±2% of control with puromycin and 14±2% of control with cycloheximide). Asterisks represent a significant difference (P<0.05).
The effect of changing pretreatment conditions was also investigated. When pretreatment with 0.4% ethanol was prolonged from 2 to 6 h, the effect of pretreatment increased significantly (Fig. 5). After deciliation, numbers of motile cells in the group without pretreatment were 5±1% of control. Numbers of motile cells were 11±1% of control after 2 h pretreatment and 15±1% of control after 6 h pretreatment. Further, treatment with puromycin (10 μM) significantly decreased numbers of motile cells after deciliation even in the groups with pretreatment (4±1% and 7±0% of control after 2 and 6 h pretreatment, respectively) (Fig. 5). Again, puromycin treatment apparently inhibited synthesis of a protein or proteins required for acquisition of ethanol tolerance.

Pretreatment was performed by the addition of ethanol at a final concentration of 0.4%. After deciliation, compared to cells without pretreatment (filled bar, 5±1% of control), numbers of motile cells were greater with pretreatment (11±1% of control after 2 h and 15±1% of control after 6 h). The effect of pretreatment was attenuated by the treatment with the protein synthesis inhibitor, puromycin (4±1% of control after 2 h and 7±0% of control after 6 h). Asterisks represent a significant difference (P<0.05).
Paramecium cells were also pretreated with different concentrations of ethanol ranging from 0.2 to 1.2% for 2 h before deciliation by the treatment with 4% ethanol. Although there was no significant difference between the group without pretreatment (8±0% of control) and the group with 0.2% pretreatment (10±0% of control), numbers of motile cells pretreated with ethanol concentrations greater than 0.4% were significantly higher than that of the group without pretreatment (17±2%, 23±2% and 26±1% of control after pretreatment with 0.4%, 0.8% and 1.2% ethanol, respectively) (Fig. 6). The effect of pretreatment on the acquisition of tolerance thus depends on the duration of pretreatment exposure and pretreatment ethanol concentration.

After deciliation, no significant difference was observed between cells without pretreatment (filled bar, 8±0% of control) and cells pretreated with 0.2% ethanol (extreme left shaded bar, 10±0% of control). Numbers of motile cells pretreated with ethanol concentrations greater than 0.4% were significantly higher than without pretreatment (17±2% of control with 0.4%, 23±2% of control with 0.8% and 26±1% of control with 1.2% pretreatment group). Asterisks represent a significant difference (P<0.05).
When being treated with ethanol at a concentration of 4%, as mentioned above, Paramecium cells are deciliated and become non-motile. Higher concentrations of ethanol are lethal. Numbers of motile cells after exposure to lethal concentrations of ethanol ranging from 5 to 9% without pretreatment were extremely low (1±0%, 1±1%, 2±1%, 1±0% and 0±0% of control after treatment with 5%, 6%, 7%, 8% and 9% ethanol, respectively, Fig. 7). Almost all cells were killed in these tests. In contrast, numbers of motile cells after pretreatment were significantly high even after treatment with lethal concentrations of ethanol (14±4%, 11±3%, 8±2%, 5±2% and 1±0% for 5%, 6%, 7%, 8% and 9% ethanol, respectively, Fig. 7). The effect of pretreatment gradually decreased with increased ethanol concentrations, yet pretreatment conferred cells with some tolerance to even the highest lethal concentration of ethanol.

Pretreatment was performed by the addition of ethanol at a final concentration of 0.4%. In cells without pretreatment (filled bars), numbers of motile cells were extremely low (1±0% of control in 5%, 1±1% of control in 6%, 2±1% of control in 7%, 1±0% of control in 8% and 0±0% of control in 9% ethanol-treated group) possibly because almost all cells were nonviable. However, numbers were significantly higher after pretreatment (shaded bars, 14±4% of control in 5%, 11±3% of control in 6%, 8±2% of control in 7%, 5±2% of control in 8% and 1±0% of control in 9% ethanol-treated group). Asterisks represent a significant difference (P<0.05).
To determine if ethanol tolerance extends to other toxic agents, deciliation was induced by treatment with an excess of calcium. Short treatment with a high concentration of calcium is termed calcium shock and is known to cause the detachment of cilia in Paramecium (Gibbons, 1965; Nelson, 1995). After calcium shock-induced deciliation, numbers of motile cells were significantly higher after pretreatment (23±1% of control) than without pretreatment (13±2% of control) (Fig. 8). Pretreatment with a low concentration of ethanol confers cells with tolerance to deciliation caused by another agent.

