2022 Volume 2 Issue 1 Pages rev-64-rev-73
2022 Volume 2 Issue 1 Pages rev-64-rev-73
Zinc is a critical trace element that is important for cellular function in both female and male reproductive organs. Zinc imbalance and/or altered zinc signaling causes multiple disorders in the reproductive process, including oogenesis, spermatogenesis, fertilization, and embryogenesis. Extracellular and intracellular dynamics of zinc ions are regulated by cell-specific transporters, i.e., Zrt-, Irt-related protein (ZIP) or zinc transporter (ZnT), which respectively transport zinc ions in or out of the cytoplasm through biological membranes. The expression and function of these transporters vary among cell types. The elucidation of the mechanisms underlying zinc homeostasis and zinc dynamics in reproductive function will lead to better infertility treatments for humans as well as the improvement of livestock production. In this review, we discuss the essential roles of zinc signaling in the key events in mammalian reproduction, with a focus on the period from gametogenesis to embryonic development.
Zinc is an essential trace mineral that is involved in many cellular processes such as cell proliferation, immune function, antioxidant defense, gene expression, and RNA polymerase activity [1-3]. Since 1963, when zinc deficiency was first reported to be associated with hypogonadism and dwarfism [4], the importance of zinc has received much attention due to the decrease in female and male reproductive functions caused by zinc deficiency. Most recently, it appears that the cytokine storm in SARS-CoV-2 infection (COVID-19 disease) may induce a depletion of zinc and increased oxidative stress in reproductive tissues, and this possibility is especially relevant to the fertility of affected couples [5].
Approximately 30%–40% of cellular zinc is present in the nucleus, with ~50% in the cytoplasm and organelles; the remaining is localized in the cell membrane [6]. The spatiotemporal zinc dynamics are tightly regulated through zinc transporters, which contains two of the solute carrier protein families; SLC30A (Zinc transporter, ZnT) and SLC39A (Zrt-, Irt-related protein, ZIP), to provide crucial cellular signaling [7, 8]. The influx and efflux of zinc ions are regulated by ZIP and ZnT, respectively (Fig. 1A) [9-11]. In mammals, a total of 23 zinc transporters have been identified: ZIP1–14 and ZnT1–9 (Fig. 1B) [12-14]. These zinc transporters are expressed at various locations in the cells and tissues [15, 16]. Although the detailed mechanisms of cellular zinc dynamics are not fully understood, recent studies have highlighted zinc homeostasis and its function in essential reproductive events. In this review, we discuss the relationship between zinc and mammalian reproduction, including oogenesis, spermatogenesis, fertilization, and embryo development.
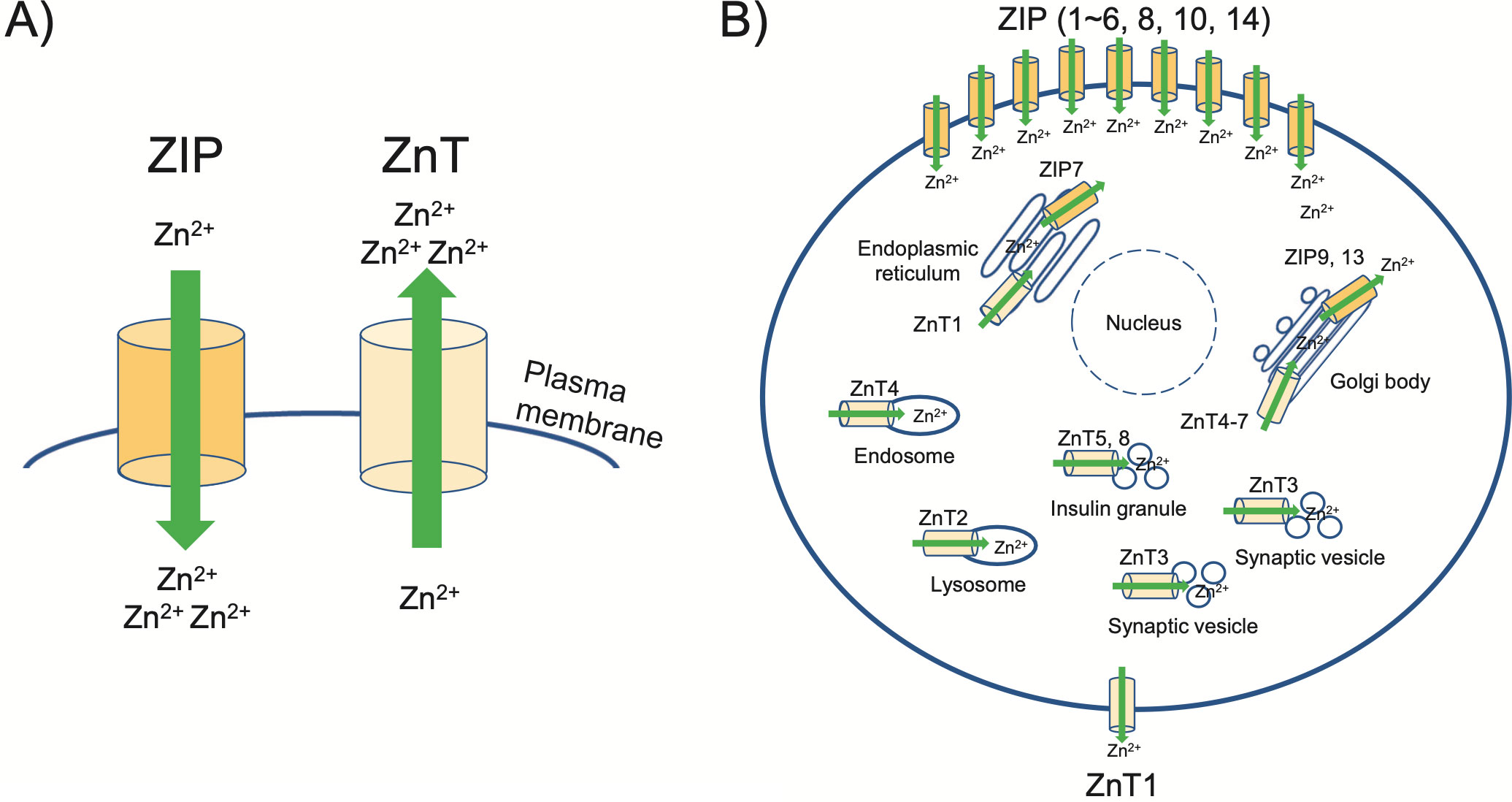
A: Zinc transporters are localized to various intracellular compartments and the plasma membrane. Green arrows indicate the direction of zinc by ZIP and ZnT, respectively. ZIP functions in zinc influx into the cytoplasm, while ZnT moves zinc in the opposite direction.
B: The localization of ZIP and ZnT transporters is shown referring to the information available in many reports.
2.1. Ovarian function
In the ovary, follicular development is controlled by multiple factors and physiological events [17]. The regulation of primordial follicle dormancy and activation is important for reproductive sustainability [18]. For example, the primordial follicles remain in a resting and dormant state until they are “activated” to begin follicle growth, and the follicle activation involves a complex interaction between germ cells and somatic factors to ensure the steady maintenance of the growing follicle pool. There is no information to date on the role of zinc in the early follicular or germ cell development in mammals. However, in the early and late germ cell development of Caenorhabditis elegans, zinc deficiency has been reported to cause reduced fertility due to impaired oocyte development [19, 20], indicating that zinc plays an important role in early and late germ cell development in C. elegans. In mammals, the involvement of zinc in early oocyte meiosis needs to be investigated in detail.
