2022 Volume 2 Issue 1 Pages rev-20-rev-28
2022 Volume 2 Issue 1 Pages rev-20-rev-28
Zinc is an essential trace element with various physiological functions; it is a structural, catalytic, and signaling component of proteins. Owing to its wide range of functions, zinc deficiency causes various symptoms, such as taste disorders, dermatitis, hair loss, decreased appetite, growth disorders, and gonad dysfunction. The global prevalence of zinc deficiency is estimated to be about 25%; thus, its prevention is important for human health. Approximately 2–3 g of zinc is present in the adult human body. Systemic zinc homeostasis is regulated by the zinc transporters ZIP4 and ZNT1, which play major roles in regulating the absorption of food-derived zinc, primarily in the duodenum and jejunum. ZIP4 is expressed on the apical membrane of intestinal epithelial cells and allows divalent zinc ions to enter cells from the lumen. Zinc in enterocytes is subsequently transported by ZNT1 on the basolateral membrane into the portal vein, where it binds to albumin and α2-macroglobulin. In turn, zinc regulates the expression of ZIP4 and ZNT1. This review briefly describes the mechanism of dietary zinc absorption, focusing on zinc in foods and the transporters involved in zinc absorption in the intestinal tract. Moreover, we discuss the potential of dietary components to increase the efficiency of zinc absorption in the intestinal tract via zinc transporters and improve zinc nutrition.
Zinc is the second most abundantly distributed essential trace element in the body at a level of approximately 2–3 g. About 60% of total zinc in the body is stored in skeletal muscles; ~30% in bones; ~5% in the liver and skin, and the rest is widely distributed throughout the body, including the brain and kidneys [1]. Based on bioinformatics research, approximately 2,800 human proteins are potentially zinc-binding proteins in vivo [2]. Zinc functions as an important cofactor for the activity of as many as 300 zinc-containing enzymes, including alcohol dehydrogenase, carbonic anhydrase, and superoxide dismutase [3, 4]. Zinc enzymes are present in all six major classes of enzymes: oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases. In addition, zinc is essential for certain protein structures, including zinc finger transcription factors and the insulin hexamer. Moreover, zinc functions as a signaling mediator through changes in the concentration of zinc ions inside and outside of the cell in response to various stimuli [1, 5]. Because zinc has such a wide range of functions, zinc deficiency can cause a variety of symptoms, including taste disorders, dermatitis, hair loss, growth retardation, anorexia, gonad dysfunction, decreased immunity, and delayed wound healing [6].
Recently, zinc deficiency has been reported in both developing countries and developed countries [7-9], including Japan, and it is estimated that approximately 20–30% of the Japanese population is zinc deficient [10]. Under these circumstances, a zinc preparation for hypozincemia was approved for the first time in Japan in March 2017, and the Japanese Society of Clinical Nutrition issued Japan’s Practical Guideline for Zinc Deficiency 2018 [11].
There has been accumulating interest in efficient prevention of zinc deficiency. Here, we discuss zinc content in foods, the mechanism of intestinal zinc absorption, factors affecting zinc absorption, and foods that can help increase zinc absorption and prevent zinc deficiency.
2.1. Zinc consumption in Japan
Zinc deficiency is common in Japan and the recommended daily allowance (RDA) of zinc in adult males and females, based on intake standards of the United States and Canada, is 11 mg and 8 mg, respectively [12]. For pregnant and lactating women, an additional dose of 2 mg and 4 mg is recommended, respectively, as serum zinc levels decrease as the pregnancy period progresses. However, the actual intake of zinc is approximately 9 mg for adult males in their 20s to 70s, and for females in numerous age groups, intake does not reach the recommended level, especially for those in their 20s to 30s, which is only approximately 7–7.5 mg [13]. Furthermore, it has been reported that approximately 60–70% of men and women over 20 years in Japan consume less than the recommended amount of zinc [14]. Pregnant women and lactating mothers also consume only 7.4 mg and 8.0 mg of zinc, respectively, which raises concerns about insufficient zinc intake and deficiency. Breast milk, especially colostrum, generally contains much higher concentrations of zinc than found in serum [15], and the demand for zinc in newborns is thought to be high; thus, it is important for nursing mothers to obtain sufficient zinc.