The pretreatment was performed by the addition of ethanol at a final concentration of 0.4%. Compared to a group without pretreatment (filled bar, 13±2% of control), numbers of motile cells were significantly high in a group with pretreatment (shaded bar, 23±1% of control) after deciliation by a calcium shock. An asterisk represents a significant difference (P<0.05).
In this study, the effect of pretreatment was retained for more than 24 h (Fig. 3). In the experiment, after deciliation treatment, numbers of motile cells in the group without pretreatment significantly increased with increased incubation time (8±1% of control in 24 h and 13±2% of control in 48 h, Fig. 3) even for cells that were not pretreated. During this time, cells were kept in buffered solution containing no nutrients. It seemed possible that starvation could confer tolerance to deciliation. Cells were starved for 24 h to 48 h, and numbers of motile cells after deciliation by ethanol treatment were compared with a non-starved group (0 h). Almost all cells in the non-starved group were deciliated (1±0% of control at 0 h). In contrast, a large number of cells in starved groups were still motile (13±1% and 16±1% of control after 24 h and 48 h of starvation, respectively) (Fig. 9), indicating that starvation confers ethanol tolerance without any particular treatment. Treatment with the protein synthesis inhibitor, puromycin (10 μM) during the starvation period, significantly decreased numbers of motile cells after deciliation in the starved groups (5±1% and 5±0% of control after 24 h and 48 h of starvation, respectively, Fig. 9), again indicating that ethanol tolerance is mediated by synthesis of a certain protein or proteins.

