2022 Volume 37 Issue 1 Article ID: ME21076
2022 Volume 37 Issue 1 Article ID: ME21076
Pseudomonas syringae pv. tabaci 6605 (Pta6605) is a foliar plant pathogen that causes wildfire disease on tobacco plants. It requires chemotaxis to enter plants and establish infection. While chemotactic signals appear to be the main mechanism by which Pta6605 performs directional movement, the involvement of aerotaxis or energy taxis by this foliar pathogen is currently unknown. Based on domain structures and similarity with more than 50 previously identified putative methyl-accepting chemotaxis proteins (MCPs), the genome of Pta6605 encodes three potential aerotaxis transducers. We identified AerA as the main aerotaxis transducer and found that it possesses a taxis-to-serine-and-repellent (Tsr)-like domain structure that supports a periplasmic 4HB-type ligand-binding domain (LBD). The secondary aerotaxis transducer, AerB, possesses a cytosolic PAS-type LBD, similar to the Aer of Escherichia coli and Pseudomonas aeruginosa. Aerotaxis ability by single and double mutant strains of aerA and aerB was weaker than that by wild-type Pta6605. On the other hand, another cytosolic PAS-type LBD containing MCP did not make a major contribution to Pta6605 aerotaxis in our assay system. Furthermore, mutations in aerotaxis transducer genes did not affect surface motility or chemotactic attraction to yeast extract. Single and double mutant strains of aerA and aerB showed less colonization in the early stage of host plant infection and lower biofilm production than wild-type Pta6605. These results demonstrate the presence of aerotaxis transducers and their contribution to host plant infection by Pta6605.
Pseudomonas syringae is a foliar Gram-negative pathogenic bacterium that comprises more than 50 pathovars and, thus, causes diseases in various plant species (Mansfield et al., 2012). One pathovar that causes wildfire disease in tobacco plants, P. syringae pv. tabaci 6605 (Pta6605), is exceptionally motile and destructive to its host plants (Ichinose et al., 2013). Many factors involved in Pta6605 virulence have been identified, including flagellar motility, effector proteins, phytotoxins, extracellular polysaccharides, and a multidrug resistance efflux pump (Ichinose et al., 2013). However, as a foliar pathogen, Pta6605 needs to navigate the leaf surface and enter apoplastic spaces through natural openings, such as open stomata or wounds. Flagella-mediated motility and chemotaxis play major roles in this process.
Motile bacteria perform chemotaxis as one of their sophisticated abilities to adapt to a hostile and ever-changing environment. Chemotaxis is the directed movement of cells toward a favorable life-sustaining condition (positive chemotaxis) and away from harmful substances (negative chemotaxis) (Sourjik and Wingreen, 2012). Chemotaxis requires the binding of chemical signals to the ligand-binding domain (LBD) of a chemoreceptor, a methyl-accepting chemotaxis protein (MCP), and this triggers downstream signaling that is relayed to a two-component system comprising the histidine kinase, CheA, and the response regulator, CheY, which regulates flagellar rotation (Bi and Lai, 2015). Bacterial chemotaxis has been extensively examined in model organisms such as Escherichia coli and P. aeruginosa (Parkinson et al., 2015; Sampedro et al., 2015). We recently investigated the role of genes encoding the core chemotaxis proteins (CheA and CheY) for Pta6605 virulence, and found that chemotaxis-defective mutants were less motile and unable to establish an infection (Tumewu et al., 2021b).
Aerotaxis, often called energy taxis, is performed by bacterial cells in response to the ever-changing environment (Schweinitzer and Josenhans, 2010). Although aerotaxis signaling induces a behavioral response to oxygen, its complex signaling mechanisms have not yet been elucidated. When bacteria inhabit a microenvironment, the level of oxygen will continue to decrease during bacterial multiplication. The use of oxygen triggers electron transport, which, in turn, regulates the proton motive force. To guide bacterial cells toward oxygen, aerotaxis transducer proteins sense increasing levels of electron transport or the proton motive force (Rebbapragada et al., 1997; Edwards et al., 2006). Similar to chemotaxis, an aerotaxis signaling pathway was reported to require a signaling relay mediated by CheA, CheY, the coupling protein CheW, and a dedicated MCP-like transducer protein (Rowsell et al., 1995). Downstream signaling upon ligand sensing is highly similar to chemotaxis, as described above.
MCPs function as specific sensors for various physicochemical signals. An MCP typically has an LBD, transmembrane domains (TMDs), a histidine kinase, adenyl cyclase, methyl-accepting chemotaxis protein and phosphatase (HAMP) domain, and a signaling domain (SD) (Ud-Din and Roujeinikova, 2017). The ligand-sensing specificity of an MCP is generally influenced by the type of LBD. Chemoreceptor proteins may be grouped into seven types based on the localization of the LBD and the presence and number of TMDs (Ud-Din and Roujeinikova, 2017). The aerotaxis transducer in E. coli, Aer, which senses the redox status of the electron transport system, has been intensively investigated. Aer contains a cytoplasmic PAS-type LBD and is embedded in the cell membrane by TMDs (Edwards et al., 2006; Fig. 1D). This topological organization is common in other aerotaxis transducers, such as Aer of Pseudomonas putida (Nichols and Harwood, 2000) and P. aeruginosa (Hong et al., 2004), Aer-1 of Vibrio cholerae (Boin and Häse, 2007), Aer-1 and Aer-2 of Ralstonia solanacearum (Yao and Allen, 2007), and Aer1-1 and Aer1-2 of Pseudomonas chlororaphis (Arrebola and Cazorla, 2020). On the other hand, atypical Aer transducers were also found in AerC of Azospirillum brasilense (Xie et al., 2010) and Aer-2 of V. cholerae (Boin and Häse, 2007) and P. aeruginosa (Hong et al., 2004). Due to the absence of TMDs, these Aer transducers are soluble proteins that are expected to localize in the cytoplasm. This type of Aer consists of a HAMP domain, PAS domain, and SD. The PAS domain of the Aer transducers described above is associated with a flavine adenine dinucleotide (FAD) cofactor, which allows them to sense changes in the redox status of the electron transport system. On the other hand, the taxis to serine and repellent (Tsr) of E. coli does not possess a PAS-type LBD; it supports a periplasmic 4-helix-bundle (4HB)-type LBD. Tsr presumably senses the proton motive force directly or indirectly (Rebbapragada et al., 1997).