2.2. Zinc deficiency in Japan
According to the analysis of serum zinc concentration data in the Japanese population, serum zinc concentrations decrease as people age, and the percentage of people over 60 years with low serum zinc levels is reported to be as high as ~40% [16]. A study evaluating zinc nutrition by measuring zinc concentration in Japanese hair reported that the risk of zinc deficiency is particularly high in children aged 0–4 years, suggesting that ~40% are zinc deficient [17]. While the rate of zinc deficiency tends to increase with age, the rate decreases in men over 80 and women over 90, suggesting that people with longevity may be zinc sufficient [17]. This suggests that zinc is an essential nutrient for achieving a long and healthy life.
Serum zinc levels are commonly used as an indicator of zinc nutrition; however, serum zinc is only approximately 0.1% of the total zinc in the body and is known to exhibit diurnal variations; it tends to be higher in the morning and lower in the afternoon and it can be affected by stress and hormonal status [16]. Therefore, it is necessary to perform measurements early in the morning on an empty stomach. In addition to serum zinc levels, other biomarkers for zinc have been reported, such as the zinc concentration in hair and urine and plasma alkaline phosphatase activity [18,19]. It is hoped that future studies will develop noninvasive, simpler, and more sensitive bioindicators for zinc nutrition.
2.3. Causes of zinc deficiency
Zinc deficiency may be caused by insufficient zinc intake, factors inhibiting zinc absorption such as phytate and polyphosphate, consumption of medicines that inhibit zinc absorption or increase zinc excretion, or congenital zinc deficiency. Congenital zinc deficiency is known as acrodermatitis enteropathica (AE) [20,21], which is caused by reduced intestinal zinc absorption, and transient neonatal zinc deficiency [22-25], which is caused by low zinc concentration in breast milk.
In Japan’s super-aged society (defined as >20% of the population over 65 years old), several people suffer from chronic diseases and consume multiple medications. Zinc deficiency may increase the risk of undernutrition due to taste disorders and anorexia, and delay the healing of bedsores; therefore, prevention of zinc deficiency is important.
3.1. Food items having high zinc content
The absorption rate of zinc in the gastrointestinal tract varies depending on the amount consumed. Although zinc absorption is approximately 90% in zinc-deficient diets [26, 27], it is typically 30% under normal conditions [28]. Therefore, sufficient zinc intake and improvement in absorption efficiency are important for maintaining good zinc nutrition. Zinc is primarily found in animal foods and is known to have a higher bioavailability efficiency than plant foods. Oysters are particularly rich in zinc, with one serving (three oysters) containing approximately 8.4 mg, which is most of the RDA for adults [12, 29]. In addition, meat, especially liver, is rich in zinc (Table 1). Among plant foods, soybeans and seeds are relatively high in zinc. The Japanese population has a high zinc intake derived from staple cereals, particularly rice [13].
3.2. Factors inhibiting zinc absorption
Plant foods are one of the major sources of zinc. However, plant foods, especially the starch layer and germ of cereals, endosperm, and cotyledons of legume seeds also contain a large amount of phytic acid (myo-inositol hexaphosphate), which inhibits zinc absorption; thus, the bioavailability of their zinc may not be high [30]. Phytic acid is negatively charged under physiological conditions, indicating its potential to form complexes with positively charged multivalent cations, especially iron, zinc, magnesium, and calcium. Given that these complexes are soluble under acidic conditions in the stomach and precipitate at neutral pH in the intestine, these minerals in the gastrointestinal tract are poorly absorbed and are excreted in the feces [31]. Therefore, decreasing phytic acid is expected to improve mineral absorption. Phytase, which hydrolyzes phytic acid, is found in the small intestine; however, its activity in the human intestine is much lower than in that of sheep and pigs, which are able to degrade phytate with their own intestinal phytase [31]. Moreover, phytase of plant or microbial origin is widely applied to reduce phytate content in foods to improve the bioavailability of minerals and trace elements during food processing and preparation, predominantly during soaking, malting, germination, fermentation, and bread making [30,32]. Therefore, the use of fermented foods, such as natto (fermented soybeans), miso (fermented soybean paste), and germinated brown rice, among other plant-based foods, is considered efficient in terms of mineral nutrition.