Compared to a non-starved group (extreme left filled bar, 1±0% of control in 0 h), numbers of motile cells were significantly high in a starved group (13±1% of control in 24 h and 16±1% of control in 48 h starvation). The effect of starvation was attenuated by the treatment with protein synthesis inhibitor, puromycin (5±1% of control in 24 h and 5±0% of control in 48 h starvation). Asterisks represent a significant difference (P<0.05).
The surface of the ciliated protozoan, Paramecium, is covered with thousands of cilia that allow the organism to move about in its environment. Deciliation thus renders cells with reduced or absent motility. Deciliated cells can reproduce cilia and return to a normal state (reciliation). This reciliation process takes several hours (Ogura, 1981). In this study, acquisition of tolerance to deciliation was assessed by counting the numbers of non-deciliated and motile cells just after deciliation. Deciliated cells sedimented to the bottom of centrifuge tubes. Sedimented cells were examined and were largely deciliated (Fig. 1b). After deciliation treatment, some motile cells remained in medium above the bottom of the centrifuge tubes because Paramecium swims preferentially upwards and accumulates toward the top of the water column. This phenomenon is termed negative geotaxis or gravitaxi (Jennings, 1906). Motile cells were examined and were completely ciliated similar to an untreated cell shown in Fig. 1a and seemed to have no morphological damage (data not shown). Thus, non-deciliated cells could be distinguished from deciliated cells and precisely counted to determine numbers of ethanol tolerant cells.
Deciliation is not a novel technique in biological research. To study transcriptomic and proteomic changes during ciliogenesis, deciliation has been performed on ciliated protozoa including Paramecium (Arnaiz et al., 2009; Arnaiz et al., 2010). Deciliation has also been used for studies of epidermal cells of planarians (Stevenson & Beane, 2010), early sea urchin embryos (Chakrabarti et al., 1998), and mammalian olfactory and rod photoreceptor cells (Mitchell et al., 2009). Cells may undergo deciliation (or deflagellation) under stress conditions induced by infection, mechanical stress, and acid or other chemical treatment (Rannestad, 1974; Iomini et al., 2004; Overgaard et al., 2009). Further, an increase in cytosolic calcium by an influx of extracellular calcium or a release from internal calcium stores may trigger ciliary detachment (Quarmby & Hartzell, 1994; Chakrabarti et al., 1998; Wu et al., 2018). The exact mechanism underlying deciliation (or deflagellation) is, however, still unknown. In the present study, why deciliation occurs when cells were treated with 4% ethanol and why cells pretreated with low concentrations of ethanol resisted deciliation was not clarified. The effect of ethanol on membrane structure and function has been extensively studied with both yeast and bacteria (Thomas et al., 1978; Dombek & Ingram, 1984; Alexandre et al., 1994). It has been shown that low concentrations of ethanol trigger alterations in the fluidity, permeability and components of cytoplasmic membrane. However, a causal relationship between these alterations in membrane and an increase of intracellular calcium during ethanol-induced deciliation is unknown. In the present study, the effect of pretreatment was attenuated by protein synthesis inhibitors (Figs. 4 and 5), suggesting the synthesis of a protein or proteins might be involved in tolerance acquisition. However, the identity of protein or proteins newly synthesized during pretreatment and how it or they contribute to tolerance acquisition was not elucidated. Proteomic analysis in cells pretreated with a low concentration of ethanol will be needed to define relationships between acquisition of ethanol tolerance and protein synthesis. Understanding the mechanism of deciliation may also help clarify the molecular mechanism behind tolerance acquisition.
Numbers of motile cells after deciliation treatment gradually increased with increases in ethanol pretreatment concentrations (Fig. 6). Thus, the effectiveness of pretreatment depends on the intensity of the stimulus. The lower threshold concentration of ethanol required for induction of deciliation was unclear, yet pretreatment with concentrations of ethanol higher than 1.2% would likely have a greater effect on tolerance acquisition. Cells previously deciliated are more resistant to subsequent deciliation (data not shown), which shows that the higher the intensity of pretreatment, the greater its effect.
Effects of pretreatment also depend on duration. Numbers of motile cells after deciliation treatment significantly increased after pretreatment for 6 h compared to 2 h (Fig. 5). This increase did not occur in the presence of the protein synthesis inhibitor, puromycin, possibly indicating that a newly synthesized protein or proteins during pretreatment period might be necessary for acquisition of ethanol tolerance.
Ethanol tolerance in Paramecium has much in common with ischemic preconditioning (IPC), an experimental technique for producing resistance in many tissues to loss of oxygen supply (Murry et al., 1986). Tolerance acquisition in Paramecium resembles IPC in some characteristics: 1) mild stimuli induces tolerance to moderate or severe stimuli, 2) one stimulus induces tolerance to other types of stimuli, and 3) tolerance is induced by chemical agents. The molecular mechanism behind IPC is not fully understood, but adenosine molecules produced during ischemia have been implicated in protecting cells from ischemic insult (Tsuchida et al., 1994). Conceivably, mitochondria may be involved in signaling processes for preconditioning (Correia et al., 2010). Further study is needed to clarify the involvement of adenosine molecules and mitochondria in the mechanism of tolerance acquisition in Paramecium.
A decade ago, Sidiropoulou et al. (2009) demonstrated that a single prefrontal cortical neuron in mouse brain might be able to store a memory. More recently, Kobayashi et al. (2016) demonstrated that a single neuron in the nematode, Caenorhabditis elegans, is capable of memorizing information without input from other cells. Further, learning in Paramecium was suggested as early as 1911 (Day & Bentley, 1911). Attempts to demonstrate learning in Paramecium revealed that they can learn stimuli such as light intensity, temperature gradients and electrical shock (Mendelssohn, 1895; Jennings, 1906; Nakaoka & Oosawa, 1977; Armus & Montgomery, 2001; Armus et al., 2006; Mingee, 2013). The accumulation of cyclic adenosine monophosphate (cAMP) molecules in cytosol was recently proposed as the basis of learning in Paramecium (Alipour et al., 2017; Alipour et al., 2018). Development of models of learning and memory in unicellular organisms has implications beyond basic neuroscience research. In the present study, ethanol-induced ethanol tolerance was demonstrated for the first time in Paramecium. This tolerance acquisition is proposed as a primitive model of short-term cellular memory that is evolutionarily conserved from the common ancestor of all life. Elucidating the mechanism of ethanol tolerance acquisition in Paramecium will shed light on the ability of this organism to memorize and retain the environmental information and identify a new avenue for research field on single-cell memory function in multicellular organisms.
We thank Dr. Md. Monjurul Ahasan for his critical reading and helpful comments on the manuscript.