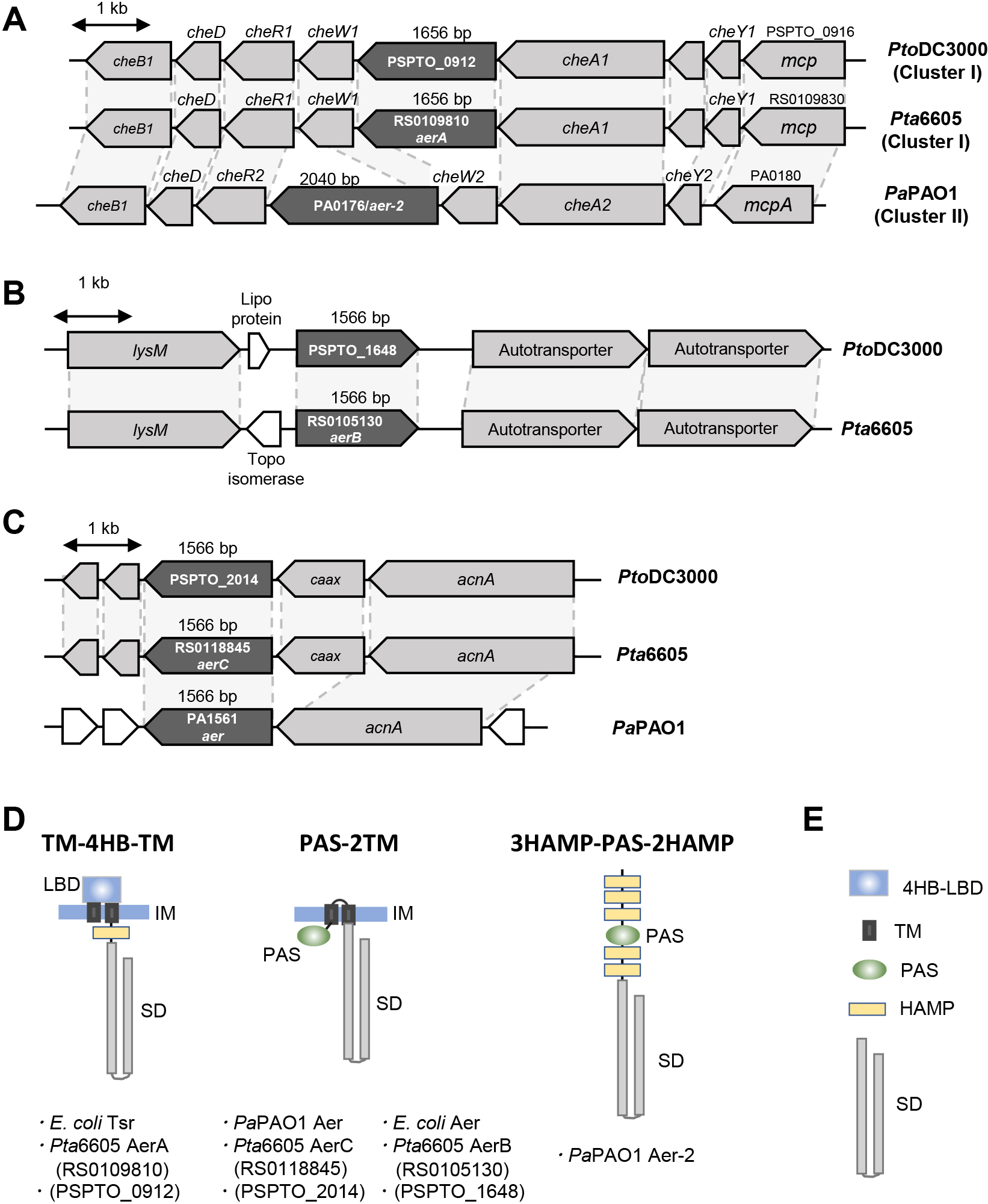
Predicted aer transducer genes of Pta6605 and their homologs in the closely related plant pathogen PtoDC3000 and animal pathogen PaPAO1. Potential aer transducer genes were depicted by dark gray pentagons and each ortholog is connected with a shadow background. Schematic organization of che gene clusters including RS0109810 (aerA) (A), RS0105130 (aerB) (B), RS0118845 (aerC) (C), and the surrounding genes in Pta6605, and their orthologs in PtoDC3000 and PaPAO1. (D) Schematic model of the domain organization of predicted Aer transducer proteins in Pta6605 and reference proteins in E. coli and PaPAO1. Each domain is explained in E. (E) Blue rectangles indicate the 4HB-type LBD; small black rectangles indicate transmembrane domains (TMDs), green ovals indicate the PAS-type LBD; cream rectangles indicate HAMP domains, and two consecutive gray rectangles indicate signaling domains (SDs).
Chemotaxis plays an important part in infection and disease development caused by animal and plant pathogens (Matilla and Krell, 2018). In plant-infecting bacteria, chemotaxis towards plant-derived compounds is considered to facilitate entry into plants through natural openings or wounds (Matilla and Krell, 2018). Aerotaxis itself has been associated with effective and efficient attachment and colonization by the biocontrol bacteria A. brasilense (Greer-Phillips et al., 2004) and P. chlororaphis (Arrebola and Cazorla, 2020) and the soil-borne pathogen R. solanacearum (Yao and Allen, 2007). We assume that Pta6605, as a foliar plant pathogen, also performs aerotaxis to reach a potential niche that supports its survival on the harsh foliar plane. However, there is currently no information on aerotaxis by foliar plant pathogens. Therefore, we herein identified two functional aerotaxis transducers in Pta6605, AerA and AerB. AerA showed similarity to the Tsr of E. coli, while AerB resembled the typical PAS-domain-containing transmembrane Aer proteins. Aerotaxis is also required for early colonization in host plants and plays a role in the formation of biofilms by this foliar plant pathogen.
