2022 Volume 37 Issue 1 Article ID: ME21061
2022 Volume 37 Issue 1 Article ID: ME21061
Pea wilt disease, caused by the soilborne and seedborne fungal pathogen Fusarium oxysporum f. sp. pisi (Fop), first appeared in Japan in 2002. We herein investigated the molecular characteristics of 16 Fop isolates sampled from multiple locations and at different times in Japan. The 16 isolates were divided into three clades in molecular phylogenic analyses based on both the TEF1α gene and the rDNA-IGS region. All of the Fop isolates harbored a PDA1 gene, which encodes the cytochrome P450 pisatin demethylase (Pda1), and also carried one or both of the SIX6 and SIX13 genes, which encode secreted in xylem (Six) proteins. Other forms of F. oxysporum and other species of Fusarium did not carry these sets of genes. Based on these results, a PCR method was developed to identify Fop and differentiate it from other forms and non-pathogenic isolates of Fusarium spp. We also demonstrated that the PCR method effectively detected Fop in infected pea plants and infested soils.
Pea (Pisum sativum L.) is one of the most commonly and widely cultivated Fabaceae plants. In 2019, 21.8 million tons of green peas and 14.2 million tons of dry peas were harvested worldwide (FAOSTAT, 2019). In Japan, 20,000 tons of podded green peas, 6,300 tons of green peas, and 900 tons of dry peas were harvested in 2019 (e-Stat, 2019). Similar to many other crops and vegetables, diseases threaten pea cultivation by decreasing production. Harveson et al. (2020) listed 27 pea diseases caused by fungi, bacteria, viruses, and nematodes, and The Phytopathological Society of Japan (2021) listed 30 pea diseases.
Fusarium oxysporum Schlecht. emend. Snyd. et Hans. f. sp. pisi (Lindf.) Snyd. et Hans. (Fop) causes Fusarium wilt, one of the most destructive diseases of pea (Kraft, 1994; Haglund and Kraft, 2001). Pea plants infected by this pathogen present with leaf yellowing, browning of the vascular tissues, and blight and ultimately die (Matsusaki et al., 2003). Pea wilt was initially reported in the USA in 1925, and also occurs in Europe, and Asia (Kraft, 1994). However, it was not detected in Japan until 2002 (Sakoda et al., 2018, 2019). The Plant Protection Station of the Japanese Ministry of Agriculture, Forestry and Fisheries (MAFF) has listed Fop as one of the pathogens that needs to be monitored to prevent invasion (MAFF, 2021). Since its first appearance in Aichi Prefecture in 2002, sporadic outbreaks of Fop have been reported in several areas of Japan, including Shizuoka, Hokkaido, and Wakayama Prefectures (Sakoda et al., 2018). Countermeasures for eradication have been taken in each area. It is extremely important to develop methods for the specific identification of Fop so that it may be identified and eradicated from infested fields.
F. oxysporum is a ubiquitous ascomycete fungus that is widely distributed in the environment, and many strains are known to be soilborne and/or seedborne pathogens of plants. The range of plant species that may be infected (the host range) of each isolate is strictly and clearly defined for this fungus, and more than 120 forms (formae speciales; ff. spp.) have been identified based on their host ranges (Michielse and Rep, 2009; Kashiwa et al., 2016; Arie, 2019). One of the forms that causes pea wilt is f. sp. pisi (Fop), which never causes disease in other plant species. The detection of Fop in soil or plant tissues and its differentiation from other forms and non-pathogenic isolates of F. oxysporum and Fusarium spp. are important for its eradication.
Although the specific identification of Fop is possible using in planta bioassays based on the inoculation of pea plants, this process requires too much time, space, and labor. Therefore, faster, easier, and more accurate techniques to identify Fop are needed. Recent molecular and genomic studies have begun to reveal the mechanisms underlying host specificity in F. oxysporum as well as the factors influencing host specificity, such as secreted proteins called effectors, which may be employed to discriminate between pathogenic forms (Arie, 2019, 2020). For example, PCR, real-time PCR, and Loop-mediated isothermal amplification (LAMP) methods that target effector genes have been developed for the specific identification of F. oxysporum f. sp. lycopersici, which is the form that causes tomato wilt (Lievens et al., 2009; Inami et al., 2010; Ayukawa et al., 2016, 2017; Kashiwa et al., 2016).
Effectors are proteins secreted by plant pathogens during host colonization and are essential for pathogenicity. The presence/absence patterns of effector genes may sometimes influence host specificity in F. oxysporum (van Dam et al., 2016). Lineage-specific (LS) chromosomes, which are not necessary for fungal growth, are rich in genes encoding effectors. LS chromosomes have been identified in F. oxysporum ff. spp. lycopersici and radicis-cucumerinum, the cucumber root and stem rot pathogen (Ma et al., 2010; van der Does et al., 2016; Ayhan et al., 2018). Some of the Secreted in xylem (Six) proteins (Six1 to Six14) have been identified as effectors, and their encoding genes, SIX1 to SIX14, are located on LS chromosomes in F. oxysporum f. sp. lycopersici (Schmidt et al., 2013; Vlaardingerbroek et al., 2016). Moreover, homologs of the SIX genes have been identified in various pathogenic forms of F. oxysporum, including Fop (Houterman et al., 2009; Gawehns et al., 2014; Taylor et al., 2016; Jenkins et al., 2021).
Plants produce antibiotic chemicals such as phytoanticipins and phytoalexins, which are involved in innate and acquired resistance against pathogens (VanEtten et al., 2001). Some pathogenic forms of Fusarium spp. harbor enzymes that detoxify phytoanticipins or phytoalexins (VanEtten et al., 1994; Curir et al., 2005; Coleman et al., 2011; Milani et al., 2012). The pea root rot pathogen F. solani f. sp. pisi (Fsp) possesses the PDA1 gene, which encodes the cytochrome P450 pisatin demethylase (Pda1) that detoxifies pisatin, a phytoalexin produced by pea. Pda1 is a crucial factor that influences both the pathogenicity and host specificity of Fsp (VanEtten et al., 1998; Miao et al., 1991; Bani et al., 2014). Coleman et al. (2011) reported that Fop also carries a PDA1 gene. The isolate NRRL 26761 of F. oxysporum f. sp. phaseoli, the yellow pathogen of common bean (Phaseolus vulgaris L.), harbors a PDA1 homolog and is pathogenic to pea (Coleman et al., 2011). These findings suggest the importance of PDA1 for the pathogenicity of Fop in pea.
In the present study, we performed a phylogenetic analysis of Japanese Fop isolates using two genetic regions: the translation elongation factor 1α gene (TEF1α) and the ribosomal DNA intergenic spacer (rDNA-IGS) region. We used PCR to investigate the presence/absence of the SIXs and PDA1 genes, and developed a PCR-based method to identify Fop and distinguish it from other forms of F. oxysporum and other Fusarium isolates.
