2023 Volume 11 Issue 1 Pages 11-15
2023 Volume 11 Issue 1 Pages 11-15
A 23-year-old woman (gravida 3, para 2) was referred to our hospital at 29 weeks of gestation by a nearby doctor due to hydramnios and suspected umbilical cord hernia. Ultrasound and magnetic resonance imaging indicated hydramnios, umbilical cord hernia, and a dilated stomach, with suspicion of upper gastrointestinal atresia. She was hospitalized at 33 weeks of gestation owing to membrane rupture. Fetal bradycardia was observed during delivery, and an emergency cesarean section was performed. A female infant weighing 1,799 g was delivered (Apgar score: 1/1); hemorrhagic ulceration of the umbilical cord was observed. Pancreatic phospholipase A2, trypsin, lipase, and bile acid levels in the amniotic fluid were elevated. The infant died of hemorrhagic shock and circulatory failure on the second day after birth. Based on these findings, we hypothesize that umbilical cord ulceration or hemorrhage may occur in congenital upper gastrointestinal atresia due to discharge of fetal gastrointestinal enzymes into the amniotic fluid.
Umbilical cord hemorrhagic ulcerations are rare and considered one of the most significant conditions associated with fetal perinatal death. In 1991, Bendon et al. reported the relationship between umbilical cord ulceration and intestinal atresia, given that all three fetuses with hemorrhage from umbilical cord ulcerations had congenital intestinal atresia.1) The pathogenesis and pathophysiology of umbilical cord ulceration and hemorrhage associated with intestinal atresia are not well understood, but the excretion of fetal gastrointestinal enzymes into the amniotic fluid is considered a contributing factor. Intestinal atresia might produce reflux of gastric or duodenal contents, which could initiate an ulcer. Here, we report a case of umbilical cord hemorrhagic ulceration, which was thought to be caused by amniotic drainage of fetal gastrointestinal enzymes due to upper gastrointestinal atresia.
The patient was a 23-year-old woman (gravida 3, para 2) with an unremarkable medical history. Her second child had a complete transposition of the great arteries. She was referred to our hospital at 29 weeks of gestation by a nearby doctor due to hydramnios and suspected umbilical cord hernia. Abdominal ultrasound showed hydramnios (amniotic fluid index [AFI], 38 cm), hernia into the umbilical cord, and a dilated stomach (Figure 1A). Magnetic resonance imaging also revealed a dilated stomach (Figure 1B). Hydramnios was assumed to be caused by congenital upper gastrointestinal atresia due to a severely dilated stomach in the fetus. The patient was followed-up at our outpatient clinic. She was hospitalized at 33 weeks and 6 days of gestation due to high rupture of the membranes. During hospitalization, the amniotic fluid was yellow without bloodlike appearance or turbidity. Internal examination revealed cervical dilatation at 5 cm and the head of the fetus was fixed in the pelvis. The fetal heart rate chart showed a baseline of 150 bpm, transient tachycardia, and no bradycardia on cardiotocography, which suggested reassuring fetal status. Uterine contractions with pain were observed at 7 min intervals; therefore, the course of delivery was monitored as part of the vaginal delivery policy. Amniotic fluid was collected at the time of artificial membrane rupture. Three hours after admission, fetal bradycardia of <60 bpm was observed (Figure 1C). There was no evidence of abnormal placental findings, such as postplacental hematoma or thickening. Internal examination revealed uterine cervix dilated at 6 cm and bloodlike amniotic fluid. As fetal bradycardia did not improve, an emergency cesarean section was performed under general anesthesia with a diagnosis of non-reassuring fetal status. The fetus was delivered 10 minutes after fetal bradycardia was observed and had the following parameters: 1,799 g, Apgar score: 1 point at 1 min/1 point at 5 min. Umbilical vascular hemorrhage prevented umbilical artery blood collection. Therefore, umbilical artery blood pH and BE could not be measured. Hemoglobin level in the fetus after birth was 9.0 g/dl. There was no loop of the umbilical cord or external fetal malformations, other than a hernia, in the umbilical cord. There was no evidence of placental abnormalities, such as placental abruption. However, Wharton’s jelly of the umbilical cord had thinned, exposing the umbilical vessels on the surface, with disruption and bleeding of some umbilical veins (Figures 2A, 2B). Hemorrhagic ulceration of the umbilical cord was observed (Figure 2B). We measured the levels of gastrointestinal enzymes in the amniotic fluid (Table 1). The infant’s spontaneous respiration was weak; hence, she was immediately intubated and admitted to the neonatal intensive care unit. At birth, the infant’s blood pressure was 21/15 mmHg, indicating shock. Blood tests revealed a hemoglobin level of 9.0 g/dl and severe anemia. The infant died of hemorrhagic shock and circulatory failure on the second day after birth.