P. syringae pv. tabaci 6605 strains were maintained in King’s B medium supplemented with nalidixic acid (Nal) (Shimizu et al., 2003). E. coli strains used in DNA recombination and mutant construction were maintained in Luria-Bertani (LB) medium supplemented with appropriate antibiotics. The bacterial strains and plasmids used in the present study are listed in Table 1.
| Bacterial strain, plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Escherichia coli | ||
| DH5α | F–λ– ϕ80dLacZ ΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK– mK+) supE44 thi-1 gyrA relA1 | Nippon Gene |
| S17-1 | thi pro hsdR hsdR hsdM+ recA(chr::RP4-2-Tc::Mu-Km::Tn7) | Schäfer et al. 1994 |
| S17-1 λpir | λpir lysogeny of S17-1 | Simon et al. 1983 |
| P. syringae pv. tabaci | ||
| Isolate 6605 | Wild-type isolated from tobacco, fully virulent, Nalr | Shimizu et al. 2003 |
| ΔaerA | Isolate 6605 ΔRS0109810, Nalr | This study |
| ΔaerA-C | pB-aerA introduced aerA, Nalr Kmr | This study |
| aerA | Isolate 6605 insertional mutant in RS0109810, Nalr | This study |
| aerA-C | pB-aerA introduced aerA, Nalr Kmr | This study |
| ΔaerB | Isolate 6605 ΔRS0105130, Nalr | This study |
| ΔaerB-C | pD-aerB possessing ΔaerB, Nalr Kmr | This study |
| ΔaerC | Isolate 6605 ΔRS0118845, Nalr | This study |
| aerAΔaerB | Isolate 6605 double mutation in RS0109810 and RS0105130, Nalr | This study |
| Plasmid | ||
| pGEM-TEasy | Cloning vector, Ampr | Promega |
| pG-RS0109810 | RS0109810 fragment-containing pGEM-T Easy, Ampr | This study |
| pG-ΔRS0109810 | ΔRS0109810 fragment-containing pGEM-T Easy, Ampr | This study |
| pG-RS0109810m | Mutated RS0109810 fragment-containing pGEM-T Easy, Ampr | This study |
| pG-RS0105130 | RS0105130 fragment-containing pGEM-T Easy, Ampr | This study |
| pG-ΔRS0105130 | ΔRS0105130 fragment-containing pGEM-T Easy, Ampr | This study |
| pG-RS0118845 | RS0118845 fragment-containing pGEM-T Easy, Ampr | This study |
| pG-ΔRS0118845 | ΔRS0118845 fragment-containing pGEM-T Easy, Ampr | This study |
| pK18mobSacB | Small mobilizable vector, Kmr, sucrose sensitive (sacB) | Schäfer et al. 1994 |
| pK18-ΔRS0109810 | RS0109810 deleted DNA-containing pK18mobsacB, Kmr | This study |
| pK18-RS0109810m | Mutated RS0109810 fragment-containing pK18mobsacB, Kmr | This study |
| pK18-ΔRS0105130 | RS0105130 deleted DNA-containing pK18mobsacB, Kmr | This study |
| pK18-ΔRS0118845 | RS0118845 deleted DNA-containing pK18mobsacB, Kmr | This study |
| pDSK519 | Broad host range cloning vector, Kmr | Keen et al. 1988 |
| pBSL118 | Mini-Tn5-derived plasmid vector for insertion mutagenesis, Ampr, Kmr | Alexeyev et al. 1995 |
| pB-aerA | pBSL118 possessing expressible aerA, Kmr | This study |
| pD-aerB | pDSK519 possessing expressible aerB, Kmr | This study |
Nalr, nalidixic acid-resistant; Ampr, ampicillin-resistant; Kmr, kanamycin-resistant.
To evaluate the function of aer gene products in Pta6605, several series of mutant strains were constructed. The genetic regions of A3SK_RS0109810 (hereinafter referred to as RS0109810), A3SK_RS0105130 (RS0105130), and A3SK_RS0118845 (RS0118845), including the upstream and downstream regions, were amplified using a series of primer pairs and isolated using a TA cloning system (pGEM T-Easy; Promega) to obtain pG-RS0109810, pG-RS0105130, and pG-RS0118845, respectively. All primers used in the present study are listed in Table 2. Inverse PCR was performed to delete each open reading frame (ORF) using each set of primers. After transformation, the deleted constructs of the plasmids obtained, pG-ΔRS0109810, pG-ΔRS0105130, and pG-ΔRS011884, were confirmed by PCR and DNA sequencing. Mutated DNA fragments were excised and subcloned into pK18mobsacB (Schäfer et al., 1994), and the resultant plasmids, pK18-ΔRS0109810, pK18-ΔRS0105130, and pK18-ΔRS0118845, were introduced into E. coli S17-1. Mutations were introduced by the conjugation of Pta6605 wild-type (WT) and plasmid-possessing E. coli S17-1 with subsequent homologous recombination, and each deletion mutant was selected on King’s B agar plates supplemented with 10% sucrose. To define each mutant, we assigned names to the encoding genes as follows: aerA for RS0109810, aerB for RS0105130, and aerC for RS0118845. Each deletion mutant, ΔaerA, ΔaerB, and ΔaerC, was confirmed by PCR.
| Primer Name | Sequence (5′--3′) | Description |
|---|---|---|
| RS0109810_a | GCTGACGCTGGCGATCATC | Amplification of RS0109810 ORF for insertional mutagenesis |
| RS0109810_b | GGTGACTTTGGCGTTCTCGG | |
| RS0109810_1 | AGTACGTGATGTCAGTCAGG | Amplification of RS0109810 and the surrounding region |
| RS0109810_2 | AACCGACCACTTCCCAAGG | |
| RS0109810_3 | CTAgctagcTCCTTGGGAATTGCGAATCCG | Deletion of RS0109810 ORF |
| RS0109810_4 | CTAgctagcCGGCTTCGATAGAGACTCCA | |
| RS0109810_5 | CCAGGAAAAGGCGCAGATGGAAGCTTGAAGCAGCGGAC | Insertional mutagenesis of RS0109810 |
| RS0109810_6 | GTCCGCTGCTTCAAGCTTCCATCTGCGCCTTTTCCTGG | |
| RS0109810_7 | GGGTTCGATCCTTGAACAGTGCAGC | Amplification of RS0109810 and the promoter region for complementation |
| RS0109810_8 | TCAGGGCAGGATCAGCTTGGAAACC | |
| RS0105130_1 | TTACAGTGCGGACACGCTGG | Amplification of RS0105130 and the surrounding region |
| RS0105130_2 | CCAAATGGAGTCTGCGTTACGG | |
| RS0105130_3 | CGCggatccGGTTCAGTCGCTAAGCATGC | Deletion of RS0105130 ORF |
| RS0105130_4 | CGCggatccATAGGCATGTTGACGCGCAT | |
| RS0118845_1 | CCTCGCATTGGCCTTTCATC | Amplification of RS0118845 and the surrounding region |
| RS0118845_2 | TGGCGCAAGCAGTGCTGC | |
| RS0118845_3 | GAagatctGACTTGAGACTGTTTTCAACGCC | Deletion of RS0118845 ORF |
| RS0118845_4 | GAagatctTGGGTTTCGATCCTTCGATC |
Bold letters indicate artificial nucleotides for the insertional mutagenesis of RS0109810 ORF in RS0109810_5 and RS0109810_6. Lowercase letters indicate the artificial nucleotide sequence for NheI in RS0109810_3 and RS0109810_4, BamHI in RS0105130_3 and RS0105130_4, and BgIII in RS0118845_3 and RS0118845_4.