2.2. Oocyte maturation
A divalent ion, i.e., Ca2+, has the most important role in gametogenesis and fertilization [21-23]. Most mammalian oocytes are arrested at the prophase of the first meiosis, which is also called the germinal vesicle (GV) stage because these oocytes possess a GV. After a surge of luteinizing hormone (LH), GV oocytes resume meiosis and progress to the metaphase of the second meiosis (MII) through GV breakdown (GVBD). Oocytes that reach the MII stage are arrested again just before fertilization. The Mos/Mitogen-activated protein kinase (MAPK) pathway regulates the arrest at the first meiosis [24]. Several research groups revealed that an optimal concentration of zinc in oocytes was required for the arrest through a component of the Mos/MAPK pathway called 'cytostatic factor (CSF)' [25-27]. Treatment with a zinc chelator, i.e., N, N, N′, N′-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN), can induce meiotic resumption from the GV stage [27]. Kong et al. also reported that meiotic resumption induced by TPEN can be inhibited by an injection of Mos short interfering (si)RNA or treatment with cycloheximide, a potent inhibitor of protein synthesis [27]. Taking the above findings together, it appears that at least in immature (GV) oocytes, zinc signaling has an inhibitory role in meiotic resumption via a suppression of CSF activity (Fig. 2).
After the resumption of meiosis from the GV stage, a large fluctuation of the level of zinc occurs [26]. It is reported that TPEN treatment throughout the first meiosis resulted in a failure of progression to asymmetrical division (the extrusion of the first polar body) and in arrest at telophase I [26]. An injection of nondegradable cyclin B1, which is a component of maturation promoting factor (MPF), partially rescued the arrest of zinc-insufficient oocytes, enabling them to enter MII [28]. MPF is also thought to be another component of CSF, suggesting that zinc may have different roles at each meiotic stage via the regulation of CSF activity.
The results of an in vivo study using a zinc-deficient diet revealed that zinc plays an important role in follicle development and ovulation. Feeding a zinc-deficient diet for 10 days completely blocked ovulation and compromised cumulus expansion in vivo [29]. A more acute 3-day treatment with a zinc-deficient diet did not block ovulation but did increase the number of oocytes trapped in luteinizing follicles. Moreover, 23% of ovulated oocytes did not reach MII due to severe spindle defects [29]. These observations correspond to the results from oocytes treated with TPEN [26-28, 30]. Together these findings indicate that zinc signaling has an essential role in the meiotic progression of oocytes both in vitro and in vivo.
The complete understanding of zinc-mediated effects in cumulus oocyte complexes (COCs) during the periovulatory period is lacking. The lack of cumulus cell expansion is known to be due to an almost complete suppression of phospho-Sma- and Mad-related protein 2/3 (SMAD2/3) signaling. The chelation of zinc with TPEN causes an acute increase in steroidogenic transcripts (such as Cyp11a1 and Star mRNA) and an increase in progesterone accumulation in the culture medium [29, 31]. TPEN is also known to abolish SMAD2/3 signaling in COCs. The signaling through the zinc-binding SMAD (mothers against decapentaplegic homolog) transcriptional pathway is known to inhibit progesterone production [32-34], and TPEN treatment potently suppresses SMAD2/3 phosphorylation in COCs [29]. These results have shown that suppression of SMAD2/3 signaling also leads to other defects including a complete failure of cumulus expansion as seen in the TPEN-treated COCs.

Zinc acts as a switch to regulate CSF during the establishment, maintenance, and arrest of MII.
Upper panel: the approximate relative zinc levels in GV oocytes, MII, and early embryos.
Subsequent panels: the dynamics of key cellular activities during oocyte maturation and fertilization. In this model, as intracellular zinc increases, the MPF activity also increases, inducing the resumption of meiosis; at the AI/TI stage, when intracellular zinc exceeds a certain level, CSF is activated, leading to CSF-mediated MII arrest. After maturation, zinc sparks cause a decrease in intracellular zinc, which leads to reduced CFS and MPF activity in fertilization. AI/TI: anaphase I/telophase I, GV: germinal vesicle, GVBD: GV breakdown, MI: metaphase I, MII: metaphase II, PN: pronucleus.
2.3. The accumulation of zinc during oocyte maturation
Studies using zinc-selective indicators such as Fluozin-3 AM or Zinc BY1 have shown that zinc is accumulated by the MII phase before 'zinc spark' described below [30, 35-40]. Kim et al. (2011) revealed distinct regions of high zinc concentration that formed a polarized and hemispherical pattern [35]. The polarization of the zinc-enriched compartments mirrored that of the cortical granules (CGs), which are exocytic vesicles that participate in the cortical reaction, which involves the hardening of the zona pellucida to establish a block to polyspermy at fertilization [41]. These vesicles undergo dynamic movement during oocyte maturation and exocytosis at the time of fertilization [42].
