2020 Volume 8 Issue 2 Pages 40-46
2020 Volume 8 Issue 2 Pages 40-46
Hypertensive disorders of pregnancy (HDP) represent a major cause of maternal and neonatal morbidity and mortality. Studies conducted over the last decade have improved our understanding of the potential mechanisms underlying HDP pathogenesis. The first step in HDP is reduced uteroplacental perfusion as a result of abnormal extravillous trophoblast invasion of spiral arterioles. Subsequent placental ischemia leads to maternal vascular endothelial dysfunction that may be caused by an imbalance between pro- and anti-angiogenic factors, enhanced formation of vasocontractile factors such as endothelin and thromboxane, increased vascular sensitivity to angiotensin II, and/or decreased formation of vasodilators such as nitric oxide (NO) and prostaglandin I2. NO is one of the major mediators from the endothelium, and its production is modified by endogenous NO synthase inhibitors such as asymmetric dimethylarginine (ADMA). ADMA levels are generally higher in patients with cardiovascular and metabolic diseases and widely recognized as a prognostic marker for major cardiovascular events and mortality. Recent studies have found ADMA levels to be higher in patients with preeclampsia. In addition, multiple studies indicate that elevated ADMA in early stages of pregnancy might predict the development of preeclampsia. Finally, ADMA has been found to be associated with uterine artery flow disturbance. Collectively, these findings strongly suggest that elevated ADMA-mediated endothelial dysfunction could be a causative factor for HDP. In this review, we discuss the biology of ADMA, with a particular focus on its potential role in HDP.
Hypertensive disorders of pregnancy (HDP) are those involving hypertension (blood pressure (BP)≥140/90 mmHg) in pregnancy1) and are classified into 4 groups: preeclampsia (PE), gestational hypertension, superimposed preeclampsia, and chronic hypertension.2) Recently, the American College of Obstetricians and Gynecologists has broadened the definition of PE to a BP > 140/90 after 20 weeks of pregnancy and either proteinuria ≥300 mg/24 h or protein/creatinine ratio ≥0.3 g/g creatinine or one of the following complications: thrombocytopenia, elevated liver transaminases, pulmonary edema, newly onset renal insufficiency, or cerebral or visual disturbance.3) Even though PE is a leading contributor to maternal and perinatal morbidity and death worldwide, the underlying mechanisms for its development remain unelucidated and treatment options are very limited.4,5,6,7)
Several hypotheses regarding the pathogenesis of PE are well established; one of these is “the two-step theory”.7,8) It is also well known that the presence of additional constitutional risk factors for PE, such as age, obesity, and pre-existing hypertension, contribute to vascular endothelial dysfunction, vasoconstriction, and hypertension in PE.9) In addition to monitoring these factors that could affect endothelial function, observation of uterine artery blood flow by doppler ultrasound is an effective and frequently performed screening method.10) These observations strongly indicate that the impaired build-up and function of the placental vasculature could be critical for HDP development.9) Nitric oxide (NO) is essential to the proper development and function of the placental vasculature.11) In addition, many clinical and experimental studies have found that the incidence of PE is strongly associated with the degree of endothelial dysfunction.10,12,13) This is also supported by studies that have demonstrated the critical relevance of endothelial mechanisms in the increased maternal tissue blood flow and reduced maternal BP.14,15,16)
Endothelial dysfunction may be a pathophysiological link among PE, recurrent pregnancy loss, and future cardiovascular events.17) The molecular mechanisms for endothelial dysfunction in preeclampsia could be multifactorial, and may include an imbalance between pro- and anti-angiogenic factors, enhanced formation of vasocontractile factors such as endothelin and thromboxane, increased vascular sensitivity to angiotensin II, and decreased formation of vasodilators such as NO, prostaglandin I2, and prostacyclin. Among these factors, the present study focuses on a circulating endogenous NO synthase (NOS) inhibitor known as asymmetric dimethylarginine (ADMA), for the following reasons: (1) numerous studies have revealed elevated ADMA levels in patients with PE,18) (2) elevated ADMA in early stages of pregnancy reportedly predicts development of HDP,19) and (3) ADMA is associated with uterine artery flow disturbances.20) These findings strongly indicate that elevated ADMA-mediated endothelial dysfunction could be one of the culprits for HDP. In this review, we discuss the biology of ADMA, with a particular focus on its potential role in HDP.