Regarding the aerA gene, we generated not only a deletion mutant, but also a deoxyadenosine monophosphate (A) insertion mutant. Using pG-RS0109810 as a template and two complementary mutated oligonucleotides, as shown in Table 2, the site-directed mutation of RS0109810 was introduced by PCR and pG-RS0109810m was generated. Conjugation and homologous recombination were performed using Pta6605 WT and E. coli S17-1 possessing pK18-RS0109810m. After 10% sucrose selection, we performed colony PCR to amplify RS0109810 genomic DNA and digested the PCR product using HindIII. The HindIII site was newly generated by the insertion of an “A”. This insertion caused a frameshift mutation, and the 87th–88th amino acids were changed to alanine and a stop codon (Fig. S1).
To generate the double mutant of aerA/ΔaerB, a mutation in ΔaerB was introduced into an aerA-defective mutant strain. To complement this mutation, the full-length aerA gene was subcloned into the transposon vector pBSL118 at the EcoRI site (Alexeyev and Shokolenko, 1995). The expression vector pDSK519 (Keen et al., 1988) was used to subclone the aerB gene at the EcoRI site. Constructs were transformed into the E. coli S17-1 λpir strain (Simon et al., 1983) for conjugation to the respective mutant strains. The resulting strains were screened by PCR and were resistant to Nal and kanamycin (NalrKmr).
Aerotaxis assayAerotaxis was assayed based on the air trap scheme of Arrebola and Cazorla (2020) with several modifications. Before the experiment, modified Pasteur pipettes and 3 mL of LB medium containing 0.1% agar in sterile glass test tubes were prepared. Bacterial strains were precultured in KB liquid medium supplemented with Nal for approximately 20 h. Bacterial cells were washed twice by centrifugation and resuspended in 10 mM of HEPES buffer (pH 7.4) at an OD600 of 1.0. The bacterial suspension (150 μL) was injected into the LB broth outside the Pasteur pipette. To create an air trap inside the Pasteur pipette, 1 mL of paraffin oil was carefully overlaid on the LB broth outside the Pasteur pipette. A schematic representation of the experimental set-up is shown in Fig. 2A. Test tubes were incubated at 27°C for 24 h without any agitation. Photographs were taken after the incubation, 150 μL of the medium inside the Pasteur pipette was then collected, and bacterial populations were counted.

Aerotaxis assay of WT, aer mutant, and complemented strains of Pta6605. (A) Experimental set-up for the aerotaxis assay. Using a test tube and modified Pasteur pipette containing dense LB broth, a bacterial suspension was inoculated outside the pipette in addition to liquid paraffin to create an air-trap environment. (B) Qualitative observation of aerotaxis assay results after a 24-h static incubation at 27°C. Red squares refer to dense bacteria in LB medium that are in contact with air inside the Pasteur pipette. (C) Quantitative analysis of bacterial populations moved to LB medium in contact with air inside the Pasteur pipette. The bacterial population was expressed as log CFU mL–1. Asterisks indicate significant differences between WT and mutant strains at ***P<0.001 or **P<0.01 by Dunnett’s multiple comparison test. Error bars represent standard errors from two independent experiments conducted in triplicate. ns: not significant.
To investigate the surface motility of aer mutant strains, motility assays were performed on semisolid agar medium as previously described (Tumewu et al., 2020). Bacterial strains were cultured overnight in LB broth supplemented with 10 mM MgCl2 and resuspended in 10 mM of MgSO4 at an OD600 of 0.1. In the swimming assay, 3 μL of the bacterial suspension was spotted onto the center of 0.25% agar minimal medium (MM; 50 mM potassium phosphate buffer, 7.6 mM (NH4)2SO4, 1.7 mM MgCl2 and 1.7 mM NaCl, pH 5.7) supplemented with 10 mM each of mannitol and fructose (MMMF) and then incubated at 23°C for 72 h. The same amount of the bacterial suspension was spotted onto SWM plates (0.45% agar, 0.5% peptone, and 0.3% yeast extract; Difco) and incubated at 27°C for 48 h for a swarming assay. Photographs were taken at the end of the incubation.
Chemotaxis assayA quantitative chemotaxis assay was performed as described in our previous study (Tumewu et al., 2020). To prepare bacterial cells, each strain was cultured in LB medium supplemented with 10 mM MgCl2 overnight. After washing with MM, bacterial cells were resuspended in MMMF broth and incubated for another 5 h to starve cells. After the incubation, cells were washed and resuspended in 10 mM of HEPES buffer (pH 7.4) at an OD600 of 0.05. To prepare attractant-containing capillaries, one end of a glass capillary (5 μL; Drummond Scientific) was sealed with a flame and dipped in attractant solution. Prepared capillaries were submerged in 200 μL of the bacterial suspension in a 96-well microtiter plate. After a 30-min incubation at 27°C, capillaries were washed, and the content was pushed out using a dedicated plunger. Bacterial colony-forming units (CFUs) were then counted after serial dilutions and plating on KB with Nal plates.
Biofilm formation assayTo quantify the ability of cells to attach to a polypropylene surface, a biofilm assay was performed. Each strain was cultured overnight in LB broth with 10 mM MgCl2. Bacterial cells were washed and resuspended in MMMF broth at an OD600 of 0.1. The bacterial suspension (200 μL) was dispensed in a polypropylene microtiter plate (Corning) and incubated at 27°C for 48 h without shaking. Cells that adhered to the polypropylene microtiter plate were stained with crystal violet, and the pigment in stained cells was extracted by 95% ethanol. Absorbance was measured at 595 nm using an iMark™ Microplate Absorbance Reader (Bio-Rad Laboratories) (Tumewu et al., 2021a).