The Fusarium isolates used in the present study are listed in Table 1. The total number of Japanese F. oxysporum f. sp. pisi (Fop) isolates examined herein was 16. Among these isolates, 15 were obtained from the Yokohama Plant Protection Station (YPPS), MAFF, Yokohama, Japan, which included five isolates from Aichi Prefecture, three from Shizuoka, one from Hokkaido, and six from Wakayama. The isolate (200929a) examined in the present study was isolated from a pea plant with wilt symptoms in a Wakayama field. All Fop isolates were obtained through single colony selections. K3-1, K3-3, K4-1, K4-2, and K5-2 were isolated from pea seeds at the YPPS. Since they did not exhibit pathogenicity in peas, they were defined as non-pathogenic isolates (Table 1). Isolates were cultured and maintained on potato dextrose agar (PDA) medium plates at 28°C under dark conditions. Isolates were also stored in 25% (v/v) glycerol at –80°C.
| Species Form Isolate |
Year | Place | Origin Plant | Sourcea | Reference | Pathogenicity in pea cv. Misasab |
Mating typec |
GenBank Accession No.d | |
|---|---|---|---|---|---|---|---|---|---|
| TEF1α | rDNA-IGS | ||||||||
| Fusarium oxysporum | |||||||||
| f. sp. pisi | |||||||||
| 1-1-M | 2002 | Aichi, Japan | Pea | YPPS | Sakoda et al. (2018) | + | 1-2 | LC648692* | LC648663* |
| 1-2-1-5 | 2002 | Aichi, Japan | Pea | YPPS | + | 1-2 | LC648693* | LC648664* | |
| 1-5-2-M | 2002 | Aichi, Japan | Pea | YPPS | Sakoda et al. (2019) | + | 1-2 | LC648694* | LC648665* |
| 2-4-2-M | 2002 | Aichi, Japan | Pea | YPPS | + | 1-2 | LC648695* | LC648666* | |
| 2-9-M | 2002 | Aichi, Japan | Pea | YPPS | + | 1-2 | LC648696* | LC648667* | |
| 9-1-M-2 | 2003 | Shizuoka, Japan | Pea | YPPS | + | 1-1 | LC648697* | LC648668* | |
| 10-1 | 2003 | Shizuoka, Japan | Pea | YPPS | + | 1-2 | LC648698* | LC648669* | |
| 12-1 | 2003 | Shizuoka, Japan | Pea | YPPS | + | 1-2 | LC648699* | LC648670* | |
| KKB31 | 2015 | Hokkaido, Japan | Pea | YPPS | Sakoda et al. (2019) | + | 1-2 | LC648691* | LC648662* |
| 215B | 2016 | Wakayama, Japan | Pea | YPPS | Sakoda et al. (2019) | + | 1-2 | LC648685* | LC648656* |
| 219A | 2016 | Wakayama, Japan | Pea | YPPS | + | 1-2 | LC648686* | LC648657* | |
| 22a | 2016 | Wakayama, Japan | Pea | YPPS | + | 1-2 | LC648687* | LC648658* | |
| 28a | 2016 | Wakayama, Japan | Pea | YPPS | + | 1-2 | LC648688* | LC648659* | |
| 39b | 2017 | Wakayama, Japan | Pea | YPPS | Sakoda et al. (2019) | + | 1-2 | LC648689* | LC648660* |
| 49b | 2017 | Wakayama, Japan | Pea | YPPS | + | 1-2 | LC648690* | LC648661* | |
| 200929a | 2020 | Wakayama, Japan | Pea | TUAT | This study | + | 1-2 | LC648700* | LC648671* |
| f. sp. adzukicola | |||||||||
| 241054 | Unknown | Hokkaido, Japan | Adzuki bean | MAFF | Kondo et al. (2009) | – | 1-1 | LC648701* | LC648672* |
| f. sp. apii | |||||||||
| 1017 | Unknown | Japan | Celery | SUF | NT | 1-2 | LC648702* | AB106048 | |
| f. sp. conglutinans | |||||||||
| Cong:1-1 | Unknown | Japan | Cabbage | TUAT | Kashiwa et al. (2013) | NT | 1-1 | LC648703* | AB106051 |
| f. sp. coriandrii | |||||||||
| 1709C2 | 2017 | Ibaraki, Japan | Coriander | TUAT | NT | 1-1 | LC648704* | LC648673* | |
| f. sp. cubense race 1 | |||||||||
| 160527 | 2016 | Okinawa, Japan | Banana | TUAT | Nitani et al. (2018) | NT | 1-2 | LC648705* | LC648674* |
| f. sp. cubense tropical race 4 | |||||||||
| FOC-BR | Indonesia | Banana | TUAT | NT | 1-1 | LC648706* | LC648675* | ||
| f. sp. lycopersici race 1 | |||||||||
| 103036 | Unknown | Japan | Tomato | MAFF | Inami et al. (2014) | NT | 1-1 | LC648707* | AB106020 |
| f. sp. lycopersici race 2 | |||||||||
| 103038 | Unknown | Japan | Tomato | MAFF | Inami et al. (2014) | NT | 1-1 | LC648708* | AB106031 |
| 12575 | Unknown | Tochigi, Japan | Tomato | JCM | Inami et al. (2014) | NT | 1-1 | LC648709* | AB106027 |
| 4287 | Unknown | Spain | Tomato | Di Pietro | Di Pietro et al. (1998) | NT | 1-1 | KP693888 | AB120973 |
| f. sp. lycopersici race 3 | |||||||||
| Chz1-A | Unknown | Kumamoto, Japan | Tomato | TUAT | Inami et al. (2014) | NT | 1-2 | LC648710* | AB373819 |
| KoChi-1 | Unknown | Kochi, Japan | Tomato | TUAT | Inami et al. (2012) | NT | 1-1 | LC648711* | AB675382 |
| f. sp. spinaciae | |||||||||
| 170612b | 2017 | Ibaraki, Japan | Spinach | TUAT | – | 1-2 | LC648712* | LC648676* | |
| Other plant pathogenic isolates | |||||||||
| 860926a | 1986 | Ibaraki, Japan | Mitsuba | TUAT | NT | 1-1 | LC648713* | LC648677*e | |
| 1709M | 2017 | Ibaraki, Japan | Mitsuba | TUAT | NT | 1-1 | LC648714* | LC648678*e | |
| Non-pathogenic isolates | |||||||||
| K3-1 | 2017 | Unknown | Pea | YPPS | – | 1-1 | LC648715* | LC648679* | |
| K3-3 | 2017 | Unknown | Pea | YPPS | Sakoda et al. (2018) | – | 1-1 | LC648716* | LC648680* |
| K4-1 | 2017 | Unknown | Pea | YPPS | – | 1-1 | LC648717* | LC648681* | |
| K4-2 | 2017 | Unknown | Pea | YPPS | – | 1-1 | LC648718* | LC648682* | |
| K5-2 | 2017 | Unknown | Pea | YPPS | Sakoda et al. (2018) | – | 1-1 | LC648719* | LC648683* |
| Fo304 | Unknown | Japan | Tomato | TUAT | Inami et al. (2014) | NT | 1-1 | LC648720* | AB373828* |
| F. commune | |||||||||
| f. sp. rapae | |||||||||
| ne-1 | 2017 | Ibaraki, Japan | Potherb Mustard | TUAT | NT | 1-1 | LC648721* | LC648684* | |
| Non-pathogenic isolate | |||||||||
| W5 | 2011 | Aomori, Japan | Rice | TUAT | Saito et al. (2021) | – | 1-1 | LC648722* | LC516582* |
| F. fujikuroi | |||||||||
| Miyagi 92-10 | Unknown | Miyagi, Japan | Rice | TUAT | Saito et al. (2021) | NT | 1-1 | LC648723* | LC649895* |
| F. sacchari | |||||||||
| 7610 | Unknown | Unknown | FGSC | Kawabe et al. (2005) | NT | 1-2 | LC648724* | AB106061 | |
| F. solani | |||||||||
| f. sp. pisi | |||||||||
| C1-2A | Unknown | Wakayama, Japan | Pea | TUAT | + | NA | LC648725* | NA | |
| Other plant pathogenic isolate | |||||||||
| 305125 | Unknown | Unknown | Sweet pea | MAFF | Tsumuki et al. (1995) | – | NA | LC648726* | NA |
a YPPS, Yokohama Plant Protection Station; TUAT, Laboratory of Plant Pathology, Tokyo University of Agriculture and Technology; MAFF, Ministry of Agriculture, Forestry and Fisheries of the Japanese government; SUF, Shinshu University Fusarium collection; JCM, Japan Collection of Microorganisms; Di Pietro, Cordoba University; FGSC, Fungal Genetic Stock Center, Kansas State University.