A knockout strain of the SPDT gene in rice, a novel SULTR-like phosphorus distribution transporter, reduced phosphorus and phytate in brown de-husked rice by 20–30%, while yield, seed germination, and seedling vigor were not affected [33]. This is expected to improve the bioavailability of minerals in grains.
Several factors inhibit the absorption of zinc in food additives. Phytic acid, as mentioned above, is used to prevent discoloration, oxidation, and pH adjustment of foods via its metal-chelating effect. Polyphosphates, used as binding agents and emulsifiers, and sodium ethylenediaminetetraacetate, used as an antioxidant, have zinc chelating effects [34]. Consuming a well-balanced diet of various foods may reduce the effect of these factors on zinc absorption; however, care should be taken to avoid an extremely unbalanced diet, which may lead to zinc deficiency.
3.3. Zinc-based food additives
In Japan, zinc gluconate and zinc sulfate are designated as food additives [35]. In 1983, the use of zinc gluconate for nutritional enhancement was limited to “breast milk substitute foods.” However, it was later approved for use in “food for specified health use” and “food with nutrient function claims,” with the amount of zinc contained in the recommended daily intake not exceeding 15 mg; zinc gluconate could also be used in “food with special dietary use” with permission or approval (limited to those for sick people). Thus, zinc gluconate can also be used in comprehensive nutritional foods as a meal replacement for sick people who may be at risk of zinc deficiency [35].
Zinc sulfate is approved only for use in “breast milk substitutes” for nutritional enhancement, and for use as a manufacturing agent in the production of “sparkling liquor,” such as beer. The addition of zinc sulfate to foaming liquors maintains good yeast nutrition during the fermentation process; since most zinc ions are consumed by yeast, zinc sulfate has little effect on the amount of zinc in the product after manufacture [35]. Zinc gluconate, zinc sulfate, zinc acetate, zinc carbonate, zinc chloride, and zinc oxide are regarded as generally recognized as safe substances in the U.S., and are recognized as compounds that can be added to foods in the EU [36,37]. They are also added to supplements, candies, and beverages.
| Food | Zinc concentration (mg/100 g) *1 |
Zinc content per dish (mg) *2 |
|---|---|---|
| Oysters (raw) | 14.0 | 8.4 (3 pieces 60 g) |
| Pork liver (raw) | 6.9 | 4.8 (1 meal 70 g) |
| Beef chuck eye roll (red meat) | 5.6 | 3.9 (1 meal 70 g) |
| Scallop (raw) | 2.7 | 2.7 (1 piece 100 g) |
| Beef liver (raw) | 3.8 | 2.7 (1 meal 70 g) |
| Grilled eel | 2.7 | 2.2 (1/2eel 80 g) |
| Snow crab (boiled) | 3.1 | 1.2 (2 legs 40 g) |
| Rice (brown rice) | 0.8 | 1.2 (1 bowl 150 g) |
| Rice (polished rice) | 0.6 | 0.9 (1 bowl 150 g) |
| Natto | 1.9 | 0.8 (1 pack 40 g) |
| Whole egg | 3.6 | 0.7 (1 piece 60 g) |
| Egg yolk | 1.1 | 0.7 (1 piece 20 g) |
| Processed cheese | 3.2 | 0.6 (1 piece 20 g) |
| Yogurt | 0.4 | 0.4 (1 meal 100 g) |
| Cashew nuts | 5.4 | 0.4 (5 grains 8 g) |
| Firm tofu | 0.6 | 0.4 (1 meal 70 g) |
| Cocoa | 7.0 | 0.4 (1tbsp 6 g) |
| Soybean (boiled) | 1.9 | 0.4 (1 meal 20 g) |
| Almond | 3.6 | 0.2 (5 grains 6 g) |
*1 Calculated using the data in Standard Tables of Food Composition in Japan 2020 (Eighth revised edition).