Abdominal ultrasound, magnetic resonance imaging, and cardiotocography findings.
A) Abdominal ultrasound showed hydramnios, hernia into the umbilical cord, and a dilated stomach. The arrowhead indicates the hernia into the umbilical cord.
B) Magnetic resonance imaging also revealed a dilated stomach. The arrowhead indicates the dilated stomach.
C) Cardiotocography immediately before emergency cesarean section. Fetal bradycardia of <60 bpm was noted.
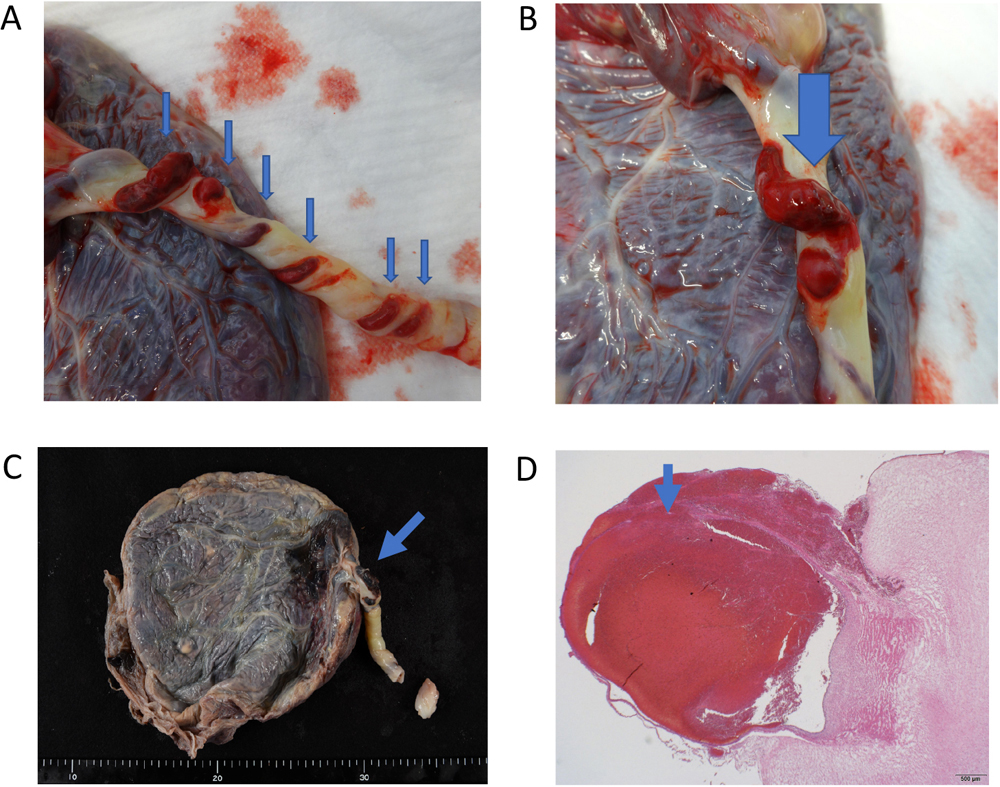
Gross and pathological findings.
A) Gross findings of the umbilical cord. Arrowheads indicate thinning of the Wharton’s jelly of the umbilical cord, exposing the umbilical vessels on the surface. Some of the umbilical veins were disrupted and bleeding.
B) The arrowhead indicates the hemorrhagic ulceration of the umbilical cord.
C) Pathological findings of the umbilical cord. The arrowhead indicates the hemorrhagic ulceration of the umbilical cord.
D) The arrowhead indicates degeneration of the Wharton’s jelly, thinning of the vein wall, and disruption of the vessel.
| Bile acid | 23.5 μmol/L |
| Trypsin | 23,521 ng/ml |
| Phospholipase A2 | 15,424 ng/dl |
| Lipase | 340 U/L |
Congenital intestinal atresia occurs in approximately 1 case per 2,000–5,000 births.2) Umbilical ulcers occur in 6.5–13.6% of upper gastrointestinal obstructions,3) and when they develop, fragile umbilical vessels break down, resulting in sudden hemorrhage, deterioration of fetal well-being, and high mortality rates. Bendon et al. considered vascular reactivity, gastric reflex, and epithelial abnormality as causes of umbilical cord ulcers, and reported that they occur due to their single and combined involvement.1) In our case, congenital upper gastrointestinal atresia and high levels of gastrointestinal enzymes, such as pancreatic phospholipase A2, trypsin, lipase, and bile acids, were present in the amniotic fluid (Table 1), but no vascular reactivity or epithelial abnormalities were observed. This suggests that gastric reflex may have occurred. In addition, there was a decrease in Wharton’s jelly around the umbilical vessels, which was confirmed by gross and pathological findings indicating exposed umbilical vessels and thinned surfaces (Figures 2A–2D). These results suggest that, in our case, gastrointestinal enzymes in the amniotic fluid were elevated due to fetal gastric reflex vomiting caused by congenital upper gastrointestinal atresia, resulting in the development of an umbilical cord ulcer.