b +, pathogenicity; –, no pathogenicity; NT, not tested.
c NA, no amplicon was obtained with EF1/EF2 primers for TEF1α and FIGS11/FIGS12 primers for rDNA-IGS.
d Asterisks represent data obtained in this study. NA, no amplicon.
e Not used for the phylogenetic analysis due to a deletion of ca. 300 bp.
Regarding in planta pathogenicity assays, we used 26 isolates of Fusarium spp. including the 16 Fop isolates (Table 1). Each isolate was cultured in potato dextrose broth (PDB) medium for 5 days at 28°C with reciprocal shaking at 120 rpm. The bud cells that formed were filtered through a double layer of sterilized cheese cloth to remove mycelia, collected by centrifugation at 3,000×g for 10 min, and suspended in sterilized water at a concentration of 1.0×107 cells mL–1. This suspension was used as the inoculum.
To test the pathogenicity of each isolate, we employed the soil drenching method with the pea cultivar Misasa (Asahi Noen Seed), which is susceptible to Fop (Sakoda et al., 2018). Two seeds were sown in each plastic pot with a diameter of 7 cm containing autoclaved (121°C, 40 min) soil (Kumiai Nippi Engeibaido No. 1; Nihon Hiryo). The roots of each 10-day-old pea plant were wounded by inserting a plastic peg into the soil five times, and the inoculum was then added to the soil at a rate of 1 mL plant–1. After inoculation, plants were maintained in a greenhouse at 28°C. Tests were conducted using four or six plants with three biological replications. Disease severity in each plant at 28 days post-inoculation was evaluated as follows: 0, no symptoms; 1, yellowing or wilting of the lower leaves; 2, yellowing or wilting of the upper and lower leaves; 3, wilting of the entire plant; 4, death.
Extraction of fungal genomic DNA (gDNA)gDNA was extracted from mycelia that had been cultured on a PDA plate using the procedure of Saitoh et al. (2006). A Nanodrop One Spectrophotometer (Thermo Fisher Scientific) was employed to assess the concentration and quality of gDNA.
Identification of mating types by PCRThe mating type of each isolate was identified by PCR using a MiniAmp Thermal Cycler (Thermo Fisher Scientific) with the primers listed in Table S1. The method and PCR conditions employed were identical to those described by Inami et al. (2012). Isolates from which an approximately 280-bp fragment was amplified with the primer set Gfmat1a/Gfmat1b were identified as MAT1-1. Isolates from which an approximately 220-bp fragment was amplified with the primer set GfHMG1/GfHMG2 were identified as MAT1-2.
PCR amplification of the TEF1α gene fragment and the rDNA-IGS regionIn the molecular phylogenetic analysis, we amplified fragments of the TEF1α gene (ca. 700 bp) and the rDNA-IGS region (ca. 600 bp) from each isolate using the EF1/EF2 primers for TEF1α (O’Donnell et al., 2009) and the FIGS11/FIGS12 primers for the rDNA-IGS region (Kawabe et al., 2005) (Table S1). We used a MiniAmp Thermal Cycler (Thermo Fisher Scientific), and 10 μL of the PCR mixture contained 30 ng gDNA, 1×Ex Taq Buffer (Takara Bio), 0.5 mM of each dNTP (Takara Bio), 0.2 μM of each primer, and 0.5 U TaKaRa Ex Taq (Takara Bio). Reactions consisted of three steps: 94°C for 1 min; 30 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min; and 72°C for 7 min.
Sanger sequencingPCR amplicons were sequenced directly and after cloning. Regarding direct sequencing, each amplicon was purified with ExoSAP-IT (Thermo Fisher Scientific) and sequenced in a 3710xl Genetic Analyzer (Thermo Fisher Scientific) using the BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific) with the primers used for amplification. Concerning cloning, each amplicon was ligated into the pGEM-T Easy vector (Promega). The inserted DNA fragments were then sequenced with the M13F/M13R primers (5′-CGCCAGGGTTTTCCCAGTCACGAC-3′/5′-AGCGGATAACAATTTCACACAGGA-3′). Data were processed using GENETYX-Mac version 11.2.1 software (Genetyx) and deposited in GenBank (Table 1 and 2).