Disturbed bioavailability of NO, a characteristic feature of endothelial dysfunction, could be induced by increased generation of reactive oxygen species (ROS),21) decreased tetrahydrobiopterin, an important cofactor for NOS,22) reduced L-arginine bioavailability,23) and/or increased endogenous NOS inhibitors such as ADMA.23,24,25) Among these, ADMA is thought to play a major role in endothelial dysfunction.25) In fact, a positive correlation between endothelial dysfunction and ADMA levels was observed in patients with essential hypertension,26) hypercholesterolemia,27) and chronic kidney disease (CKD).28) In women with PE, maternal endothelial function assessed by flow mediated vasodilation of the brachial artery has been found to be impaired concomitantly with increases in ADMA levels.20) One study found that exogenously administered ADMA impairs endothelial function in vivo,28) while another revealed that short-term reduction of circulating ADMA by hemodialysis is associated with amelioration of endothelial dysfunction in patients with end-stage renal disease.29,30) These observations clearly indicate that endogenous ADMA may play a major role in the development of endothelial dysfunction in various diseases including HDP.
Humans generate approximately 300 μmol of ADMA per day in normal condition.31) Various types of cells including those of the placenta can generate methylated arginines.23,25) Dimethylarginines are derived from the degradation products of post-transcriptionally arginine-methylated proteins, which have been produced by protein arginine methyltransferases (PRMTs) (Figure 1).32) S-adenosylmethionine works as a methyl donor in the reactions mediated by PRMTs.32) After proteolysis of arginine-methylated proteins, free dimethylarginines are released from the cells (Figure 1). A direct synthetic pathway for methylated arginine from free arginine has not yet been identified.33) The modification of protein arginine methylation is involved in various cellular functions such as signal transduction, protein subcellular localization, transcriptional regulation and protein-protein interactions.34) For example, Takahashi et al. found that PRMT-1 induces methylation of forkhead transcription factor DAF-16, which regulates stress tolerance and fat storage in C. elegans.35) Further, loss of PRMT-1 markedly decreases ADMA production, which results in a shorter lifespan relative to the wildtype; this phenotype is restored by PRMT-1 expression in an enzymatic activity-dependent manner,35) suggesting the potential importance of PRMT-mediated methylated arginine in maintaining cellular homeostasis. Oxidized low-density lipoprotein (LDL) is reported to increase the generation of ADMA by endothelial cells (ECs) via up-regulation of PRMT genes.32) Furthermore, ADMA release from ECs is also enhanced by shear stress through the induction of PRMT genes.36) These observations suggest that ADMA generation may be regulated in part by PRMT in vivo.
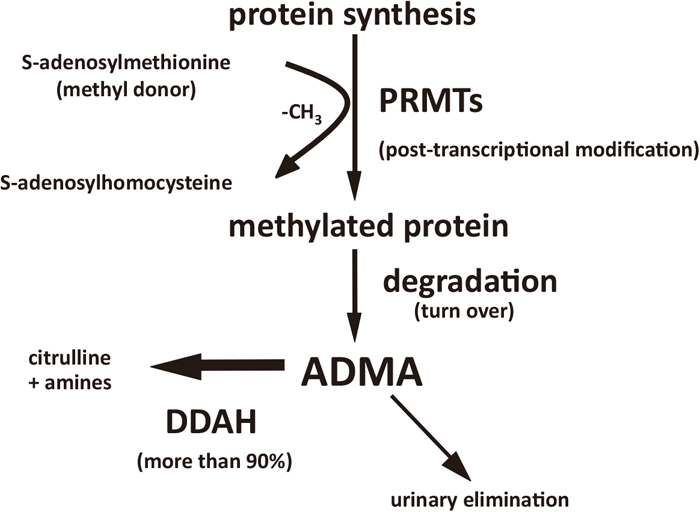
Synthesis and metabolism of ADMA.
ADMA, asymmetric dimethyl arginine; PRMT, protein arginine methyltransferase; DDAH, dimethylarginine dimethylaminohydrolase.
