2023 Volume 11 Issue 1 Pages 1-6
2023 Volume 11 Issue 1 Pages 1-6
Aim: This study aimed to investigate the effects of relaxin (RLX) on hypertensive disorders of pregnancy (HDP) and fetal growth restriction (FGR) using a nitric oxide synthase (NOS) inhibitor-induced HDP murine model.
Methods: Pregnant C57BL/6NCrSlc mice were divided into three groups at 6 days post coitum (dpc). The control group (control) received saline by subcutaneous injection at 6–10 dpc. The NOS inhibitor group (L-NAME) received 50 mg/kg/day L-NG-nitro arginine methyl ester (L-NAME) by subcutaneous injection at 6–10 dpc. The RLX combination group (L-NAME+RLX) received the same dose of L-NAME plus 0.5 μg/g/day human relaxin-2 by subcutaneous injection at 6–10 dpc. Maternal systolic blood pressure (SBP) and urinary protein were assessed at 16 dpc. At 17 dpc, fetal weight was recorded, and placentas, maternal serum, and kidneys were collected.
Results: Maternal SBP and fetal weight were improved in L-NAME+RLX mice compared with L-NAME mice. No significant differences were observed in abortion rate, urinary protein, placental weight, serum placental growth factor concentration, and glomerular open capillary area.
Conclusion: RLX improved maternal SBP and FGR, suggesting that RLX may be a candidate drug for prophylaxis of HDP with FGR.
Hypertensive disorders of pregnancy (HDP) affect 3–5% of all pregnant women and cause serious perinatal complications for both the mother and fetus, including HELLP syndrome, eclampsia, and fetal growth restriction (FGR).1) Although the exact etiology of HDP is still unknown, it has been shown to be closely related to placental dysplasia in early pregnancy, as well as fetal placental circulatory insufficiency.2,3,4)
Hormonal replacement cycle frozen-thawed embryo transfer has recently been shown to be associated with an increased risk of HDP compared with natural cycle frozen-thawed embryo transfer.5,6) This may be explained by the absence of the corpus luteum and hormones it secretes.7) Relaxin (RLX) is a peptide hormone secreted by the corpus luteum and released into the maternal circulatory system; its effects are exerted through nitric oxide (NO) signaling.8,9,10) RLX has an adjuvant effect on pregnancy and delivery by inducing the relaxation of the pubic ligament, softening of the cervix, and relaxation of the vagina.11) In addition to effects on the reproductive system, RLX has been shown to have vasodilatory, antifibrotic, and anti-cytokine effects, and is thus considered a promising novel therapeutic agent for hypertensive heart failure in the cardiovascular field.9,12,13,14)
This study aimed to investigate the effects of RLX on HDP with FGR during the period from implantation to the completion of placental circulation using a murine model of HDP induced by a nitric oxide synthase (NOS) inhibitor.15)
This study was conducted in accordance with the Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan) with approval from the Ethics Committee for Animal Experiments of Niigata University Graduate School (experimental plan approval number SA00896).
As a preliminary experiment, we examined the toxicity of RLX in pregnant mice prior to starting the study. Between the control group (n=6) and the RLX group (n=8), there were no significant differences in abortion rate, systolic blood pressure (SBP), urine protein, fetal body weight, placental weight, maternal serum placental growth factor (PLGF) concentration, and glomerular open capillary area (methods of administration are described below). We also confirmed that the dose of human relaxin-2 used in this study caused no apparent adverse events in pregnant mice.
AnimalsTwenty-four pregnant C57BL/6NCrSlc mice (Japan SLC, Tokyo, Japan) were purchased 5 days post coitum (dpc) and were housed individually at 20–26°C, humidity of 40–60%, and a ventilation frequency of 6–15 times/hour, with lighting at 150–300 lux brightness in a 12/12-hour light/dark period, a noise level below 60 dB, and food and water available ad libitum. We monitored the health of mice daily.