| Isolatea | PCR detectionb | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SIX | PDA1 | ||||||||||||||
| SIX1 | SIX2 | SIX3 | SIX4 | SIX5 | SIX6 | SIX7 | SIX8 | SIX9 | SIX10 | SIX11 | SIX12 | SIX13 | SIX14 | ||
| F. oxysporum | |||||||||||||||
| f. sp. pisi | |||||||||||||||
| 1-1-M (P1) | –c | – | – | – | – | LC648752c | – | – | – | – | – | – | LC648766 | – | LC648734 |
| 1-2-1-5 (P3) | – | – | – | – | – | LC648753 | – | – | – | – | – | – | LC648767 | LC648781 | LC648735 |
| 1-5-2-M (P1) | – | – | – | – | – | LC648754 | – | – | – | – | – | – | LC648768 | – | LC648736 |
| 2-4-2-M (P3) | – | – | – | – | – | LC648755 | – | – | – | – | – | – | LC648769 | LC648782 | LC648737 |
| 2-9-M (P1) | – | – | – | – | – | LC648756 | – | – | – | – | – | – | LC648770 | – | LC648738 |
| 9-1-M-2 (P2) | – | – | – | – | – | – | LC648775 | LC648776 | – | LC648777 | LC648778 | LC648779 | LC648771 | LC648780 | LC648739 |
| 10-1 (P1) | – | – | – | – | – | LC648757 | – | – | – | – | – | – | LC648772 | – | LC648740 |
| 12-1 (P1) | – | – | – | – | – | LC648758 | – | – | – | – | – | – | LC648773 | – | LC648741 |
| KKB31 (P2) | – | – | – | – | – | LC648751 | – | – | – | – | – | – | – | – | LC648733 |
| 215B (P1) | – | – | – | – | – | LC648745 | – | – | – | – | – | – | LC648760 | – | LC648727 |
| 219A (P1) | – | – | – | – | – | LC648746 | – | – | – | – | – | – | LC648761 | – | LC648728 |
| 22a (P1) | – | – | – | – | – | LC648747 | – | – | – | – | – | – | LC648762 | – | LC648729 |
| 28a (P1) | – | – | – | – | – | LC648748 | – | – | – | – | – | – | LC648763 | – | LC648730 |
| 39b (P1) | – | – | – | – | – | LC648749 | – | – | – | – | – | – | LC648764 | – | LC648731 |
| 49b (P1) | – | – | – | – | – | LC648750 | – | – | – | – | – | – | LC648765 | – | LC648732 |
| 200929a (P1) | – | – | – | – | – | LC648759 | – | – | – | – | – | – | LC648774 | – | LC648742 |
| f. sp. adzukicola | |||||||||||||||
| 241054 | – | – | – | – | – | – | – | – | – | – | – | – | +c | – | – |
| f. sp. apii | |||||||||||||||
| 1017 | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – |
| f. sp. conglutinans | |||||||||||||||
| Cong:1-1 | – | – | – | + | – | – | – | + | – | – | – | – | – | – | – |
| f. sp. coriandrii | |||||||||||||||
| 1709C2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| f. sp. cubense race 1 | |||||||||||||||
| 160527 | – | – | – | – | – | + | – | – | – | – | – | – | + | – | – |
| f. sp. cubense tropical race 4 | |||||||||||||||
| FOC-BR | + | – | – | – | – | + | – | – | – | – | – | – | + | – | – |
| f. sp. lycopersici race 1 | |||||||||||||||
| 103036 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | – |
| f. sp. lycopersici race 2 | |||||||||||||||
| 103038 | + | + | + | – | + | + | + | + | + | + | + | + | + | + | – |
| 12575 | + | + | + | – | + | + | + | + | + | + | + | + | + | + | – |
| 4287 | + | + | + | – | + | + | + | + | + | + | + | + | + | + | – |
| f. sp. lycopersici race 3 | |||||||||||||||
| Chz1-A | + | + | + | – | + | + | + | + | + | + | + | + | + | + | – |
| KoChi-1 | + | + | + | +d | + | + | + | + | + | + | + | + | + | + | – |
| f. sp. spinaciae | |||||||||||||||
| 170612b | – | – | – | – | – | – | – | + | – | – | – | – | – | – | LC648743 |
| Other plant pathogenic isolates | |||||||||||||||
| 860926a | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – |
| 1709M | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – |
| Non-pathogenic isolates | |||||||||||||||
| K3-1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| K3-3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| K4-1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| K4-2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Fo304 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| F. commune | |||||||||||||||
| f. sp. rapae | |||||||||||||||
| ne-1 | – | – | – | – | – | – | – | + | – | – | – | – | – | + | – |
| Non-pathogenic isolate | |||||||||||||||
| W5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| F. solani | |||||||||||||||
| f. sp. pisi | |||||||||||||||
| C1-2A | – | – | – | – | – | – | – | – | – | – | – | – | – | – | LC648744 |
| Other plant pathogenic isolate | |||||||||||||||
| 305125 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
a P1, P2, or P3 after the isolate number indicates a clade shown in Fig. 1.
b Primers used are listed in Table S1. +, positive; –, negative.
c +, amplicon obtained by PCR; –, no amplicon. Accession numbers indicate that the amplicon was present and sequenced, and data were deposited in GenBank.
d This amplicon contains a transposon insertion (Inami et al., 2012).
The phylogenic relationships between the Japanese and non-Japanese Fop isolates were analyzed using TEF1α sequences (Table 1 and S2). To clarify the phylogenic positions of the Japanese Fop isolates among various other forms of F. oxysporum, we performed a phylogenetic analysis using rDNA-IGS sequences (Table 1). Multiple sequences were aligned using ClastalW version 2.1 (Larkin et al., 2007), and phylogenetic analyses were performed using the maximum likelihood method. We adopted the Hasegawa-Kishino-Yano model (Hasegawa et al., 1985) with 1,000 replicates of bootstrap values. The outgroup for TEF1α was the root rot pathogen of pea, F. solani f. sp. pisi isolate C1-2A (Table 1), while that for rDNA-IGS was F. sacchari isolate FGSC 7610 (Table 1). All evolutionary analyses were performed using MEGA X software (Kumar et al., 2018; Stecher et al., 2020).
Detection of SIX and PDA1 genes by PCRAll 16 Japanese Fop isolates were subjected to PCR analyses aimed at detecting homologs of the F. oxysporum f. sp. lycopersici SIX genes and PDA1 using previously designed primer sets (Table S1; van der Does et al., 2008; Lievens et al., 2009; Meldrum et al., 2012; Milani et al., 2012; Taylor et al., 2016). Ten microliters of the PCR mixture contained 30 ng gDNA, 1×Ex Taq Buffer, 0.5 mM of each dNTP, 0.2 μM of each primer, and 0.5 U Ex Taq. The reaction conditions for SIX1 to SIX5, SIX7, and SIX9 to SIX14 were as follows: 94°C for 1 min; 30 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min; and 72°C for 7 min. The conditions for SIX6 were as follows: 94°C for 1 min; 30 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 45 s; and 72°C for 7 min. The conditions for SIX8 were as follows: 94°C for 1 min; 30 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s; and 72°C for 7 min. The conditions for PDA1 were as follows: 94°C for 1 min; 30 cycles of 94°C for 30 s, 63°C for 30 s, and 72°C for 1 min; and 72°C for 7 min.
In reactions designed to detect Fop isolates using the primers listed in Table 3, 10 μL of the PCR mixture contained 30 ng of fungal gDNA, 1×PCR Buffer for KOD Fx Neo (Toyobo), 0.4 mM of each dNTP, 0.1 μM of each primer, and 0.2 U KOD Fx Neo (Toyobo). PCR conditions were 94°C for 1 min, 30 cycles of 98°C for 10 s, 60°C for 30 s, and 68°C for 30 s, followed by 68°C for 7 min.
| Primer set | Primer name | Sequence (5′-3′) | Expected amplicon |
|---|---|---|---|
| piPDA | piPDAF | GGTCATTCTGAAAGAAGAGCTTCAGC | 841 bp |
| piPDAR | CCGTTGACACCAACCTCAGTCTGTTATC | ||
| piSIX6 | piSIX6F | GCTCCGTCTGCTATAAAGCCAATA | 349 bp |
| piSIX6R | GTCGATCCACCAATACCTTCATTC | ||
| piSIX13 | piSIX13F | ATCAGGCCTTCAACGAAGAG | 739 bp |
| piSIX13R | ATGGCGTTATGCTCATTGACACT |
To clarify the detection limits of the primers listed in Table 3, the gDNA of Fop isolate 39b was serially diluted with water, with concentrations ranging between 3 ng μL–1 and 30 fg μL–1. Diluted samples (1 μL per reaction) were used as templates in PCR reactions set up as described above.
Detection of Fop in infected plants and infested soilsPea plants (cv. Misasa) were inoculated with Fop isolate 39b as described above for the pathogenicity tests. A sterilized toothpick was inserted into the basal stem tissues 28 days after the inoculation and then soaked in the PCR mixture for 5 s. Two samples from two individual plants each were used. Healthy pea plants were employed as the control.