Host plant and virulence assayThe virulence of WT and mutant strains was analyzed using a flood inoculation method as described in our previous study (Tumewu et al., 2020). Briefly, gas-sterilized tobacco seeds (Nicotiana tabacum L. cv. Xanthi NC) were sown on Murashige-Skoog (MS) plates supplemented with 1% sucrose and vitamin solution (thiamin hydrochloride 3 mg L–1, nicotinic acid 5 mg L–1, and pyridoxine hydrochloride 0.5 mg L–1). After a 2-week incubation, seedlings were transplanted into 0.1% sucrose MS plates and grown for a further 3 d. The inoculum was prepared in 10 mM MgSO4 at an OD600 of 0.004 (8×106 CFU mL–1) supplemented with 0.025% (v/v) Silwet L-77 (OSI Specialties) to promote cell attachment. Tobacco seedlings were flooded by the inoculum for approximately 10 s. The inoculum was decanted, and the plate was left to dry inside a clean bench for 15 min. After 6 h and 3 d of the incubation at 22°C (with a long photoperiod), 2 leaf disks (Ø 4 mm) were collected and ground in 1 mL of sterilized water. After serial dilutions, the suspension was spotted on KB agar in Nal plates. Recovered bacterial CFUs were assessed after a 2-d incubation at 27°C. The fresh weight of the seedlings (mg) was also measured at 6 d post-inoculation (dpi). The aerial part of each seedling was cut and gently blotted with a paper towel to remove excess moisture and then immediately weighed. Photos of seedlings were taken at 3 and 6 dpi.
Data analysisThe significance of differences in aerotaxis ability, chemotaxis ability, biofilm formation, and bacterial populations between mutant strains and WT was examined using an analysis of variance (ANOVA), followed by Dunnett’s test at the 95% confidence level. Analyses of all data were performed using GraphPad Prism ver. 9 (GraphPad Software). P<0.05 was considered to be significant.
Pta6605 has more than 50 genes encoding putative MCPs in its genome. Since P. aeruginosa and P. syringae strains conserve high DNA sequence synteny, we searched for the corresponding orthologs of the aer and aer-2 genes of P. aeruginosa PAO1 (PaPAO1) (Hong et al., 2004) in Pta6605. The aer gene (PA1561) in PaPAO1 was located downstream of acnA (gene encoding aconitate hydratase 1), and the mcp genes PSPTO_2014 in P. syringae pv. tomato DC3000 (PtoDC3000) and RS0118845 in Pta6605 were located in similar loci; however, a single gene encoding the CAAX amino terminal protease family protein was located between the mcp and acnA genes in P. syringae (Fig. 1C). Amino acid identities among Aer in PaPAO1, MCPs encoded by PSPTO_2014, and RS0118845 were more than 77%, and MCPs encoded by PSPTO_2014 and RS0118845 showed 96.4% amino acid identity. Aer also showed 50% amino acid similarity to MCPs encoded by PSPTO_1648 and RS0105130, and MCPs encoded by PSPTO_1648 and RS0105130 showed 96.0% amino acid identity to each other. Furthermore, PSPTO_1648 and RS0105130 were identified at the same gene locus (Fig. 1B). The MCPs described above had a similar overall structure. Although Aer of PaPAO1 showed homology to many MCPs in PtoDC3000 and Pta6605, homology was restricted in the SD. The deduced structures of the above proteins belongs to the “PAS-2TM-SD” type of the MCP family, which, in turn, belongs to topology type II by the classification of Ud-Din and Roujeinikova (2017). Originally named PAS for three different eukaryotic proteins, Per-ARNT-Sim (period, hydrocarbon receptor nuclear translocator, single-minded) is cytosolic and diverse in many organisms, and includes a transducer molecule that senses light, oxygen, and redox signals (Schweinitzer and Josenhans, 2010; Ortega et al., 2017). Aer in E. coli was also shown to conserve the PAS-2TM-SD structure (Rebbapragada et al., 1997); therefore, the structure of Aer of PaPAO1 resembles that of Aer of E. coli (Bibikov et al., 2000), although the latter has a HAMP domain before the SD (Fig. 1D). There were no other genes encoding “PAS-2TM-SD” type MCP in PtoDC3000 or Pta6605.
PaPAO1 has been shown to possess another aerotaxis transducer gene, aer-2 (Hong et al., 2004). The aer-2 gene (PA0176) was located on the chemotaxis gene cluster as one ORF of a large operon (Fig. 1A). This gene cluster in PaPAO1 was well conserved in PtoDC3000 and Pta6605, and the deduced amino acid sequences in each ortholog were very similar, except for one; however, gene names (number) and cluster numbers were defined in a different manner. In the corresponding locus of the aer-2 gene of PaPAO1, we found the mcp genes, PSPTO_0912 in PtoDC3000 and RS0109810 in Pta6605 (Fig. 1A). However, the predicted structures of Aer-2 of PaPAO1 and MCPs encoded by PSPTO_0912 and RS0109810 were very different, and neither PtoDC3000 nor Pta6605 had similar genes to the aer-2 gene in any genomic area. Aer-2 of PaPAO1 was classified as a topology type IVa MCP (Ud-Din and Roujeinikova, 2017), and the structure was very unusual. Aer-2 of PaPAO1 is a cytosolic protein composed of three HAMP domains followed by a PAS domain, two HAMP domains, and the SD (3HAMP-PAS-2HAMP-SD) (Watts et al., 2011). PSPTO_0912 and RS0109810 both encoded topology type Ia, and the LBD type was 4HB (Fig. 1D). The topology type Ia MCP harbored a periplasmic LBD, two TM domains, and a cytosolic SD. Since the topology types of Aer-2 of PaPAO1 and MCPs encoded by PtoDC3000 and Pta6605 differed, amino acid identity was restricted in only the SD to approximately 40%. The structures of MCPs encoded by PSPTO_0912 and RS0109810 resembled the Tsr of E. coli. Tsr is an energy taxis sensor that responds to serine, carbon sources, pH, temperature, and oxygen (Schweinitzer and Josenhans, 2010). Therefore, in addition to RS0105130 (aerB) and RS0118845 (aerC), RS0109810 (aerA) may be a gene that encodes a potential aerotaxis transducer in Pta6605.
