2023 Volume 2 Issue 3 Pages rev-28-rev-39
2023 Volume 2 Issue 3 Pages rev-28-rev-39
The high toxicity of soluble selenium (Se) has led to the establishment of environmental standards in Japan. Consequently, various methods for recovering Se from wastewater and contaminated soil have been developed and applied. Despite the recovery and recycling potential of Se after wastewater and/or soil treatment, Se recycling has rarely been mentioned in previous studies. Therefore, a recycling method for Se proposed by the authors is outlined here. Briefly, the selenate contained in wastewater was converted into solid elemental Se or volatile dimethyl diselenide via Pseudomonas stutzeri NT-I metabolism, recovered, purified to high-purity elemental Se, and recycled. The advantages and disadvantages of this recycling method are discussed, as well as those of other recovery and recycling treatment. Overall, Se in soils and wastewater can be recovered by choosing a treatment method suitable for each condition and Se species.
A recycling-oriented society in which the environment is considered, aiming at the elimination of fossil fuel dependence and reduction of global greenhouse gas effects, is required internationally. In line with this, in September 2015, the United Nations Summit adopted several Sustainable Development Goals (SDGs). As a result, research and development efforts have focused not only on reducing the cost of energy while improving its use, but also on saving resources and energy. Rare metals have supported both high-performance and environmentally compatible technologies, such as regenerative energy use (SDG 7 - Affordable and Clean Energy) and production (SDG 12 - Responsible Consumption and Production) with the premise of recycling. Unlike the common metals used in large quantities, rare metals are often used in small amounts to improve technological performance and designated as “industrial vitamins” because of their distinctive properties and essential role in electronic devices.
Selenium (Se) is a rare metal and an essential trace element for both plants and animals [1]. The antioxidant effect of Se in the human body has driven its use as a raw material in therapeutic drugs such as anticancer drugs [2]. However, Se may be toxic to the human body if taken in excess. It is known that chronic ingestion of Se at 400 μg/day or more causes gastrointestinal disorders and peripheral neuropathy in adult human, and acute toxic effects, such as severe gastrointestinal disorders and dyspnea, when ingested at higher dosages (e.g., in g/day) [3]. As an important industrial raw material for advanced industries, Se is mainly smelted as a by-product from electrolytic slimes of copper [4]. Previously used as a semiconductor with rectifying effects, Se is now used as a material for copper indium gallium selenide (CIGS) photovoltaic panels. Recent studies have developed CIGS photovoltaic cells with conversion efficiencies exceeding 34%, and the practical application of photovoltaic cells with such high conversion efficiencies has become more realistic despite their low-cost and low-resource use [5].
| Target | Low name | reference value |
|---|---|---|
| water | Basic Environment Law Water Supply Act Sewerage Law |
0.01 mg/L |
| Water Pollution Control Law | 0.1 mg/L | |
| soil | Soil Contamination Countermeasures Law | 0.01 mg/L (soil elution standard), 150 mg/kg (soil content standard) |
Thus, Se has a variety of uses and an increase in its demand is expected in the future. With the expansion of Se production and application, industrial wastewater containing this metal at concentrations high enough to be considered non-natural [6] and the increasing possibility of Se leak into the environment have to be considered. However, it is difficult for purifying, recovering, and reusing the Se contained in industrial wastewater. This is because industrial wastewater contains impurity ions such as As, Fe, Cu and S and the others in addition to selenium [7, 8]. In industrial wastewater, Se occurs mostly in the form of selenate [Se(VI)] or selenite [Se(IV)] oxyanions, which are detrimental to organisms at low levels. For instance, the half-lethal dose (LD50) of oral administration in rats is 1.6 mg/kg for selenate and 10 mg/kg for selenite, which is comparable to the LD50 of arsenic, regarded as a severe venom [9–11]. The Japanese government has focused on the toxicity of Se and has legislated on the Se standards for water, soil, and air (Table 1). Currently, Se refining processes and thermal power plants generate wastewater containing high concentrations of soluble seleno-oxyanions [12]. Physicochemical treatments, such as coprecipitation, have been implemented to treat wastewater containing Se and allowed achieving Se levels in solution under 0.1 mg/L before discharge (Figure 1). For soluble Se, physicochemical treatment methods for selenate in particular are still being investigated but a treatment combining ion exchange and precipitation methods for high Se concentration has been proposed [13–17]. Although the purpose of these treatment methods is to remove Se, there are few reports on the recovery, purification, and recycling of removed Se.