SBP was measured at 6, 10, 13, and 16 dpc using an indirect tail-cuff method (MK-2000ST; Muromachi Kikai Co., Tokyo, Japan). Urine was obtained by applying gentle abdominal pressure at 16 dpc, and urinary protein was semi-quantitatively assessed on a scale ranging from 0 to 3 based on results of a dipstick test (Ames urinalysis test strip; Siemens Healthcare Diagnostics K.K., Tokyo, Japan). Mice were euthanized at 17 dpc by whole heart blood collection under isoflurane inhalation anesthesia. Maternal serum, maternal kidneys, fetuses, and placentas were collected for further investigation. Fetal body weight and placental weight were measured immediately after delivery.
Administration of L-NG-nitro arginine methyl ester and RLXThe vasodilatory effect of RLX is inhibited by L-NG-nitro arginine methyl ester (L-NAME), a NOS inhibitor. In this study, we created a murine model of HDP by administering L-NAME. At 6 dpc, mice were randomly divided into three groups: the control group (control, n=9) received saline by subcutaneous injection at 6–10 dpc; the NOS inhibitor group (L-NAME, n=7) received 50 mg/kg/day L-NAME (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) by subcutaneous injection at 6–10 dpc; and the RLX combination group (L-NAME+RLX, n=8) received the same dose of L-NAME as in the NOS inhibitor group plus 0.5 μg/g/day human relaxin-2 (RLX; product number 332–45091; PEPTIDE INSTITUTE, INC., Osaka, Japan) by subcutaneous injection at 6–10 dpc.
Biochemical measurement of blood samplesMaternal serum samples were stored at −80°C until use for in vitro assays. PLGF concentrations were measured using the PLGF ELISA Kit (ab197748; Abcam plc, Cambridge, UK) according to the manufacturer’s recommended protocol.
Histological examinationFetal kidneys were formalin-fixed, embedded in paraffin, thinly sliced into 2 μm thick sections, and stained with hematoxylin-eosin. Endotheliosis was identified as a decrease in glomerular open capillary area, expressed as a percentage of the glomerular tuft area. Forty randomly selected glomeruli from each mouse were analyzed by calculating the area of the ellipse using the major and minor diameters, in accordance with previously described methods.16)
Statistical analysisData were collected and entered into a spreadsheet (Excel spreadsheet, Microsoft, Tokyo, Japan) and statistically analyzed using EZR version 1.55 (Free statistical software; Saitama Medical Center, Jichi Medical University, Saitama, Japan) and GraphPad Prism 9 (GraphPad, San Diego, CA). The Mann-Whitney U test, chi-squared test, and ANOVA with Tukey-Kramer test were used for comparisons among three or more groups, with P-values <0.05 considered statistically significant.
RLX improved L-NAME-induced hypertension and decreased L-NAME-induced increase in urine protein in pregnant mice. The maternal body weight gain (Table 1) and abortion rate (Table 2) from 5 to 16 dpc were similar between the L-NAME and L-NAME+RLX groups but differed from the control group.
| Control (n=9) | L-NAME (n=7) | L-NAME+RLX (n=8) | |
|---|---|---|---|
| Body weight at 5 dpc (g) | 22.1±1.6 | 21.3±1.2 | 21.5±1.0 |
| Body weight at 11 dpc (g) | 25.3±1.7 | 24.8±1.0 | 25.3±1.3 |
| Body weight at 16 dpc (g) | 32.9±2.3 | 32.6±1.8 | 31.8±1.4 |
| Maternal weight gain from 5 to 16 dpc (g) | 10.8±0.9 | 11.3±1.3 | 10.3±1.3 |
dpc, days post coitum; L-NAME, L-NG-nitro arginine methyl ester; RLX, relaxin.
| Abortion | Production | Total pregnancies | |
|---|---|---|---|
| Control (n=9) | 11 (14.1%) | 67 | 78 |
| L-LAME (n=7) | 13 (14.4%) | 77 | 90 |
| L-NAME+RLX (n=8) | 9 (10.3%) | 69 | 78 |
Pearson’s chi-squared test, P=0.84
L-NAME, L-NG-nitro arginine methyl ester; RLX, relaxin.