To prepare an artificially infested soil with Fop isolate 39b, 5 g of autoclaved soil was mixed with 1 mL of the bud cell suspension (1.0×107 cells mL–1) in a Petri dish (90 mm in diameter). The infested soil was dried at room temperature overnight. A similar sample was prepared with distilled water as a negative control. Soil DNA was extracted from 0.5 g of each soil sample using the FastDNA SPIN Kit for Soil (MP Biomedicals) with 10% skim milk (w/v) in a Fastprep-24 grinder (MP Biomedicals), as previously described by Kashiwa et al. (2016). Fifty nanograms of soil DNA was used as the template for PCR. Two replicates were prepared for each treatment.
Soil samples (original) were collected from two different pea-growing fields (No. 28 and 49) in May 2020. Both fields had histories of pea wilt disease; however, the occurrence of pea wilt was not confirmed in the 2019 crop season (between September 2019 and May 2020). Fields No. 28 and 49 were both subsequently disinfested using soil solarization and chloropicrin-fumigation, and soil samples were again collected (disinfested; September 2020). Five grams of soil sampled from three locations in each field were mixed well and 15 g of soil was subjected to soil DNA extraction as described above.
All 16 Fop isolates showed pathogenicity in pea cv. Misasa (Table 1 and Fig. S1). As expected, the isolate C1-2A of F. solani f. sp. pisi (Fsp) exhibited strong pathogenicity in peas (Table 1 and Fig. S1). It was not possible to distinguish between the symptoms presented by Fop and Fsp. We also tested F. oxysporum f. sp. adzukicola (the pathogen of adzuki bean wilt) isolate 241054, F. oxysporum f. sp. spinaciae (the pathogen of spinach wilt) isolate 170612b, non-pathogenic F. oxysporum isolates K3-1, K3-2, K4-1, K4-2, and K5-2 from pea, F. solani isolate 305125 from sweet pea (Lathyrus odoratus L.), and F. commune isolate W5 from rice (Oryza sativa L.). None of these isolates exhibited any pathogenicity in peas (Table 1 and Fig. S1).
Mating typeWe identified the mating type of each isolate and found that 15 out of the 16 Japanese Fop isolates were MAT1-2 (Table 1). Only one isolate, 9-1-M-2 from Shizuoka Prefecture, was MAT1-1 (Table 1 and Fig. 2). All isolates were MAT1-1 or MAT1-2, which suggested that all of the tested isolates were heterothallic (Table 1; Arie et al., 2000).
PhylogenyWe constructed a phylogenetic tree based on TEF1α sequences from the 16 Japanese Fop isolates and 24 Fop isolates from other countries whose sequences were available in the NCBI database (Table S2). The tree supported three clades, P1–P3 (Fig. 1). Clade P1 comprised 18 isolates, including all seven isolates from Wakayama, three from Aichi, and two from Shizuoka, along with three isolates from the USA, two from the UK, and one from the Czech Republic. Clade P2 was composed of two isolates, one from Shizuoka and one from Hokkaido. Clade P3 included two Japanese isolates, 16 isolated from the USA, and two from the UK.
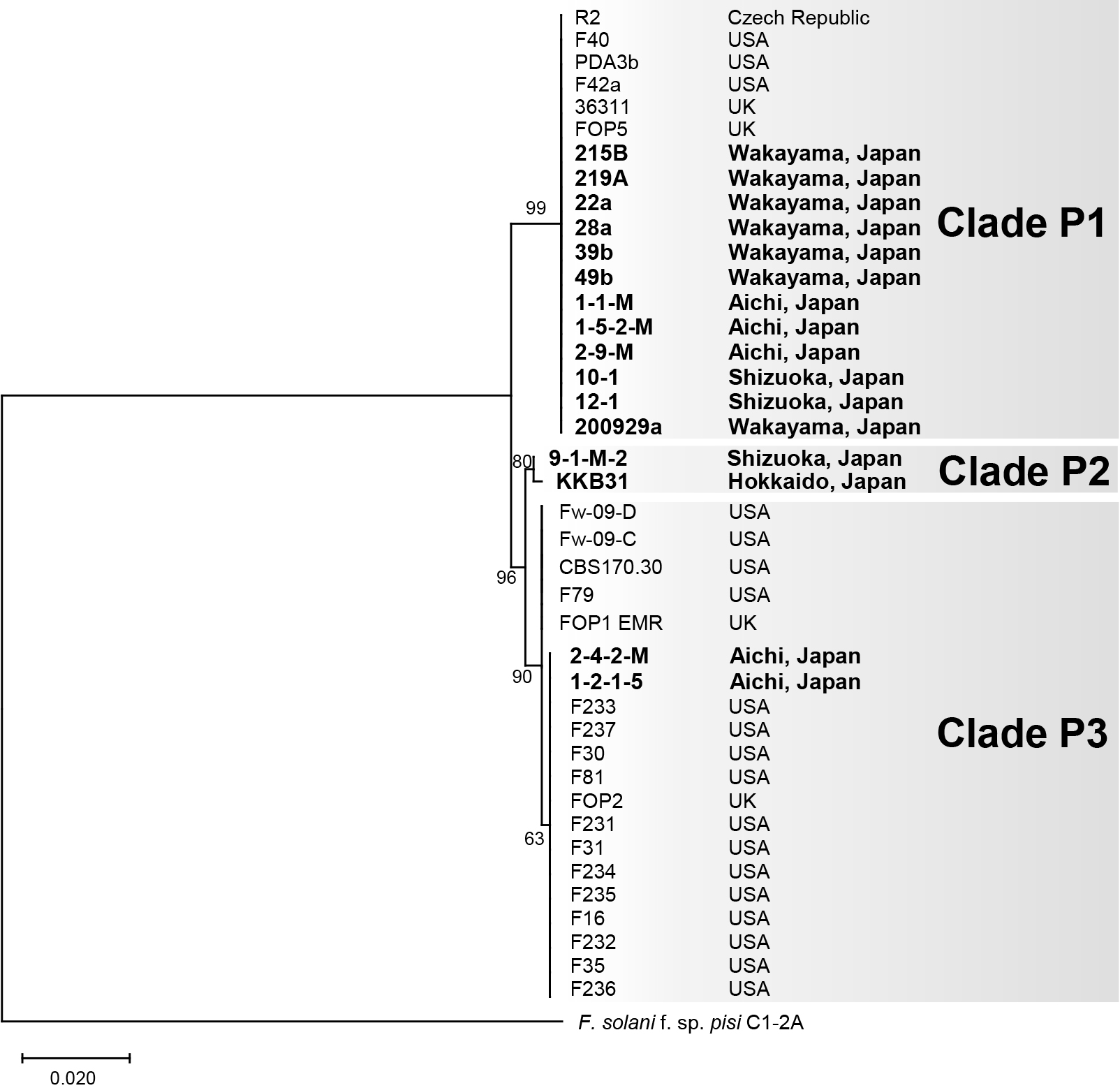
Phylogenetic tree of Fusarium oxysporum f. sp. pisi based on the TEF1α gene. The tree was constructed using the maximum likelihood method and the Hasegawa-Kishino-Yano model. Numbers on nodes represent bootstrap values estimated from 1,000 replicates, where bootstrap values are higher than 60%. Fop isolates were divided into three clades, P1, P2, and P3. F. solani f. sp. pisi isolate C1-2A was used as an outgroup. The source location of each isolate is shown on the right of the phylogenetic tree. Sequence information is presented in Table 1 and S2.