*2 Calculated based on the standard amount of food for one meal.
Zinc ingested from food is primarily absorbed in the duodenum and jejunum, bound to albumin and α2-macroglobulin in the portal vein, adsorbed into the liver, and subsequently distributed to peripheral tissues. Zinc that is not absorbed into the gastrointestinal tract or contained in gastrointestinal secretions and sloughing mucosal cells is excreted in the feces [1]. Although the excretion of zinc in urine is minor, the amount excreted in urine is reduced in cases of zinc deficiency and increased in some diseases [38].
Zinc homeostasis is regulated by several proteins, including zinc transporters and metallothioneins. Zinc transporters include the ZRT, IRT-like protein (ZIP) and Zn transporter (ZNT) families. Fourteen members of the ZIP family and 10 members of the ZNT family have been identified in humans; however, ZNT9 is thought to have no zinc transport function [1]. In general, ZIP proteins import divalent zinc ions into the cytosol of the cell from the extracellular space or intracellular compartments, whereas ZNT proteins export divalent zinc ions from the cytosol into the extracellular space or intracellular compartments.
The zinc transporter ZIP4 is expressed on the apical membrane of intestinal epithelial cells, and ZNT1 is expressed on the basolateral membrane of intestinal epithelial cells; both play a critical role in intestinal zinc absorption. ZIP4 transports zinc from the lumen into enterocytes, and ZNT1 exports zinc into the portal blood. These transporters regulate the absorption of dietary zinc in the gastrointestinal tract (Fig. 1). In 2002, ZIP4 was identified as the causative gene of AE, a rare autosomal recessive genetic disorder of zinc deficiency, demonstrating the importance of ZIP4 [20,21]. More than 30 mutations or unclassified variants of ZIP4 have been reported [39]. Analysis of intestinal-specific knockout mice confirmed that ZIP4 plays an essential role in zinc absorption in the mammalian intestine [40]. The expression of ZIP4 is regulated by zinc levels in a post-transcriptional and post-translational manner. During zinc deficiency, ZIP4 mRNA is stabilized [41], whereas the ZIP4 protein accumulates on the cell surface and functions in the uptake of food-derived zinc [41,42]. Under conditions of zinc sufficiency, ZIP4 is endocytosed and rapidly degraded by both the lysosomal and proteasomal pathway [41,43] (Fig. 2). Potential zinc-sensing elements and specific motifs for ZIP4 endocytosis have been proposed in the ZIP4 sequence [44-49].
ZNT1 is expressed in various tissues; in the intestinal tract, it is expressed in the basolateral membrane and is thought to function in the transport of zinc from the intestinal epithelial lining to the bloodstream [50]. Unlike ZIP4, ZNT1 transcription is induced by MTF-1, a metal response element binding transcription factor 1, during zinc sufficiency [51,52]. Under conditions of zinc deficiency, ZNT1 expression is also regulated by zinc levels; however, surface ZNT1 is endocytosed and degraded (Fig. 2). Thus, ZIP4 and ZNT1 function cooperatively in zinc absorption in the intestine.
In addition, ZIP5 and ZIP14 are expressed on the basolateral membrane of intestinal epithelial cells and are thought to transport zinc from the blood to intestinal epithelial cells [41,53,54]. In contrast to ZIP4, ZIP5 mRNA levels do not change in response to zinc treatment. However, the translation of ZIP5 is zinc-responsive. When zinc is deficient, ZIP5 mRNA translation is arrested; when zinc is adequate, ZIP5 mRNA is translated, and the protein accumulates in the basolateral membrane [41]. Some zinc transporters may be involved in the regulation of zinc homeostasis in vivo; however, their specific roles in zinc absorption have not been elucidated [29,55].
Moreover, the systemic hormone hepcidin, which is produced in response to iron loading and regulates iron homeostasis, appears to attenuate ZNT1 expression and influence its export from small intestinal epithelial cells [56].