More than 90% of circulating ADMA is metabolized by the action of dimethylarginine dimethylaminohydrolase (DDAH),37) specifically into citrulline and dimethylamine (Figure 1). DDAH is widely distributed throughout the body.38,39) One study found that S-2-amino-4(3-methylguanidino)butanoic acid, an inhibitor of DDAH, inhibits methylarginine metabolism and increases ADMA sufficiently enough to inhibit endothelial NOS.40) In addition, we previously reported that DDAH overexpression decreases ADMA production by cultured vascular smooth muscle cells, which enhances NO generation via inducible NOS.41) In DDAH-I+/− mice, endothelial dysfunction associated with increases in plasma ADMA and BP levels was observed,42) which strongly suggests active participation of endogenous ADMA-DDAH system in regulating vascular function. Furthermore, it has been reported that impaired ADMA metabolism by DDAH is associated with elevated ADMA levels in hypercholesterolemia, diabetes, CKD,43,44,45) and PE.46,47) Taken together, these observations suggest that decreased DDAH action may be one of the main mechanisms underlying the elevation of ADMA levels in high-risk patients, including those with HDP.
Compared to nonpregnant women, healthy pregnant women were found to have significantly lower ADMA levels.48) One longitudinal study also revealed that ADMA levels decreased over the course of a normal pregnancy, concomitant with reduced systemic BP,49) suggesting that ADMA might play a role in hemodynamic adaptation in pregnancy. Pregnant women who do not exhibit a physiological reduction in ADMA level during gestation are more likely to develop PE,18,19,20) and elevated ADMA levels observed in PE patients normalize quickly after delivery.20) A positive association has been reported between the degree of increase in ADMA levels and the severity of PE.50,51) Moreover, Savvidou et al. assessed the uterine artery flow patterns at 23–25 weeks of gestation to identify patients with impaired uterine perfusion, and reported that impaired flow was significantly associated with circulating levels of ADMA.20) They also found that women who developed PE in later phases of pregnancy were characterized by abnormal flow patterns of uterine arteries and elevated ADMA levels. Besides the vascular flow, NO is also well known to be essential for the proper development and function of the placental vasculature.11) Therefore, ADMA-elicited poor NO availability could disturb proper placentation in HDP patients. In addition to these observations, a recent meta-analysis confirmed significant ADMA elevation in PE patients,18) which may suggest the involvement of ADMA in the development of PE.
Mechanisms for ADMA elevation in HDPAs mentioned, we observed a marked decrease in enzymatic activity of placental DDAH, while PRMT levels were not altered in PE patients,46) suggesting that decreased ADMA degradation, but not enhanced ADMA generation, could account for ADMA elevation in PE. Although the mechanism underlying DDAH dysregulation in PE is not fully understood, some PE-related conditions such as hypoxia, renin angiotensin system (RAS) activation, and increased oxidative stress could explain this phenomenon, in that both RAS and oxidative stress reportedly decrease the activity of DDAH,52,53) which results in ADMA accumulation. One study found that hypoxia itself reduces DDAH expression and subsequently increases ADMA levels in a model of pulmonary hypertension.54) Accordingly, HDP-mediated placental ischemia and enhanced RAS and oxidative stress could be involved in DDAH inactivation and subsequent ADMA elevation, leading to endothelial dysfunction in patients with PE. Since DDAH enzymes can be inactivated by oxidation of an active site cysteine residue,55) some clinically utilized agents with antioxidative properties have been found to improve DDAH activity and subsequent decreases in ADMA in various conditions.56,57,58) Indeed, inhibition of the renin angiotensin system was found to decrease elevated ADMA and ameliorate endothelial dysfunction in patients with chronic kidney disease.59) In addition, some reports have demonstrated that increased transcription of DDAHs genes can decrease ADMA and increase NO production. For instance, all-trans retinoic acid60) as well as PPAR-γ ligand61) increase DDAH expression and subsequently decrease ADMA levels. Furthermore, the farnesoid X receptor has been identified within intron 1 of DDAH gene, and its antagonist has been shown to increase DDAH expression and reduce circulating ADMA.62) Although it is still controversial, some studies have demonstrated the possibility that antioxidant or L-arginine treatment could prevent PE and intrauterine growth restriction.63) Moreover, given that infusion of synthetic inhibitors of NOS that mimic the effects of ADMA causes PE-like symptoms in rodents, but can be reversed by infusion of L-arginine,64,65,66) we surmise that ADMA is actively involved in the development of HDP. Therefore, counteracting ADMA through the enhancement of DDAH activity may be a novel therapeutic option for HDP.