The mean SBP, measured by the tail-cuff method at 13 dpc, was significantly higher in the L-NAME group than in the control and L-NAME+RLX groups (control, 103.2±8.8 mmHg; L-NAME, 115.7±4.1 mmHg; L-NAME+RLX, 98.5±7.3 mmHg; control vs. L-NAME, P=0.011; control vs. L-NAME+RLX, P=0.43; L-NAME vs. L-NAME+RLX, P=0.00085) (Figure 1). Similarly, at 16 dpc, the L-NAME group had a significantly higher mean SBP than the control and L-NAME+RLX groups (control, 98.3±6.6 mmHg; L-NAME, 115.1±7.2 mmHg; L-NAME+RLX, 101.9±6.6 mmHg; control vs. L-NAME, P=0.00056; control vs. L-NAME+RLX, P=0.60; L-NAME vs. L-NAME+RLX, P=0.0064) (Figure 1).

SBP at 6, 10, 13, and 16 dpc for the control (n=9), L-NAME (n=7), and L-NAME+RLX (n=8) groups. SBP, systolic blood pressure; dpc, days post coitum; L-NAME, L-NG-nitro arginine methyl ester; RLX, relaxin.
The mean urine protein score as assessed by the dipstick method at 16 dpc was significantly higher in the L-NAME group than in the control group and was also significantly higher in the L-NAME+RLX group than in the control group (control, 0.3±0.3; L-NAME, 1.9±0.8; L-NAME+RLX, 1.3±0.7; control vs. L-NAME, P=0.005; control vs. L-NAME+RLX, P=0.026; L-NAME vs. L-NAME+RLX, P=0.21) (Figure 2).
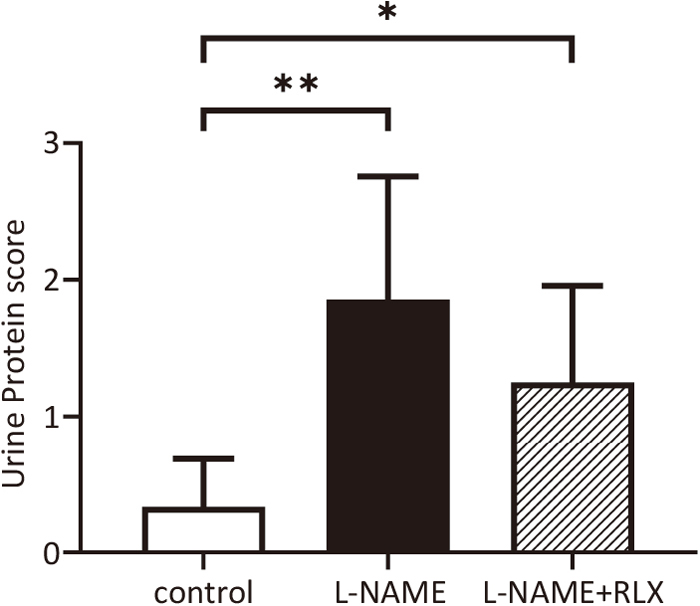
Urine protein score at 16 dpc for the control (n=9), L-NAME (n=7), and L-NAME+RLX (n=8) groups. dpc, days post coitum; L-NAME, L-NG-nitro arginine methyl ester; RLX, relaxin.
The mean fetal body weight at 17 dpc was significantly lower in the L-NAME group than in the control group. Conversely, the mean fetal body weight was significantly higher in the L-NAME+RLX group than in the L-NAME group (control, 0.76±0.07 g; L-NAME, 0.72±0.07 g; L-NAME+RLX, 0.75±0.06 g; control vs. L-NAME P=0.015; control vs. L-NAME+RLX, P=0.55; L-NAME vs. L-NAME+RLX, P=0.003) (Figure 3).

Fetal body weight at 17 dpc for the control (n=78), L-NAME (n=90), and L-NAME+RLX (n=78) groups, and gross appearance of the fetus in each group at 17 dpc. dpc, days post coitum; L-NAME, L-NG-nitro arginine methyl ester; RLX, relaxin.