Because Se is present at trace concentrations not only in wastewater but also in the natural environment (e.g., in volcanic sediments), naturally-derived Se is widely distributed in Japan, which has many volcanos [18–21]. Japan’s soil contamination controls and environmental basic laws establish the standard levels for Se and the countermeasures to be applied when the levels are exceeded, even for naturally-derived Se. Excavation muck, such as that from tunnel construction, is likely to contain Se [22, 23] leading to increased construction costs and delayed construction periods due to the countermeasures that need to be applied. In the natural environment, Se is found at low concentrations, unlike that observed for industrial wastewater, and thus the recycling of environmental Se has not been examined. If no regulated substances, such as Se, are included in the materials discharged from tunnel construction, the soil is reused (e.g., embankment materials) however, if Se is present in the soil, even after it has been treated, it may leach back into the environment and worsen the health damages to the soil. Therefore, the soil itself is not reused and is treated as waste.

| Reaction No. | Starting material | Valence | Purification method | Reaction | Refined product |
|---|---|---|---|---|---|
| 1 | Se(VI) | VI | Addition of Barium (II) | Ba2+ + SeO42- → BaSeO4 | BaSeO4 |
| 2 | Boil in concentrated HCl | H2SeO4 + 2HCl → H2SeO3 + Cl2 + H2O | Se(IV) | ||
| 3 | Se(IV), SeO2 | IV | Addition of Barium (II) | Ba2+ + SeO32- → BaSeO3 | BaSeO3 |
| 4 | Addition of Ascorbic acid | Ascorbic acid + SeO32- →Dehydroascorbic acid + Se(0) | Se(0) | ||
| 5 | Addition of Na2S2O4 | H2SeO3+Na2S2O4→Se(0)+Na2SO4 + SO2 + H2O | Se(0) | ||
| 6 | Addition of KI | H2SeO3 + 4KI + 4HNO3 → Se(0) + 2I2 + 4KNO3 + 3H2O | Se(0) | ||
| 7 | Bioselenium | 0 | Oxidizing roasting | Se(0) → SeO2 | SeO2 |
In Japan, most of the Se produced is exported as elemental Se or selenium dioxide to foreign countries. In this review, "recycling" is defined as the conversion to pure Se compounds that are on the market. Therefore literature on Se compounds obtained by conventional treatment methods and Se recycling methods are outlined, and the authors’ recycling method is introduced.
Se recovery using physicochemical treatment
Ion exchange [13, 17] and adsorbent [16, 24] methods have been studied as physicochemical treatments for Se. Shi et al. reported Se removals of 96% in 100 μg/L Se-containing wastewater by using strongly basic anion-exchange resins [13]. Although Se in an ion exchange membrane after treatment has not been mentioned, a solution that has been membrane-concentrated as a selenate or a selenite solution can be recovered by desorption from an ion exchange membrane.
A method for recovering barium selenate (BaSeO4) is via precipitation from a selenate solution [25]; it can then be further reduced to selenite by using a reducing agent[26]. BaSeO4 can be recovered from a selenate solution containing little contaminants by adding barium chloride as a precipitate (Table 2, Reaction 1). Moreover, BaSeO4 can be recycled because it is distributed at a price equivalent to elemental Se. However, it should be noted that the products of these reactions may compete with Se and considered as impurities if arsenic and sulfate ions are contaminants in the solutions. Selenate reduction to selenite (Table 2, Reaction 2) is also effective because it increases Se purification (Table 2, Reactions 3-6).
There is also a method by which a selenite solution is precipitated to recover barium selenite (BaSeO3) and further reduced to elemental Se. Like selenate solutions, selenite solutions can be purified to BaSeO3 by the addition of barium chloride [27]. However, when arsenic and sulfate ions are also contained as contaminants, this reaction may lead to impurities in the final product. It is also possible to reduce BaSeO3 to elemental Se by adding ascorbic acid and sodium sulfite [28]. The reduction and recovery of selenite at room temperature yields amorphous Se, which exhibits the same red color as elemental Se. Amorphous Se and iodine are precipitated by adding potassium iodide to a selenite solution under acidic conditions. As the precipitated iodine volatilizes as hydrogen iodide when sodium sulfite is added, elemental Se can be recovered by solid-liquid separation.