ADMA and hypertensionRegarding hypertension, a growing body of evidence suggests that ADMA plays an important role in the regulation of vascular tonus and BP in health and disease.25,45,67) There may be two possible mechanisms by which ADMA causes BP elevation: 1) ADMA may exert vasoconstrictor/pressor effects by inhibiting endothelial NOS activity68,69) and activating RAS;70,71) and 2) ADMA inhibits renal sodium excretion by reducing NO bioavailability in the kidney.72,73,74) Increased urinary ADMA levels were observed in Dahl salt sensitive rats, which was associated with BP elevation.75) Furthermore, BP levels were reported to be lower in DDAH transgenic mice than in wild type mice,76) while BP levels were higher in DDAH-I-deficient mice.42) In addition, we previously found that ADMA levels are associated with mean BP levels in healthy subjects.67) These observations strongly indicate the pathological relevance of ADMA with regard to BP elevation in patients with HDP.
ADMA and renal involvementProteinuria is also one characteristic feature of PE. Although the precise mechanism is still unclear, an increasing body of evidence suggests endothelial dysfunction is linked to albuminuria.77,78,79) Therefore, it is conceivable that ADMA-elicited endothelial dysfunction in HDP might play a pivotal role in the development of proteinuria. This assumption is supported by a report that ADMA injures the glomerular filtration barrier and subsequently enhances glomerular permeability to albumin.80) In addition, we previously found that ADMA reduction by manipulation of DDAH expression significantly reduces proteinuria levels in experimental animal models of CKD and diabetes.81,82)
Several epidemiological studies have indicated the possible involvement of ADMA in the pathogenesis of renal injury. High ADMA levels have been found consistently to predict impaired renal function in CKD patients or the progression of diabetic nephropathy in diabetes.83,84,85) Moreover, we previously found that ADMA-induced disturbed bioavailability of NO could be involved in the loss of peritubular capillaries and/or impaired capillary flow, which could contribute to tubulointerstitial ischemia and renal scarring processes.81,82)
In conclusion, dysregulation of DDAH (likely due to placental ischemia), increased RAS, or oxidative stress could elevate ADMA levels in PE. Elevated ADMA-elicited endothelial dysfunction may lead to BP elevation and the development of albuminuria and renal injury, thus contributing to the pathophysiology of HDP (Figure 2). Counteracting ADMA through the enhancement of DDAH activity may be a novel therapeutic option for HDP.
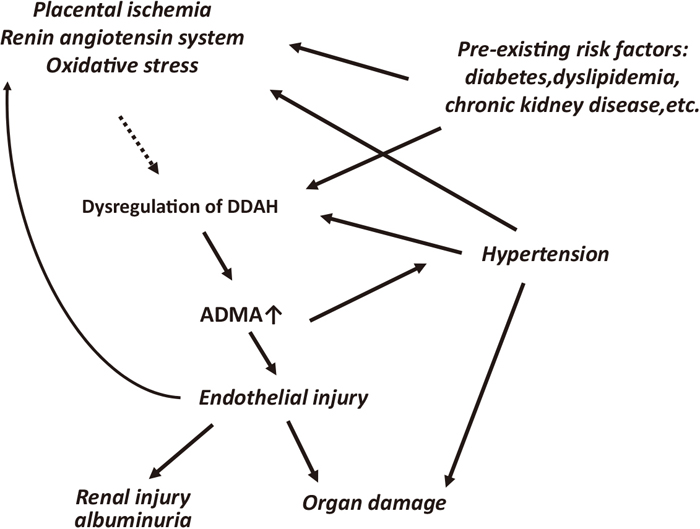
Possible roles of ADMA in HDP.
ADMA, asymmetric dimethyl arginine; DDAH, dimethylarginine dimethylaminohydrolase.
None.