As zinc exists as a divalent ion, its absorption mechanism in the gastrointestinal tract is different from that of iron and copper. Iron and copper ions present in food are reduced by their respective reductases before uptake into intestinal epithelial cells by their respective transporters, DMT1 and CTR1. Therefore, the absorption of iron and copper is markedly affected by the activity of reductases [57,58]. Alternatively, zinc does not undergo redox reactions and is absorbed in the form of a divalent cation. Thus, the presence of zinc transporters may be an important factor in determining the amount of zinc absorbed by the gastrointestinal tract.
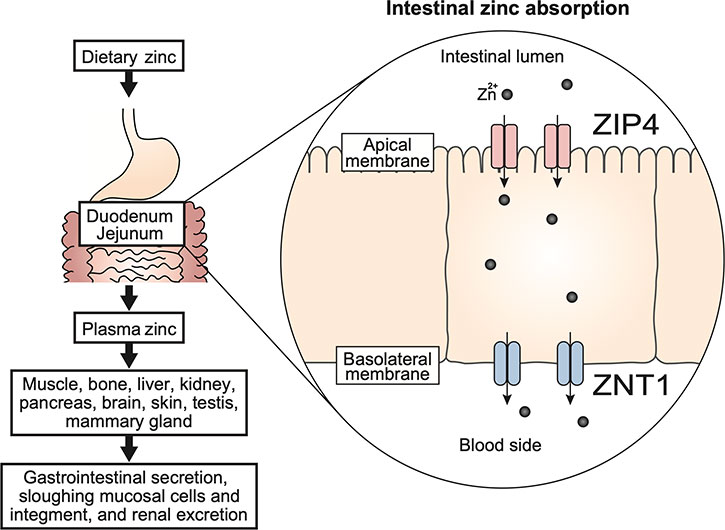
Dietary zinc is primarily absorbed by the duodenum and jejunum. Divalent zinc ions released from food are taken up intracellularly via ZIP4, which accumulates on the luminal side of intestinal epithelial cells. Zinc is transported into the blood via ZNT1, which is present on the basolateral membrane. Zinc in plasma is bound to albumin and α2-macroglobulin and delivered to the whole body. Zinc that is not absorbed by the gastrointestinal tract or contained in detached intestinal cells, pancreatic juice, or bile, is excreted in feces. Zinc is also excreted in urine and sweat.
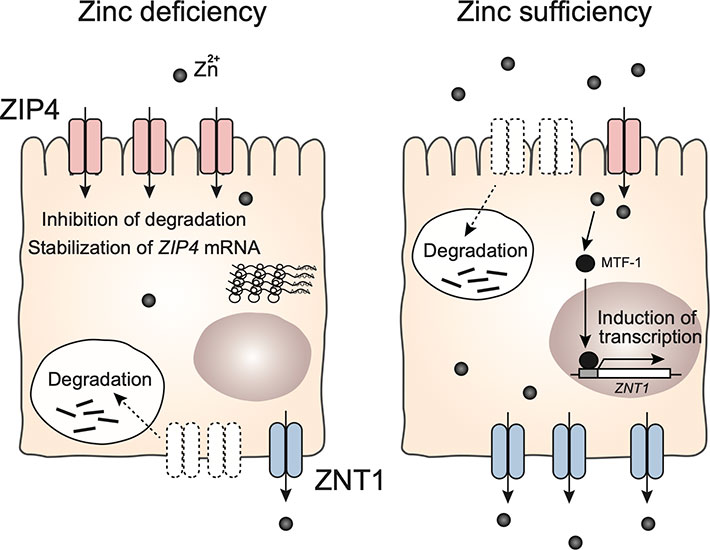
ZIP4 expression is regulated by dietary zinc in a post-transcriptional, and post-translational manner. Under conditions of zinc deficiency, ZIP4 accumulates on the apical membrane through mRNA stabilization and inhibition of protein degradation. When there is sufficient zinc, ZIP4 is rapidly endocytosed and undergoes ubiquitin-mediated degradation. In contrast, ZNT1 undergoes endocytosis and intracellular degradation during zinc deficiency, whereas it accumulates on the basolateral membrane under zinc-sufficient conditions, regulated by metal response element binding transcription factor 1 (MTF-1). MTF-1 senses cytoplasmic zinc concentration in enterocytes and regulates ZNT1 expression.