Zinc also has critical roles in spermatogenesis, sperm maturation, and capacitation. The experimental restriction of zinc intake in adult men for 24–40 weeks was shown to result in oligospermia [43]. This was caused by a decrease in testosterone levels with impaired Leydig cell function, which was subsequently restored after 2–32 months of zinc supplementation. The administration of a severe zinc-deficient diet to rats resulted in decreased weight of the testis and paragonadal gonads (seminal vesicles and prostate) and increased abnormal spermatozoa with flagella that were shorter by ~25% compared to controls, which is associated with modulated fatty acid composition and interrupting essential fatty acid metabolism [44]. Zinc deficiency was also observed to cause testicular atrophy accompanied by the loss of spermatozoa and spermatocytes [45], increased apoptotic degeneration [46], and high rates of oxidative damage to lipids, proteins, and DNA in the testes of male rats [47]. Marginal zinc deficiency also sensitized the prostate to oxidative stress in rats [48]. These results indicate that zinc plays an important role in male fertility, regulating multiple cellular processes.
Zinc is present mainly in Leydig cells, late differentiating spermatocytes, and spermatozoa in testicular tissues [49]. The developing spermatocytes contain high levels of zinc since zinc is required for DNA condensation and meiosis [50]. The chromatin structure of a spermatozoon is completely different from that of somatic cells, since each spermatozoon needs to transport the haploid genome to eggs. The zinc-dependent chromatin stability model has been proposed as the chromatin structure is packed in order to be extremely resistant to DNA damage in the sperm and rapidly decondensed to make the DNA available in the ooplasm after fertilization [51]. Thus, zinc dynamics are responsible for fertilization competency in sperm cells.
The prostate gland, the seminal fluid, and ejaculated sperm have much higher zinc content than testicular tissues [6], indicating that sperm accumulate zinc as they are transported through a seminal duct. The zinc concentration in semen is positively correlated with the sperm count and normal sperm morphology, and poor zinc nutrition may be associated with a low quality of sperm and male infertility [52]. It is pointed out that reduced seminal zinc levels associated with cigarette smoking is an important risk factor for poor sperm quality and idiopathic male infertility [53, 54]. Semen, which contains a high concentration of zinc, plays an important role in preventing premature sperm fertilization and exerts antioxidant activity; low concentrations of zinc may be a prerequisite for successful acrosomal exocytosis [55].
Few studies have examined how zinc is transported into the maturing gametes during spermatogenesis. In mice, different types of ZIP and ZnT are expressed in the testes at each stage from spermatogonia to spermatogenesis [49]. Investigations using that murine model have shown that ZIP5 imports zinc into Sertoli cells and spermatocytes, augmented by ZIP10 in primary spermatocytes and ZIP8 in secondary spermatocytes [49]. Round spermatids express ZIP6, ZIP8, and ZIP10, whereas elongating spermatids express ZIP1 and ZIP6. ZIP14 was detected in undifferentiated spermatogonia and Leydig cells. ZnT1 is dominantly expressed in Sertoli cells [56], indicating its important role in the export of zinc from Sertoli cells to the developing germ cells. In addition, the expressions of ZIP6 and ZIP10 were greatly reduced in testes of mice fed a zinc-deficient diet [49], suggesting that intracellular zinc dynamics activate zinc signaling through the expression of stage-specific transporters.
4.1. Fertilization and oocyte activation
In most mammalian species, oocytes are arrested at the MII stage before ovulation [57-59]. 'Oocyte activation' requires the progressive initiation of several events: cortical granule exocytosis, the inactivation of CSF, the resumption of meiosis and exit from MII arrest, the extrusion of the second polar body, and the formation of the pronucleus (PN) [59-61]. The completion of these events ensures the initiation of early embryo development [62]. In all species studied to date, oocyte activation requires a fertilization-associated increase in the intracellular concentration of Ca2+ [63]. While oocyte activation in many invertebrates and vertebrates is triggered by a transient increase in calcium ions [64], oocyte activation in mammalian species causes a repeated Ca2+ rise and fall, i.e., calcium oscillation [65-71].