| Processing method | Additive | Initial Se concentration (mM) |
Recovery Se | Se purity of the recovered product | Selenium purity after refined | reference |
|---|---|---|---|---|---|---|
| Batch reactor | rice straw | 0.01 | Bioselenium | 208μg/L | ‒ | [54] |
| Sludge-blanket reactor | Thauera selenatis | 0.01 | Bioselenium | 237 μg/L | ‒ | [30] |
| Upflow Anaerobic Sludge Bed reactor | Sulfurospirillum barnesii | 0.01 | Bioselenium in polyacrylamide | 1194 mg/kg | ‒ | [31] |
| Membrane biofilm reactor | methane oxidizing consortium | 0.01 | Bioselenium | ‒ | ‒ | [55] |
| Membrane biofilm reactor | anaerobic biofilm | 0.03 | Bioselenium | ‒ | ‒ | [56] |
| Membrane biofilm reactor | methane oxidizing consortium | 0.06 | Bioselenium | ‒ | ‒ | [57] |
| Biotrickling filter | activated sludge | 0.09 | Bioselenium | ‒ | ‒ | [58] |
| Upflow Anaerobic Sludge Bed reactor | granularbsludge | 0.13 | Bioselenium | 437mg/g | ‒ | [59] |
| Batch reactor | Pseudomonas stutzeri NT-I | 0.5 | Bioselenium | 30% | 99% | [33], [34] |
| Mehtlseleninic adic | 0.04% | 99% |
Se recovery via microbial metabolism
By using the metabolism of bacteria to recover Se, a specific reaction is used, and as a result, the recovered product has fewer contaminants than the product obtained by physicochemical means. In addition to elemental selenium, the sludge mixture also contains bacteria cells, hereafter referred to as "bioselenium". The elemental Se that can be recovered by microbial metabolism also has antimicrobial activity inhibiting the viability of an Escherichia coli [29]. However, if it cannot be purified elemental selenium from bioselenium, it will not be reused. Selenium treatments utilizing specific Se metabolic pathways and recycling methods from recovered materials are discussed below.
Recovery and recycling by biomineralization
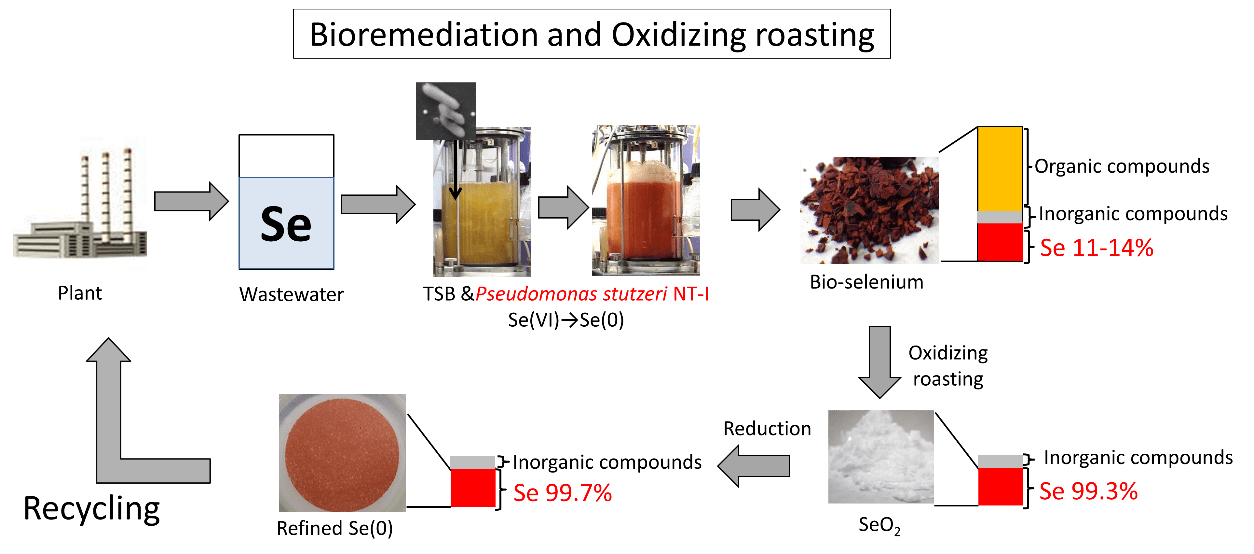
Reports of selenium wastewater treatment using microbial mineralization are shown in Table 3. Cantafio et al. recovered elemental selenium in a 186 day operation in selenium wastewater treatment using a sludge-blanket reactor by Thauera selenatis. The collected sludge mixture at this time contained selenium at a concentration of 0.237 mg/L (0.0000237%) [30]. Lenz et al. immobilized Sulfurospirillum barnesii with acrylamide and recovered elemental selenium in an Upflow Anaerobic Sludge Bed reactor. Elemental selenium was concentrated in acrylamide gels with a maximum Se concentration of 1194 mg/kg (0.1194%) in the sludge mixture after 58 days of incubation. Lenz et al. has proposed a purification process that uses the melting point difference between selenium and acrylamide gels to thermally dissolve the gel and purify Se from the gel [31]. A report using Pseudomonas stutzeri NT-I reports a series of steps in selenium wastewater treatment and selenium recycling from the recovered material [32–34]. This bacterium, which was isolated from the wastewater ditch-bottom sewage of a metal-recycling plant, reduced the Se in solution to elemental Se. In fact, P. stutzeri NT-I can reduce selenate and selenite in aqueous solution to non-toxic and insoluble elemental Se. Dimethyl diselenide (DMDSe) was biosynthesized from elemental Se with further P. stutzeri NT-I cultivation [32]. Taking advantage of the Se biomineralization ability of P. stutzeri NT-I, we recovered bioselenium at 87.8% and 78.8% of the initial concentration from simulated and real wastewater, respectively.