Aerotaxis of WT and aer mutant strains in Pta6605We initially generated deletion mutants of the aerA, aerB, and aerC genes. In the aerotaxis assay, although a high density of the WT strain was observed inside the Pasteur pipette in contact with air, the ΔaerA mutant was not present, indicating that the positive aerotaxis activity of the WT strain was lost in the ΔaerA mutant (Fig. S2A). Furthermore, the ΔaerA mutant abolished both surface swarming and swimming motilities and did not cause disease. However, when the intact aerA gene was introduced into the ΔaerA mutant, the complemented strain did not restore the WT phenotype in any experiment (Fig. S2A). We also conducted a chemotaxis assay to a known attractant, yeast extract, and found that neither the ΔaerA mutant nor ΔaerA-C exhibited chemotaxis activity (Fig. S2B). Therefore, the cause of the altered phenotype in the ΔaerA mutant was not the loss of AerA function; this phenotype may be due to a polar effect of the deletion of the aerA gene. Therefore, the aerA mutant was regenerated by the introduction of a stop codon in the aerA ORF.
Aerotaxis by the aerA, ΔaerB, and ΔaerC mutant strains was investigated. As shown in Fig. 2A, dense bacteria were observed in the upper medium in contact with air for the WT and ΔaerC mutant strains, but not for the aerA, ΔaerB, or aerA/ΔaerB mutant strains. Furthermore, the accumulation of bacterial cells was noted for the complemented strains aerA-C and ΔaerB-C (Fig. 2A). To quantitatively analyze the level of aerotaxis, the bacterial population was measured (Fig. 2B). The level of impairment in aerotaxis was more severe for the aerA mutant and aerA/ΔaerB mutant strains, while that for the ΔaerB mutant was mild. The bacterial population of the ΔaerC mutant was similar to that of the WT strain, and the bacterial populations of aerA-C and ΔaerB-C had almost recovered to the WT level.
Aerotaxis and chemotaxis signal transduction pathwayIt currently remains unclear whether Pta6605 needs a general chemotaxis signal transduction pathway to perform aerotaxis. Therefore, we examined the requirement for a two-component system (CheA and CheY) using four mutant strains (ΔcheA1, ΔcheA2, ΔcheY1, and ΔcheY2; Tumewu et al., 2021b) from the two major chemotaxis clusters in the aerotaxis assay. Aerotaxis to atmospheric air was examined using the air trap scheme described in the Methods section. ΔcheA2 and ΔcheY2 mutants, lacking a functional cluster II two-component system, did not move into the Pasteur pipette, in which the surface of the LB medium was in contact with air (Fig. 3A). Aerotaxis by the cluster I mutants, ΔcheA1 and ΔcheY1, was reduced, but not to the same extent as that by cluster II mutants. This result was also quantitatively supported. Significantly fewer bacterial CFU were recovered from medium inside the Pasteur pipette for the ΔcheA2 and ΔcheY2 strains than for the WT strain (Fig. 3B). In contrast, more bacterial CFU were recovered for the ΔcheA1 and ΔcheY1 mutants than for the ΔcheA2 and ΔcheY2 mutants, but less than WT. These results suggest that aerotaxis by Pta6605 requires the signal to be processed by the general chemotaxis pathway.

Aerotaxis assay of the WT strain and che mutant strains of Pta6605. (A) Qualitative observation of aerotaxis assay results after a 24-h static incubation at 27°C. Red squares refer to dense bacteria in the LB medium that are in contact with air inside the Pasteur pipette. (B) Quantitative analysis of bacterial populations that moved to LB medium in contact with air inside the Pasteur pipette. The bacterial population was expressed as log CFU mL–1. Asterisks indicate significant differences between the WT and mutant strains at ***P<0.001 by Dunnett’s multiple comparison test. Error bars represent standard errors from two independent experiments conducted in triplicate.
To rule out the possibility that general motility by the mutants was impaired, we examined their surface motility and chemotactic attraction to yeast extract. Swarming and swimming motilities were investigated on 0.45% agar SWM plates and 0.25% agar MMMF plates, respectively. As shown in Fig. 4A, WT, the aerA mutant, ΔaerB mutant, and double mutant showed similar swarming patterns on SWM plates, while the ΔaerC mutant produced a rather smooth pattern without the formation of tendrils. On swimming MMMF plates, all strains showed similar motility, except for the ΔaerC mutant, which had a larger swimming diameter (Fig. 4A). A chemotaxis assay to 1% yeast extract was conducted using a quantitative capillary method (Tumewu et al., 2021b). All mutant strains were attracted to yeast extract, similar to the WT strain (Fig. 4B), suggesting that mutations in aerotaxis transducer genes did not significantly affect general motility or the core chemotaxis system.

Surface motility and chemotaxis of the WT strain and aer mutant strains of Pta6605. (A) Surface swarming motility was assessed on 0.45% agar SWM plates and swimming motility in 0.25% agar MMMF plates. Photographs were taken at 48 hpi for swarming plates and at 72 hpi for swimming plates. Photographs shown are representative of three biological repeats each with three technical replicates. (B) Quantitative chemotaxis assay towards 1% yeast extract solution. Bacterial cells that move towards the attractant in capillaries were measured on KB plates supplemented with Nal. No significant differences were observed between the WT and mutant strains by Dunnett’s multiple comparison test. Error bars indicate standard errors from two independent experiments conducted in triplicate.
The Pta6605 chemotaxis system plays a significant role in virulence (Tumewu et al., 2021b). Furthermore, mutations in the mcp genes for γ-aminobutyric acid and amino acids had a prominent impact on Pta6605 fitness in host plants (Tumewu et al., 2020; 2021a). To elucidate the role of aerotaxis on virulence by Pta6605, a flood inoculation assay was performed. As shown in Fig. 5A, WT and mutant strains still caused severe disease symptoms, although the seedlings inoculated with the aerA mutant, ΔaerB mutant, and double mutant strains appeared to be slightly bigger than those inoculated with WT. Therefore, we measured bacterial populations that successfully entered the leaves as well as the fresh weight of seedlings. Fig. 5B shows that at 6 h post-inoculation (hpi), fewer bacterial populations were recovered from seedlings inoculated with mutant strains than with WT; however, these differences were not significant. Differences in the aerA mutant, ΔaerB mutant, and aerA/ΔaerB mutant were significant at 3 dpi. Except for the ΔaerC mutant, the fresh weights of seedlings inoculated with the mutant strains were approximately 2-fold those inoculated with the WT strain (Fig. 5C). This result suggests that aerotaxis plays a minor, but important role in the early stage of Pta6605 invasion into a host plant.