Phylogenetic tree of Fusarium oxysporum isolates based on the rDNA-IGS region. The tree was constructed using the maximum likelihood method and the Hasegawa-Kishino-Yano model. Numbers on nodes represent bootstrap values estimated from 1,000 replicates when bootstrap values are higher than 60%. Fop isolates were divided into three clades, Q1, Q2, and Q3. F. sacchari isolate FGSC 7610 was used as an outgroup. The form and mating type of each Fusarium isolate is shown on the right of the tree. Sequence information is presented in Table 1.
We also constructed a phylogenic tree based on the rDNA-IGS sequences from the 16 Japanese Fop isolates, 14 isolates of other forms of F. oxysporum and F. commune, and seven non-pathogenic F. oxysporum and F. commune isolates, five of which were isolated from pea (Fig. 2). In this tree, the 16 Japanese Fop isolates again formed three well supported clades, Q1–Q3, corresponding to clades P1–P3, respectively, in the TEF1α phylogeny (Fig. 1). Clades P1 and Q1 contained 12 Japanese Fop isolates that were all MAT1-2, clades P2 and Q2 contained the MAT1-2 isolate KKB31 and the MAT1-1 isolate 9-1-M-2, and clades P3 and Q3 contained the MAT1-2 isolates 1-2-1-5 and 2-4-2-M (Fig. 1, 2, and Table 1).
Presence or absence of SIX and PDA1 genesWe used PCR with primers designed to amplify the 14 SIX genes of F. oxysporum f. sp. lycopersici in order to investigate the presence or absence of SIX homologs in the 16 Japanese Fop isolates, the other forms of F. oxysporum, the non-pathogenic F. oxysporum isolates, and the other Fusarium spp. isolates listed in Table 2. In this analysis, we used the previously designed primers listed in Table S1. Among the 16 Fop isolates, 12 possessed SIX6 and SIX13 homologs (Table 2). Isolates 1-2-1-5 and 2-4-2-M had homologs of SIX14 as well as SIXs 6 and 13. Isolate 9-1-M-2 had homologs of SIXs 7, 8, and 10–14, but lacked SIX6. KKB31 possessed SIX6, but lacked SIX13. F. oxysporum f. sp. cubense isolates also had both SIX6 and SIX13, and the F. oxysporum f. sp. azdukicola isolate possessed SIX13. As expected, the F. oxysporum f. sp. lycopersici isolates had most or all of the SIX genes. The other forms and non-pathogenic isolates did not carry homologs that were detectable by the primers used (Table 2). Moreover, we did not detect SIX homologs in Fsp, the root rot pathogen of pea (Table 2). We also employed PCR and previously designed PDA1 primers (Table S1) to search for PDA1 homologs in all the isolates listed in Table 2. In this case, PDA1 homologs were only detected in the 16 Fop isolates, Fsp, and an isolate (170612b) of F. oxysporum f. sp. spinaciae (Table 2). Therefore, we found that the Fop isolates harbored SIX6 and/or SIX13 together with PDA1.
Differentiation of Fop isolates from other F. oxysporum isolates and Fusarium spp. by PCRAs demonstrated in the phylogenetic study, Fop is polyphyletic among F. oxysporum isolates (Fig. 2). Therefore, the primer sets for the rDNA-IGS sequence may not be applicable for the differentiation of Fop from other forms and non-pathogenic isolates of F. oxysporum. This is consistent with previous findings on other forms, including F. oxysporum ff. spp. lycopersici, cubense, and apii (the celery wilt pathogen) (O’Donnell et al., 1998; Kawabe et al., 2005; Epstein et al., 2017).
On the other hand, all Fop isolates tested carried either or both of the SIX6 and SIX13 homologs together with the PDA1 gene, and among the isolates tested in Table 2, Fop isolates were the only ones to carry this combination of three genes (PDA1; SIX6 and/or SIX13). Therefore, we designed specific primer sets (piPDA, piSIX6, and piSIX13) targeting these genes (Table 3). The primer sets were designed to have the same annealing temperature (60°C) in order to obtain the desired amplificons under the same reaction conditions. Regarding the specific detection of Fop by PCR, we employed KOD Fx Neo polymerase, which may be used with crude DNA samples.
The piPDA primer set amplified a fragment (841 bp) of PDA1 from all of the Fop isolates and from an isolate of F. oxysporum f. sp. spinaciae (Fig. 3 and Table 4). However, no amplicons were obtained from any of the other Fusarium isolates, including C1-2A of Fsp, which carries a PDA1 gene that is not targeted by the specific sequences of the piPDA primers (Fig. 3 and Table 4). The piSIX6 primer set amplified a fragment (349 bp) from all the F. oxysporum f. sp. lycopersici isolates, and all the Fop isolates, except for 9-1-M-2, but did not amplify the fragment from any of the other Fusarium isolates (Fig. 3 and Table 4). Similarly, the piSIX13 primer set amplified a fragment (739 bp) from an isolate of F. oxysporum f. sp. adzukicola, both of the isolates of F. oxysporum f. sp. cubense, all of the F. oxysporum f. sp. lycopersici isolates, and all of the Fop isolates, except for KKB31, but did not amplify the fragment from any other Fusarium isolates (Fig. 3 and Table 4). Therefore, as shown in Table 4, only Fop isolates showed positive results with the piPDA primers plus one or both of the piSIX6 and piSIX13 primers.

Differentiation of Fusarium oxysporum f. sp. pisi from other isolates of Fusarium species by PCR.
PCR was performed using representative isolates and the primer sets piPDA, piSIX6, piSIX13, and TEF1α (Table 3 and S1). Products of 841, 349, 739, and approximately 700 bp were generated with primer sets piPDA, piSIX6, piSIX13, and TEF1α, respectively.
| Isolate | Detection by PCR | ||
|---|---|---|---|
| piPDA | piSIX6 | piSIX13 | |
| Fusarium oxysporum | |||
| f. sp. pisi | |||
| 1-1-M | + | + | + |
| 1-2-1-5 | + | + | + |
| 1-5-2-M | + | + | + |
| 2-4-2-M | + | + | + |
| 2-9-M | + | + | + |
| 9-1-M-2 | + | – | + |
| 10-1 | + | + | + |
| 12-1 | + | + | + |
| KKB31 | + | + | – |
| 215B | + | + | + |
| 219A | + | + | + |
| 22a | + | + | + |
| 28a | + | + | + |
| 39b | + | + | + |
| 49b | + | + | + |
| 200929a | + | + | + |
| f. sp. adzukicola | |||
| 241054 | – | – | + |
| f. sp. apii | |||
| 1017 | – | – | – |
| f. sp. conglutinans | |||
| Cong:1-1 | – | – | – |
| f. sp. coriandrii | |||
| 1709C2 | – | – | – |
| f. sp. cubense race 1 | |||
| 160527 | – | – | + |
| f. sp. cubense tropical race 4 | |||
| FOC-BR | – | – | + |
| f. sp. lycopersici race 1 | |||
| 103036 | – | + | + |
| f. sp. lycopersici race 2 | |||
| 103038 | – | + | + |
| 12575 | – | + | + |
| 4287 | – | + | + |
| f. sp. lycopersici race 3 | |||
| Chz1-A | – | + | + |
| KoChi-1 | – | + | + |
| f. sp. spinaciae | |||
| 170612b | + | – | – |
| Other plant pathogenic isolates | |||
| 860926a | – | – | – |
| 1709m | – | – | – |
| Non-pathogenic isolates | |||
| K3-1 | – | – | – |
| K3-3 | – | – | – |
| K4-1 | – | – | – |
| K4-2 | – | – | – |
| K5-2 | – | – | – |
| Fo304 | – | – | – |
| F. commune | |||
| f. sp. rapae | |||
| ne-1 | – | – | – |
| Non-pathogenic isolate | |||
| W5 | – | – | – |
| F. solani | |||
| f. sp. pisi | |||
| C1-2A | – | – | – |
| Other plant pathogenic isolate | |||
| 305125 | – | – | – |
+, positive; –, negative.