The mean placental weight was significantly lower in the L-NAME group than in the control group but did not differ significantly between the control group and the L-NAME+RLX group (control, 0.144±0.04 g; L-NAME, 0.128±0.03 g; L-NAME+RLX, 0.136±0.03 g; control vs. L-NAME, P=0.015; control vs. L-NAME+RLX, P=0.33; L-NAME vs. L-NAME+RLX, P=0.35) (Figure 4).

Placental weight at 17 dpc for the control (n=78), L-NAME (n=90), and L=NAME+RLX (n=78) groups. dpc, days post coitum; L-NAME, L-NG-nitro arginine methyl ester; RLX, relaxin.
No significant differences were observed in maternal serum PLGF concentrations at 17 dpc (control, 117.8±17.6 pg/ml; L-NAME, 113.8±19.7 pg/ml; L-NAME+RLX, 107.2±20.5 pg/ml; control vs. L-NAME, P=0.91; control vs. L-NAME+RLX, P=0.51; L-NAME vs. L-NAME+RLX, P=0.78) (Figure 5).

Maternal serum PLGF concentrations at 17 dpc. PLGF; placental growth factor; dpc, days post coitum; L-NAME, L-NG-nitro arginine methyl ester; RLX, relaxin.
No significant differences were observed in glomerular open capillary area at 17 dpc (control, 31.2±9.3%; L-NAME, 29.4±6.9%; L-NAME+RLX, 34.2±7.9%; control vs. L-NAME, P=0.64; control vs. L-NAME+RLX, P=0.33; L-NAME vs. L-NAME+RLX, P=0.057) (Figure 6).

Quantitative estimates of glomerular open capillary area in the kidneys at 17 dpc. Scale bar: 10 μm.
This study showed that RLX has a suppressive effect on elevated blood pressure and an improving effect on FGR in mice with NOS inhibitor-induced HDP, suggesting that RLX may be useful against HDP with FGR.
Previous studies have shown that the inhibition of NOS with L-NAME results in HDP in an animal model, as characterized by hypertension and proteinuria with FGR.15,17,18,19) One study reported that RLX lowered systemic vascular resistance and sFlt-1 in a rat model of HDP with reduced uterine blood flow,20) while in other studies in humans it improved acute heart failure by decreasing systemic vascular resistance and blood pressure.12,21) Given its ability to lower systemic vascular resistance, RLX is considered a potential therapeutic agent for HDP with FGR. Indeed, low levels of RLX in the first trimester of pregnancy have been strongly associated with late-onset type HDP with FGR.22) Although fetal toxicity must always be considered for drugs used during pregnancy, RLX is a hormone that naturally circulates in the maternal circulation during pregnancy and is thus likely to be safe during pregnancy. In fact, maternal RLX levels as high as 2,000–8,000 pg/ml have been observed in healthy women who are pregnant or undergoing ovulation induction.23)
Since RLX is a peptide hormone secreted by the corpus luteum, and the placental circulation in mice is established at approximately 10 dpc, we administered L-NAME and RLX from 6 to 10 dpc (i.e., when the corpus luteum plays the most important role). The administration of RLX significantly suppressed the increase in SBP and improved FGR, suggesting that RLX administered during the period from implantation to the establishment of placental circulation may serve as a prophylactic agent against HDP with FGR.
NO has been suggested to play an important role in embryo implantation and placenta formation. Given that RLX also exerts its effects through NO signaling, the administration of RLX during the time of implantation was predicted to decrease the abortion rate. In fact, some reported that L-NAME administration increased the abortion rate.24) However, no significant difference was observed in the present study. This may be related to the fact that mice have multiple pregnancies, whereas humans typically have single pregnancies. Whether the dose of RLX used in the present study was appropriate will also need to be verified.
Since the gestation period differs between humans and mice, the murine model developed in this study may be used as a superimposed preeclampsia model for HDP. Future studies are warranted to examine whether RLX administered at 11 dpc, or intermittently from 6 to 17 dpc, would be more therapeutically effective.
In conclusion, we demonstrated that RLX contributes to improving maternal SBP and FGR. Our results suggest that RLX may be a candidate drug for the prophylaxis and treatment of HDP with FGR.
We thank Editage (www.editage.com) for English language editing.
The authors report no conflict of interest.