Flood inoculation assay of WT strain and aer mutant strains of Pta6605 on host tobacco seedlings. (A) Symptom development of wildfire disease on host tobacco seedlings at 3 dpi and 6 dpi. The inoculum was 8×106 CFU mL–1 of each bacterial strain, and the incubation was performed at 22°C with a long photoperiod. (B) Bacterial populations recovered from the inoculated seedlings at 6 hpi (white boxes) and 3 dpi (gray boxes). Error bars represent standard errors from two independent experiments with 3 seedlings for 6 hpi and 7 seedlings for 3 dpi. Asterisks indicate significant differences from WT at each time point by Dunnett’s multiple comparison test (*P<0.05; ***P<0.001). (C) Fresh weight of seedlings at 6 dpi. Error bars represent the standard error of the mean from 2 biological repeats each with 7 individual seedlings. Asterisks indicate significant differences from WT by Dunnett’s multiple comparison test (ns: not significant; **P<0.01; ***P<0.001).
Pta6605 forms a biofilm when transitioning from a planktonic to sessile lifestyle (Ichinose et al., 2013). As previously reported, aerotaxis contributed to pellicle formation when bacterial cells formed cell aggregates or a biofilm on medium-air contact surfaces (Hölscher et al., 2015). The present results revealed marked differences in biofilm densities between the aerA, ΔaerB, and aerA/ΔaerB mutants and the WT strain after a 48-h static incubation at 27°C (Fig. 6), indicating the role of aerotaxis in biofilm regulation in Pta6605.

Biofilm formation by WT and aer mutant strains of Pta6605. Biofilms were stained by crystal violet after a 48-h static incubation at 27°C. Quantification was performed by measuring the OD595 of a stained biofilm extracted with 95% ethanol. Error bars indicate standard errors from two independent experiments with five replicates. Asterisks indicate significant differences from the WT strain analyzed by Dunnett’s multiple comparisons test (***P<0.001). ns: not significant.
Among the approximately 50 putative chemoreceptor proteins in Pta6605, AerB and AerC are membrane-anchored MCPs with intracellular PAS-type LBD similar to the typical aerotaxis transducers Aer of E. coli, P. putida, and P. aeruginosa (Hong et al., 2004; Taylor, 2007). AerB and AerC showed 50.4 and 78.5% amino acid sequence identities, respectively, to Aer of P. aeruginosa, while AerB and AerC showed 50.2% amino acid sequence identity to each other. Although AerB exhibited aerotaxis activity, AerC did not, at least under the conditions used in the present study, and did not appear to be involved in Pta6605 aerotaxis. Nevertheless, since it is a PAS-type MCP, AerC may be another energy taxis chemoreceptor because AerC showed high identity (78.5%) to Aer of PaPAO1. Other PAS-type LBD-containing cytoplasmic MCPs have been reported, such as AerC of A. brasilense and Aer-2 of PaPAO1 (Hong et al., 2004; Xie et al., 2010). AerC of A. brasilense is a 2×PAS-SD-type MCP that functions as a redox sensor to support nitrogen fixation (Xie et al., 2010). On the other hand, as described above, Aer-2 of PaPAO1 is composed of 3HAMP-PAS-2HAMP-SD. Although Pta6605 does not have an ortholog of Aer-2 of PaPAO1, Pta6605 has four genes encoding cytoplasmic 2×PAS-SD type MCP (A3SK_RS0101665, A3SK_RS0107145, A3SK_RS0111330, and A3SK_RS0121510) and a gene encoding 4×PAS-SD type MCP (A3SK_RS0112230). Further studies are needed to clarify whether these intracellular PAS-SD-type MCPs respond to oxygen.
AerA has a periplasmic 4HB-type LBD (Fig. 1D). An MCP with 4HB-LBD is the most common MCP in Pta6605, and there are at least 16 genes encoding this type of MCP. McpS in P. putida and CtpH in P. aeruginosa are 4HB-LBD-possessing MCPs that are responsible for sensing TCA cycle intermediates (Pineda-Molina et al., 2012) and inorganic phosphate (Wu et al., 2000), respectively. Therefore, MCPs with periplasmic 4HB-type LBD appear to sense particular chemical compounds. However, Tsr with the 4HB-type LBD (Fig. 1D) is a secondary aerotaxis transducer in E. coli and directly or indirectly senses changes in proton motive force (Kim et al., 1999). Tsr not only senses oxygen (Rebbapragada et al., 1997), it responds to serine (Rebbapragada et al., 1997), carbon sources (Greer-Phillips et al., 2003), pH (Umemura et al., 2002), and temperature (Lee et al., 1988). Aer and Tsr in E. coli are both capable of sensing oxidizable carbon sources (Greer-Phillips et al., 2003). Therefore, it is plausible that AerA in Pta6605 functions as an aerotaxis transducer. The MCP of Pta6605, which has the highest amino acid identity for Tsr, is AerA. Although the identity of the amino acid sequence is not very high (32.8%), the homologous region is not only an SD, it is also the entire protein structure. Another reason why we investigated AerA is due to the location of the gene in the Pta6605 genome. As described in the Results section, the aerA gene was located in che cluster I; the homologs of che cluster II of P. aeruginosa and the locus of the aerA gene of Pta6605 and aer-2 gene of PaPAO1 were the same (Fig. 1A).
Involvement of the chemotaxis signal transduction pathway in aerotaxisChemotaxis gene clusters (che) generally comprise genes encoding proteins that are crucial for the signal transduction pathway of chemotaxis (Wuichet and Zhulin, 2010). Although Aer-mediated aerotaxis is independent of CheR and CheB methylation, the signal perceived by an Aer transducer is still able to induce CheA autophosphorylation and presumably affect downstream processes (Bibikov et al., 2004). Therefore, the presence of core chemotaxis proteins, such as CheA or CheY, may also be crucial for aerotaxis. In E. coli, CheA, CheW, and CheY are required for aerotaxis (Rowsell et al., 1995), and the presence of cheYZABW genes in che cluster I of PaPAO1 (orthologs of che cluster II in Pta6605) was confirmed to be essential for aerotaxis (Hong et al., 2004). Aerotaxis assays using four cheA and cheY mutant strains revealed that the group III chemotaxis gene cluster (cluster I in PaPAO1 and cluster II in Pta6605) was dominant in both chemotaxis and aerotaxis, and that the group II chemotaxis gene cluster (cluster II in PaPAO1 and cluster I in Pta6605) played a minor role in both chemotaxis and aerotaxis (Tumewu et al., 2021b and Fig. 3).