To clarify the detection limits of the primer pairs designed in the present study, we made serial dilutions of gDNA from isolate 39b and used them in PCRs with the three primer sets. The piPDA primer set detected as low as 3 pg μL–1 of gDNA, while the piSIX6 and piSIX13 primer sets detected as low as 300 fg μL–1 (Fig. 4).

Assessment of detection limits of piPDA, piSIX6, and piSIX13 primer sets.
gDNA from Fop isolate 39b was serially diluted from 3 ng μL–1 to 30 fg μL–1, and each dilution was used as the template in PCRs with the piPDA, piSIX6, and piSIX13 primer sets.
To investigate whether the piPDA, piSIX6, and piSIX13 primer sets are applicable for the detection of Fop in infected plants, we inoculated pea (cv. Misasa) with Fop isolate 39b, and a small amount of the infected plant was picked 28 days later by inserting a sterilized toothpick into the basal stem tissues. The toothpick was then soaked in PCR mixture to provide template DNA for the reaction. Healthy pea plants were used as controls. The pea plants inoculated with Fop produced positive bands of the expected sizes in PCRs with the three primer sets. On the other hand, no bands were produced in PCRs with the healthy pea plants. Representative data are shown in Fig. 5.

Detection of Fusarium oxysporum f. sp. pisi in infected plant tissues by direct PCR.
(A) Photographs of the pea plants used. Healthy pea plants were inoculated with sterilized water and infected pea plants were inoculated with Fop isolate 39b.
(B) PCR was performed using sterile water, the gDNA of Fop isolate 39b, and material from each plant as templates. The primer sets piPDA, piSIX6, and piSIX13 were used. To pick a small amount of material from each plant, a toothpick was inserted into basal stem tissues and soaked in the PCR mixture as a template.
We performed PCRs using the DNAs from artificially infested soil with the three primer sets piPDA, piSIX6, and piSIX13. The DNAs extracted from Fop-infested soil produced positive bands of the expected sizes with all three primer sets (Fig. 6). On the other hand, no bands were produced in PCRs with the DNAs extracted from non-infested soil. Representative data are shown in Fig. 6.
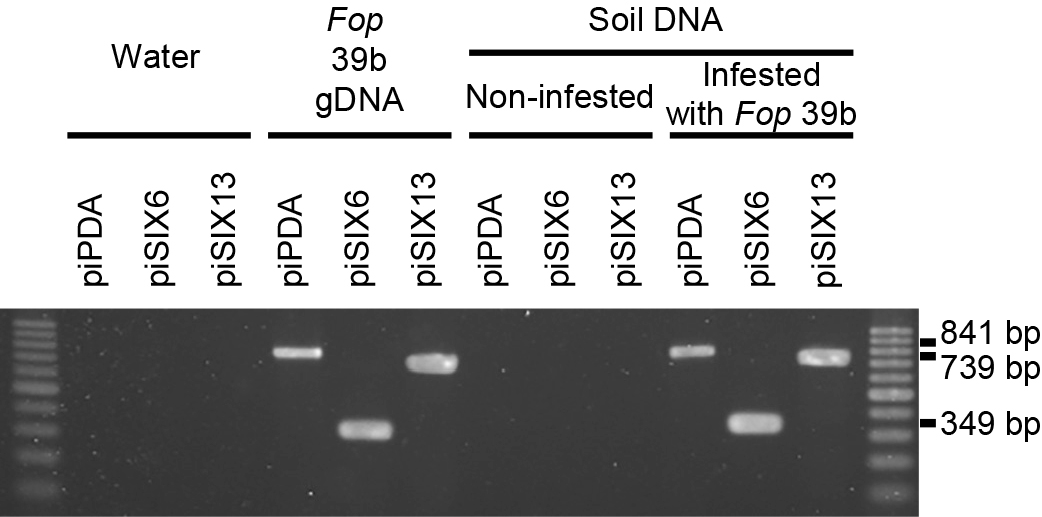
Detection of Fusarium oxysporum f. sp. pisi in artificially infested soil by PCR.
PCRs were performed using sterile water, the gDNA of Fop isolate 39b, and DNA extracted from non-infested soil and soil infested with 39b as templates. The primer sets piPDA, piSIX6, and piSIX13 were used.
DNAs from the soil samples of two pea-growing fields (No. 28 and 49) were extracted. PCRs using soil DNAs with the piPDA, piSIX6, and piSIX13 primers amplified bands of the expected sizes from the original field No. 49 sample, but not from the No. 28 sample (Fig. 7A). This result suggested that Fop existed in the field No. 49 sample.

Detection of Fusarium oxysporum f. sp. pisi in soil from pea fields by PCR.
(A) PCR was performed using soil DNA extracted from pea fields No. 28 and No. 49, before (original) and after sterilization (disinfested), as templates. As negative and positive controls, sterile water and the gDNA of Fop isolate 39b, respectively, were applied as templates. The primer sets piPDA, piSIX6, and piSIX13 were used.
(B) Colonies on F. oxysporum-selective medium plates. Photographs were taken 5 days after inoculation with diluted soil samples collected from fields No. 28 and No. 49, before (original) and after sterilization (disinfested).
(C) PCR was performed using the primer sets piPDA, piSIX6, and piSIX13 with gDNA extracted from 10 randomly selected F. oxysporum isolates from fields No. 28 and No. 49. Positive and negative amplifications are shown by + and –, respectively, in PCR. Pathogenicity, no pathogenicity and not tested (NT) are shown by +, –, and NT, respectively, for pathogenicity in pea cv. Misasa.
(D) The total numbers of colonies isolated from the diluted soil samples from fields No. 28 and No. 49, and the percentages of PCR-positive results from the 10 randomly selected isolates from each pea field.
We then applied 10-fold dilutions (w/w) of each soil mixture to plates containing the F. oxysporum-selective medium Fo-G1 (Nishimura, 2007). In total, 144 and 89 isolates were obtained from fields No. 28 and 49, respectively (Fig. 7B and D). gDNAs were extracted from the 10 isolates randomly selected as representative isolates of each field, and subjected to PCRs with the piPDA, piSIX6, and piSIX13 primers. All 10 isolates from field No. 28 were negative. On the other hand, 8 out of 10 isolates from field No. 49 were positive for the three primer sets (Fig. 7C and D). PCRs were also performed on disinfested No. 28 and 49 soil samples. No bands were obtained from the disinfested samples of either field (Fig. 7A). Moreover, no colonies were detected from disinfested soils with F. oxysporum-selective medium (Fig. 7B and D).