Ichinose et al. classified the severity of umbilical cord ulcers in patients with congenital upper gastrointestinal atresia into four grades: Grade 1, desquamation of the epithelium only; Grade 2, detachment of the basal lamina; Grade 3, thinning of Wharton’s jelly with widespread Grade 2 changes; and Grade 4, exposed umbilical artery or vein.4) They reported no association between the sites of obstruction and ulceration grades. Grade 3 umbilical cord ulcerations were observed in cases of duodenal atresia that presented with obstruction on the oral side of the major duodenal papilla. The opening of the accessory papilla, on the oral side of the major duodenal papilla, allows gastrointestinal enzymes to be expelled into the amniotic fluid. In the present case, the patient had a Grade 4 umbilical cord ulceration. Although the exact site of obstruction remained unknown, the condition was believed to be congenital upper gastrointestinal atresia due to a severely dilated stomach. Umbilical cord ulceration may occur when an upper gastrointestinal obstruction is present.
Fetuses with upper gastrointestinal atresia have been reported to vomit in the uterus.5) In this case, gastrointestinal enzymes, including bile acid, trypsin, phospholipase A2, and lipase, were elevated in the amniotic fluid. We subsequently measured the same gastrointestinal enzymes in several normal pregnant women who underwent elective cesarean section due to a previous cesarean section but found no elevation of these enzymes. The secretion of trypsin, a proteolytic enzyme, and lipase and phospholipase A2, lipolytic enzymes, is believed to begin at around 28 weeks of fetal life.6) Kimberly et al. reviewed previously reported cases of intestinal atresia complicated by umbilical cord ulceration7) and documented that all 20 previously reported cases of umbilical cord hemorrhage occurred after 30 weeks of gestation.7) The present case also exhibited umbilical cord ulceration at 33 weeks of gestation. Thus, we hypothesized that congenital upper gastrointestinal atresia resulted in discharge of gastrointestinal enzymes into the amniotic fluid and the development of umbilical cord ulceration. In several cases, umbilical cord ulceration in patients with congenital upper gastrointestinal atresia has resulted in the early onset of labor and preterm mature membrane, as in the present case. In addition to digesting the umbilical cord, gastrointestinal enzymes in the amniotic fluid may also digest the membrane, resulting in the preterm mature membrane.
Nakamura et al. reviewed previously reported cases of intestinal atresia complicated by umbilical cord ulceration8) and reported that the mean gestational age at onset was 33.3±2.7 weeks for all 27 cases examined. Various methods for the early detection of umbilical cord ulceration have been reported; however, there is currently no gold standard. It has been advocated to provide in-hospital management from gestational week 30 onward and determine the proper delivery timing on a case-by-case basis. Umbilical cord ulceration has been associated with high bile acid concentrations in amniotic fluid.9) It was also reported that trypsin levels in amniotic fluid >20,000 ng/ml tended to indicate an advanced depth of umbilical cord ulceration. In the present case, the trypsin level was 23,521 ng/ml (>20,000 ng/ml). After 30 weeks of gestation, it may be necessary to measure levels of bile acid, trypsin, phospholipase A2, and/or lipase by amniocentesis, and consider delivery if they are elevated. Especially when there are signs of preterm labor due to excessive amniotic fluid, measuring gastrointestinal enzyme levels in amniotic fluid collected by amniotic fluid aspiration may help predict umbilical cord ulceration. In another report, a case of spontaneous hemorrhage from the umbilical cord was noted at the time of ultrasonography in a 33-week-old fetus with suspected duodenal atresia, in which immediate delivery resulted in a good outcome.10) Although it may be difficult to identify an umbilical cord ulcer and hemorrhage on ultrasonography, it can be used as a diagnostic aid when these conditions are suspected.
Umbilical ulcers occur in cases of congenital upper gastrointestinal atresia, resulting in sudden hemorrhage, deterioration of fetal well-being, and high mortality rates. When congenital upper gastrointestinal atresia is diagnosed, strict follow-up is required after 30 weeks of gestation, and the timing of delivery should be determined by the measurement of gastrointestinal enzyme levels and the use of diagnostic ultrasonography.
The authors thank the pregnant woman and doctors who cooperated in the preparation of this report.
The authors declare no conflicts of interest associated with this manuscript.
AFI: amniotic fluid index
US: ultrasound