These Ca2+ oscillations play an important role in triggering key events in oocyte activation, such as cortical granule exocytosis, the resumption of meiosis, and exit from MII arrest. Although it has been known for several decades that Ca2+ is an essential trigger for egg activation, the mechanism by which sperm triggers an intracellular Ca2+ release has not been established. In addition, the details of the mechanism underlying the Ca2+ release at fertilization in vertebrates remain to be fully clarified, especially regarding the role of the interaction between the sperm and oocyte in this process [72, 73]. To solve these problems, it is necessary to clarify the fertilization mechanism at the molecular level.
Three major hypotheses have been proposed regarding the mechanism of Ca2+ release by sperm during fertilization: the membrane receptor hypothesis, the Ca2+ conduit hypothesis, and the sperm factor hypothesis [61, 64, 74-76]. The sperm factor hypothesis, which is widely supported at present, can be explained by the finding that an injection of mammalian sperm extract induces Ca2+ responses and oocyte activation events similar to those in spontaneous fertilization. An investigation of the 'sperm factor' activity in the above-mentioned mammalian sperm extract revealed that this activity appeared to be based on a sperm-specific phospholipase C (PLC) with distinctive properties such as enhanced Ca2+ sensitivity compared to the known PLC isoforms [68, 77-79]. In 2002, the sperm-specific PLC named PLCζ was discovered [80]. PLCζ appeared to have all the expected properties of an endogenous agent of oocyte activation. An important structural difference between PLCζ and other PLC isoforms is that PLCζ is smaller than all of the previously identified PLC isoforms and lacks a Pleckstrin-homology (PH) domain. It is known that upon the release of sperm-derived phospholipase C zeta (PLCζ) into the ooplasm, this enzyme hydrolyzes phosphatidyl inositol 4, 5-biphosphate (PIP2) to produce inositol 1, 4, 5-trisphosphate (IP3) and diacyl glycerol (DG) [81]. IP3 binds IP3 receptor (IP3R), which is distributed along the endoplasmic reticulum (ER; the main Ca2+ store of the cell), gating the receptor and inducing periodic intracellular Ca2+ increases [82-85] (Fig. 3).
PLCζ was subsequently identified in a number of mammalian species [86-89] and other animal species [90-92], and it was demonstrated that a microinjection of PLCζ complementary cRNA induced Ca2+ oscillations in mouse oocytes comparable to those seen during natural fertilization in mice. PLCζ is localized in the anterior and posterior somatic membrane regions [93]. Nakai et al. clarified that in pig sperm, PLCζ was present in the sperm tail in addition to its expected localization in the sperm head [94]. Thus, the localization of PLCζ in sperm may differ among animal species. Taking the above-mentioned observations together, the consensus is that PLCζ is likely to be the trigger of egg activation and embryo development — at least in mammals and probably in many other vertebrate species.

PLCζ is released from the sperm into the ooplasm. This enzyme hydrolyzes PIP2 to produce IP3 and DG. IP3 binds IP3R on the ER, stimulating Ca2+ release. This series of events causes calcium oscillations, with Ca2+ repeatedly rising and falling. Zinc into the oocyte is released to the extracellular environment following fertilization and egg activation. This phenomenon is termed a 'zinc spark'. The zinc spark occurs immediately after the first Ca2+ rise. Ca2+ oscillation is necessary for mammalian fertilization, and the zinc signal is also important for fertilization. DG: diacylglycerol, ER: endoplasmic reticulum, IP3: inositol 1, 4, 5-trisphosphate, PIP2: phosphatidyl inositol 4, 5-biphosphate, PLCζ: phospholipase C zeta.