Phylogenetic relationships among Japanese and other Fop isolates were examined in the present study, and the presence or absence of the putative effector genes SIX1–14 and PDA1 in Japanese Fop isolates was also investigated. Moreover, a PCR-based technique for identifying Fop and differentiating it from other F. oxysporum forms and other Fusarium spp. was established. The PCR method effectively detected Fop in infected pea plants and infested soils.
Both phylogenetic trees based on TEF1α and the rDNA-IGS region showed that Fop isolates fell into three independent clades (P1–3 in Fig. 1 and Q1–3 in Fig. 2). Clades P1, P2, and P3 each contained the same isolates as clades Q1, Q2, and Q3, respectively. These phylogenetic relationships suggest the polyphyletic origin of Fop. This is consistent with previous findings reported by Kawabe et al. (2005) and Epstein et al. (2017), showing that F. oxysporum ff. spp. lycopersici and apii, respectively, were polyphyletic and difficult to distinguish from other forms based on phylogenies. On the other hand, the phylogenetic tree based on TEF1α (Fig. 1), which shows relationships among Japanese and non-Japanese Fop isolates, indicated that the isolates in clades P1 and P3 were closely related to those from the USA, UK, and Czech Republic. All of the Wakayama isolates were in clade P1 together with the isolates from other countries, suggesting that the Wakayama isolates are monophyletic and arrived from other countries via seeds.
F. oxysporum has been suggested to carry functional mating type genes despite being an asexual fungus (Arie et al., 2000). Kawabe et al. (2005) reported that the F. oxysporum f. sp. lycopersici isolates belonging to each phylogenetic group carry identical mating type genes, and suggested that asexual reproduction is a major driving force for diversification. All of the Fop isolates belonging to clades Q1 and Q3 are MAT1-2, while the two isolates 9-1-M-2 and KKB31 in clade Q2 have different mating types (MAT1-1 and MAT1-2, respectively), suggesting that these two isolates reproduce sexually (Fig. 2). However, our attempts to cross these isolates (9-1-M-2 as MAT1-1 and KKB31 as MAT1-2) on carrot medium using the method described by Leslie and Summerell (2006) have so far been unsuccessful (data not shown).
All twelve Japanese isolates belonging to clade P1 carry both the SIX6 and SIX13 genes (Table 2). The two isolates in clade P3 carry SIX6, SIX13, and SIX14 (Table 2). The two isolates in clade P2 carry different combinations of SIXs as follows: KKB31 carries SIX6, and 9-1-M-2 carries SIXs 7, 8, and 10–14 (Table 2). Taken together, these results show that Fop isolates carry both SIX6 and/or SIX13. Six6 is a cysteine-rich protein with a signal peptide that functions to suppress I2 resistance in tomato (Gawehns et al., 2014). SIX6 was initially reported in F. oxysporum f. sp. lycopersici (Lievens et al., 2009) and was suggested to be involved in, but not essential for pathogenicity (Gawehns et al., 2014; Vlaardingerbroek et al., 2016). On the other hand, SIX6 disruptants in F. oxysporum f. sp. radicis-cucumerinum reduced their pathogenicity to cucumber, suggested that SIX6 played an important role in pathogenicity (van Dam et al., 2017). Six13 is also a cysteine-rich protein with a signal peptide; however, its function in pathogenicity in F. oxysporum currently remains unclear. Further studies are warranted to clarify whether SIX6 and SIX13 are involved in pathogenicity to pea in Fop and complement each other.
PDA1 encodes a pisatin demethylase that degrades pisatin, a fungicidal chemical produced by pea plants. Therefore, it is reasonable that all of the Fop isolates tested in the present study carry PDA1. This is consistent with previous findings showing that when PDA1 was introduced into F. oxysporum f. sp. lini, the flax wilt pathogen, it acquired pathogenicity to pea (Coleman et al., 2011). Fsp, the pathogen of the root rot of pea, also has a PDA1 gene (Table 2). F. oxysporum f. sp. spinaciae (170612b), the wilt pathogen of spinach, also carries a PDA1 gene with high sequence homology (Table 2); however, this isolate is not pathogenic to pea (Fig. S1). Spinach (Spinacia oleracea L.) does not produce pisatin. Therefore, the Pda1 of F. oxysporum f. sp. spinaciae may play another role other than the demethylation of pisatin.
We found that Fop isolates differentiated from other forms and non-pathogenic F. oxysporum isolates because they uniquely carry PDA1 along with SIX6 and/or SIX13. Therefore, we designed the primer sets piPDA, piSIX6, and piSIX13 (Table 3), and successfully established a PCR method to specifically identify Fop. Based on our study of the detection limits of PCR, the threshold of detection was at least 3 pg μL–1 of gDNA (Fig. 4). We demonstrated that the PCR method may be used to detect Fop in infected pea plant tissues and soils infested with Fop.
We successfully detected Fop within 3 h by simply transferring a small amount of infected plant tissues into the PCR mixture using a toothpick (Fig. 5). Therefore, it was possible to eliminate the steps of isolation, cultivation, and gDNA extraction from the fungus for the detection of Fop. This method will allow for the rapid diagnosis of pea wilt in the field.
We successfully detected Fop by PCR using soil DNAs from pea-growing fields as templates (Fig. 7). It is important to note that we detected Fop even from a field (No. 49) in which pea wilt disease was not observed in the previous crop (Fig. 7A and C). This result suggests that Fop was present in the soil at a density lower than that needed to cause disease in pea plants. After the soil solarization and chloropicrin fumigation of fields No. 28 and 49, Fop was no longer detected (Fig. 7B and D). In addition, pea wilt disease did not occur in either field in the following crop season (2020). These results suggest that our specific detection technique will also be useful for evaluating the effectiveness of soil disinfestation.
It is currently necessary to take prompt and appropriate action against the outbreak of pathogens. The detection technique established in the present study may be used to minimize the damage caused by Fop by continuously monitoring fields with a history of disease outbreaks. The present study is not only important for the epidemiology of newly emerging pathogens, but also provides important insights into management of the Fop pathogen.
Kotera, S., Hishiike, M., Saito, H., Komatsu, K., and Arie, T. (2022) Differentiation of the Pea Wilt Pathogen Fusarium oxysporum f. sp. pisi from Other Isolates of Fusarium Species by PCR. Microbes Environ 37: ME21061.
https://doi.org/10.1264/jsme2.ME21061
We deeply appreciate Dr. Antonio Di Pietro, Cordoba University (Cordoba, Spain) for providing F. oxysporum f. sp. lycopersici isolate 4287, Dr. Norio Kondo for providing MAFF 241054, and the YPPS for providing the 20 F. oxysporum isolates. Some of the results of the present study were presented as an abstract at the Symposium for the Japanese Society of Soil Microbiology (Kotera et al., 2021). This research was supported by the Science and Technology Research Promotion Program of MAFF (29031C) to T.A.; Grants-in-Aid (19H00939 and 16H02536) for Scientific Research from the Japan Society for the Promotion of Science to T.A.; The Program on Open Innovation Platform Enterprises Research Institute and Academia (OPERA; JPMJOP1833) from the Japan Science and Technology Agency (JST) for Dr. Kazuhiko Misawa and T.A.; a Project grant from the Japanese Society of Soil Microbiology to S.K.; and the Doctoral Program for World-leading Innovative & Smart Education of the Tokyo University of Agriculture and Technology, granted by the Ministry of Education, Culture, Sports, Science and Technology to S.K.