Bioselenium collected by centrifugation was red and X-ray diffraction analysis suggested the presence of amorphous Se [34]. Elemental Se in bioselenium is known to change its crystal structure depending on the drying temperature [34]. Bioselenium dried at 40 °C exhibited a red color (amorphous Se, red bioselenium) while bioselenium dried at 60 °C exhibited a black color (trigonal Se). In addition to Se, six other inorganic components are present in bioselenium: calcium, potassium, magnesium, sodium, phosphorus, and sulfur (Table 4).
Washing with hydrochloric acid and ethanol could concentrate the recovered bioselenium up to a Se concentration of 90%. However, contaminants could not be completely removed due to organic molecules derived from bacterial metabolism. For elemental Se, the retail price does not change depending on its crystalline structure but the higher the purity, the higher the price. As the purity of commercially available elemental Se is at least 99%, it is necessary to increase the purity of the recovered Se to 99% or more for recycling. Bioselenium is largely comprised of microbial-derived organic matter, which originate water and carbon dioxide by perfect combustion under pure oxygen. These organic substances can be easily removed from bioselenium; thus, if Se volatilization and separation from bioselenium is achieved, high-purity Se can be expected as well as its recycling. Therefore, selenium dioxide purification from bioselenium by oxidative roasting was attempted (Table 2, Reaction 7) [34]. Conditions of oxidized roasting were investigated, and selenium dioxide with 99% purity could be recovered when the Se in bioselenium was sufficiently oxidized (700 ℃, pure oxygen, gas flow at 100 mL/min). In addition, regardless of the Se crystal structure in bioselenium, selenium dioxide could also be recovered as amorphous and trigonal Se. The recovered selenium dioxide had a purity of 99% or more, but sulfur and calcium, which are thought to be derived from bacteria, were included as contaminants. Selenium dioxide was dissolved in water to form a selenite solution, which was then reduced to elemental Se by sodium sulfite. The sulfur and calcium contained in selenium dioxide were then removed from the purified elemental Se (Table 2, Reaction 5), and Se purity was further increased (Table 4). The purified elemental Se is equivalent in purity to the commercial elemental Se and may be reused for industrial materials and recycled. There are many reports on Se treatment methods using biomineralization (Figure 2). The Se in the sludge mixture may therefore be recovered as high-purity selenium dioxide by oxidative roasting.