The deletion of the aerA gene resulted in the loss of aerotaxis ability, chemotaxis ability, swarming and swimming motilities, and virulence (Fig. S2). Since the aerA mutant retained swarming and swimming motilities, the phenotype of the ΔaerA mutant is the result of a polar effect. The aerA gene and cheA1 gene belong to the same che1 cluster in Pta6605 (Fig. 1A). We also found that the loss of virulence in the ΔcheA1 mutant of the same che1 cluster was not recovered by complementation of the cheA1 gene, indicating that a DNA sequence containing the cheA1 gene and aerA gene is required for the transcription and/or RNA stability of the che1 cluster in Pta6605 (Tumewu et al., 2021b).
The reduced aerotactic abilities of aerA and ΔaerB mutants were not due to impaired motility or chemotaxis because these mutant strains retained the same level of surface motility and chemotaxis as the WT strain (Fig. 4).
Importance of aerotaxis in the virulence of the foliar pathogen Pta6605Yao and Allen (2007) previously demonstrated the significant role of aerotaxis by Aer1 and Aer2 in the interaction between R. solanacearum and its host tomato plant. The aer2 mutant or aer1/aer2 mutant slightly delayed wilt disease on host tomato plants, indicating that R. solanacearum needs aerotaxis for normal interactions with host plants. However, R. solanacearum is a soilborne pathogen, and the role of aerotaxis or energy taxis for foliar plant pathogens, such as Pta6605, has yet to be investigated. In the present study, we identified two functional aerotaxis transducers in Pta6605. Our air trap scheme experiment revealed that Pta6605 WT moved toward and gathered at the medium-air contact point inside the Pasteur pipette, as observed in the photograph and bacterial population in Fig. 2. Populations of the aerA mutant, ΔaerB mutant, and double mutant strains were significantly smaller than that of the WT strain, indicating that AerA and AerB both contribute to aerotaxis. The population of the aerA mutant was slightly smaller than that of the ΔaerB mutant, indicating that AerA is the predominant transducer of aerotaxis.
In plant-infecting bacteria, chemotaxis towards plant-derived compounds is considered to facilitate entry into the plant through natural openings or wounds. Flagella-mediated motility is important for Pta6605 virulence because chemotaxis-defective mutants showed a significantly weaker ability to cause disease symptoms than WT (Tumewu et al., 2021b). Some chemoreceptors are associated with virulence, such as the GABA chemoreceptor McpG (Tumewu et al., 2020) and amino acid chemoreceptors PscB and PscC2 (Tumewu et al., 2021a). We hypothesized that aerotaxis transducers also play this role in the interaction between Pta6605 and its host plants. The Pta6605 aerA mutant, ΔaerB mutant, and double mutants caused slightly different disease symptoms (Fig. 5A). The bacterial populations of the mutant strains at 3 dpi were significantly smaller than that of WT, which may have been because fewer bacterial cells had entered the seedlings at the earlier time (Fig. 5B). In comparisons with the non-motile and avirulent ΔcheA2 mutant and ΔcheY2 mutant (Tumewu et al., 2021b), the non-aerotactic aerA mutant and ΔaerB mutant were still capable of causing severe disease symptoms, as observed at 6 dpi (Fig. 5A), and the marked decrease in the fresh weight of tobacco seedlings after the inoculation with the WT strain was slightly improved after the inoculation with the aerA mutant and ΔaerB mutant (Fig. 5C). The presence of many other MCPs in Pta6605 governing other types of taxis may facilitate the entry of pathogens into tobacco seedlings. Similar cases of biocontrol ability in P. chlororaphis and virulence in R. solanacearum were observed (Yao and Allen, 2007; Arrebola and Cazorla, 2020).
Plant pathogenic Pseudomonas produces a biofilm structure to ensure survival in a harsh environment (Heredia-Ponce et al., 2021). Pta6605 forms a biofilm and attaches to the host plant surface, providing a safe space to further the infection process by producing toxins and effectors. Biofilm formation by Pta6605 is regulated through various complex pathways, including flagella and pili-mediated motilities (Ichinose et al., 2013). The non-aerotactic aerA mutant, ΔaerB mutant, and double mutant strains showed less attachment, as indicated by the lower biofilm biomass on an abiotic surface (Fig. 6). Combined with the lower bacterial population found inside the seedlings, we inferred that the ability to form a biofilm may help Pta6605 attach to the host surface and effectively cause disease. The regulation of biofilm formation appears to differ among bacterial species. An aerotaxis mutant of the soilborne pathogen R. solanacearum overproduced biofilms (Yao and Allen, 2007), whereas biofilm formation by the Δaer1-1 and Δaer1-2 mutants of the rhizobacterium P. chlororaphis was reduced (Arrebola and Cazorla, 2020).
In the present study, we demonstrated the requirement of aerA and aerB, genes for aerotaxis transducers, for the interaction of the foliar pathogen Pta6605 with its host tobacco plants. We presumed that aerotaxis helps Pta6605 to navigate the foliar surface and reach oxygen-rich stomata openings, at which gas exchange occurs. Following entry into the plant, bacterial cells attach to the cell surface, initiating the next infection process. However, the absence of aerotaxis affects bacterial entry and attachment because bacterial cells presumably do not recognize the space as a ‘suitable’ place to settle. Pta6605 has approximately 50 other MCPs with various LBD types that sense many chemical signals coming from plants, which helps bacterial cells to adapt and cause disease. Further investigations are necessary.
Tumewu, S. A., Watanabe, Y., Matsui, H., Yamamoto, M., Noutoshi, Y., Toyoda, K., and Ichinose, Y. (2022) Identification of Aerotaxis Receptor Proteins Involved in Host Plant Infection by Pseudomonas syringae pv. tabaci 6605. Microbes Environ 37: ME21076.
https://doi.org/10.1264/jsme2.ME21076
The first author was supported by the Japanese Government through a MONBUKAGAKUSHO (MEXT) Scholarship during her study at Okayama University. The Pseudomonas syringae pv. tabaci 6605 isolate was kindly provided by the Leaf Tobacco Research Laboratory of Japan Tobacco Inc. This work was supported in part by Grants-in-Aid for Scientific Research (Nos. 26660035 and 19H02956) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.