4.2. The discovery of the 'Zinc spark'
The mechanism of fertilization had been discussed mainly in terms of calcium ions, until recently, when it was shown that meiotic resumption can be artificially triggered with TPEN, and the meiotic resumption can be inhibited by overloading the oocyte with zinc ionophores [30, 95]. In mice, TPEN treatment was also shown to be sufficient to activate MII-stopped oocytes injected with "inactivated" sperm heads that did not cause intracellular calcium oscillations, resulting in live births after embryo transfer [95]. Full-term development thus seems not to be dependent on Ca2+ release during MII exit.
It remains unclear whether zinc removal is an essential physiological phenomenon in fertilization. The fertilization of a mature, zinc-enriched mouse egg triggers the transient ejection of zinc into the extracellular milieu in a series of coordinated events termed the 'zinc spark' [35, 42]. It was also demonstrated that the zinc spark occurred immediately after the first Ca2+ rise (Fig. 3). The zinc spark was also observed in human and bovine eggs following fertilization and egg activation [39, 40], indicating that the zinc spark is highly conserved (at least in several mammalian species). These results have established that zinc is an important regulator of meiosis from GV to MII [27, 28]. Bernhardt et al. proposed a model in which zinc was responsible for a concentration-dependent regulation of meiosis through the CSF component EMI2, a zinc-binding protein, and they noted that zinc sparks could ensure the rapid and efficient inactivation of EMI2 [30]. However, it is known that EMI2 inactivation results from a Ca2+-dependent mechanism [96], and zinc sparking does not occur when Ca2+ is chelated [39].
Although several studies have described a zinc spark, the significance of the zinc spark remains unclear. Zinc ions must accumulate on the oocyte in order to spark. The above-mentioned investigations revealed that immature oocytes (e.g., GV) in mouse ovaries cannot produce a zinc spark, indicating that an acute accumulation of zinc during meiotic maturation is important [26, 39]. It was also reported that zinc was accumulated in MII oocytes [97]. Kong et al. reported that Zip6 and Zip10 were highly expressed in mouse oocytes [36]. It has thus been proposed that the influx of zinc in oocytes is regulated by ZIPs (Fig. 4). Zinc spark profiles revealed that zygotes that developed into blastocysts released more zinc than those that failed to develop, and the rate of embryo development and the total cell number were higher [98]. The amount of zinc ions at the zinc sparks may therefore serve as an early biomarker of zygote quality in mouse models.

The schematic illustrates the zinc flux regulated by ZIP. Zinc flows into oocytes with low zinc levels, and meiosis is restarted, followed by an increase in the zinc concentration in the oocyte. After fertilization, the zinc-enriched oocyte causes a zinc spark.
4.3. Embryo development and zinc
Although the importance of zinc in fertilization is clear from the above-cited reports, it remains unclear how zinc functions in embryo development. Kong et al. observed that zinc was present in early mouse embryos [38]. In contrast to the rise in total zinc levels that occurs during meiotic maturation, this transition metal remained constant throughout early embryonic development, and its level was similar to the levels observed in the GV oocyte [38], suggesting the continued functional significance of this metal pool in the preimplantation embryo [26]. When TPEN, a chelator of zinc ions, was added to the early mouse embryo, the embryo development was arrested. In zebrafish embryos, zinc influxes specifically through the ZIP6/ZIP10 heteromer into cells to trigger mitosis [99]. These observations have suggested that the preimplantation embryo requires tight zinc regulation and homeostasis for the initial mitotic divisions of life many species.
Zinc is essential for both female and male reproductive tissues to create good-quality embryos. Although further studies are required to understand the relationships between the intracellular/extracellular dynamics of zinc and reproductive events, an inadequate intake of zinc is most likely to be relevant to female and male infertility or subfertility. A better understanding of zinc biology will help improve the reproductive performance of domestic animals and increase the success rate of human assisted reproductive technology.
This study was supported by Japan's Ministry of Education, Culture, Sports, Science and Technology (MEXT)-Supported Program for the Private University Research Branding Project (2016–2019) and by grants from the Japan Society for the Promotion of Science (JSPS) KAKENHI (nos. 21H02384 and 20H05373 to JI). This study was also supported by the Center for Human and Animal Symbiosis Science, Azabu University.
The authors declare no conflict of interest associated with this manuscript.
These authors Atsuko Kageyama, Jumpei Terakawa, contributed equally.