Given that zinc absorption is approximately 30%, and the absorption rate decreases with increasing zinc intake, improving intestinal zinc absorption may be an effective strategy to prevent zinc deficiency. Based on the hypothesis that food factors that increase the expression of ZIP4 have a positive effect on zinc absorption, we established a screening method for dietary components that would enable an increase in ZIP4 expression and cellular zinc levels. We examined more than 400 food extracts, and found several soybean extracts were effective in promoting the expression of ZIP4 [59]. Detailed analysis of the mechanism of increased ZIP4 expression by these extracts suggests that they inhibit ZIP4 endocytosis and subsequently increase the amount of ZIP4 present on the cell surface, which consequently contributes to increased cellular zinc levels. Furthermore, the active factor responsible for these effects was found to be soyasaponin Bb [59]. Moreover, it was recently reported that an extract of Panax ginseng induces the mRNA expression of ZIP4, which has been shown to promote zinc uptake into cells [60].
If food-derived factors that promote zinc absorption by targeting the intestinal zinc absorption mechanism, specifically by targeting ZIP4 and ZNT1, are identified, these factors may be effective in preventing zinc deficiency through the daily diet (Fig. 3).
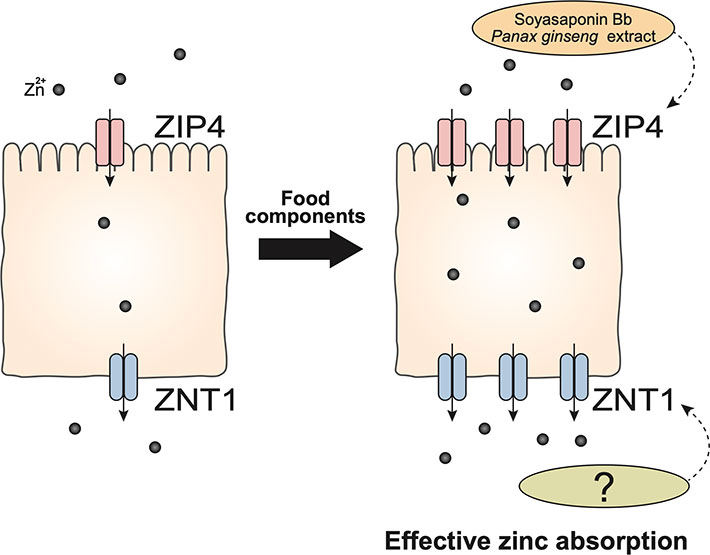
Improving the efficiency of intestinal zinc absorption can be an effective strategy to prevent zinc deficiency and improve zinc nutrition through the daily diet. Food-derived factors with the ability to increase ZIP4 or ZNT1 transporters may increase intestinal zinc absorption. Soyasaponin Bb and Panax ginseng extract are food factors that increase ZIP4 expression and cellular zinc levels. Soyasaponin Bb inhibits ZIP4 endocytosis and increases surface ZIP4 abundance. P. ginseng extract increases ZIP4 mRNA levels. Although food factors that increase ZNT1 have not yet been reported, ZIP4 and other zinc transporters, such as ZNT1, promote zinc absorption.
Here, we reviewed zinc in foods, its absorption mechanisms in the intestinal tract, factors that affect its absorption, and discussed food factors that contribute to efficient absorption. The molecular mechanisms controlling zinc absorption remain unclear, and it is necessary to elucidate the mechanisms of regulation of zinc transporters whose expression is regulated by zinc levels. In addition, the zinc trafficking process in enterocytes needs to be investigated, as well as the crosstalk between zinc and other minerals in intestinal absorption. Clarifying these points will provide new insights into improving zinc nutrition and contribute to the maintenance and improvement of human health.
The authors declare no conflict of interest associated with this manuscript.