| Element | Biomeneralization | Biovolatilization | |||
|---|---|---|---|---|---|
| Bioselenium | Refined SeO2 | Refined Se(0) | Methylseleninic Acid | Refined Se(0) | |
| Calcium | 0.3-0.4 | 0.1 | N.D. | N.D. | N.D. |
| Potassium | 0.9-1.1 | 0.2 | N.D. | N.D. | N.D. |
| Magnesium | 0.3 -0.4 | N.D. | N.D. | N.D. | N.D. |
| Sodium | 0.9 -1.1 | 0.3 | < 0.1 | N.D. | 0.4 |
| Phosphorus | 1.9 -4.2 | N.D. | N.D. | N.D. | N.D. |
| Sulfur | 1.8 -2.7 | 0.7 | N.D. | 0.03 | N.D. |
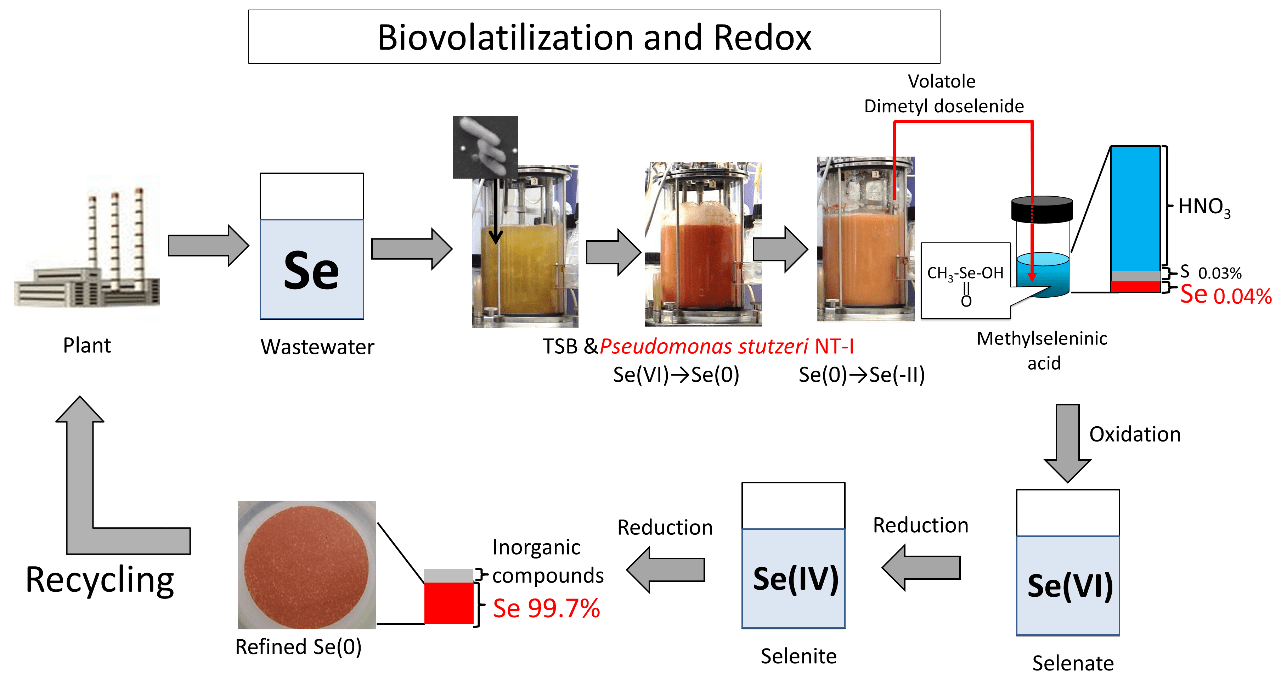
| Selenium | 11-14 | 99 | 99 | 0.04 | 99 |
N.D. : Not detected
Recovery and recycling using biovolatilization
Kagami et al. reported that P. stutzeri NT-I was able to reduce elemental Se to dimethyl diselenide (DMDSe) [35], meaning this unique bacterium carries out all reactions from selenate to DMDSe in a single cell. DMDSe is highly volatile and is easily removed from solutions. Kagami et al. also reported that Se in aqueous solution could be volatilized at 7.6 μmol/L/h in a flask and 14 μmol/L/h in Jarfarmenter culture. Gas-phase analysis showed that, in addition to DMDSe, dimethyl selenide (DMSe), dimethyl selenosulfide (DMSeS), and dimethyl disulfide (DMDS) were included in the gas phase of P. stutzeri NT-I culture. Using the Se vaporization ability of P. stutzeri NT-I, we recovered 71.2% and 38.9% of the initial Se concentration in nitric acid solution from simulated and real wastewater, respectively [33]. The only metallic elements detected in the nitric acid traps recovered in both simulated and real wastewater were Se and sulfur (Table 4). DMDSe in nitric acid becomes nonvolatile methylseleninic acid [36], which is difficult to use in the industry. Therefore, we tried to convert it into resource-valuable elemental Se (Figure 3). Methylseleninic acid recovered from the simulated wastewater contained 0.04% of Se and 0.03% of sulfur. A nitric acid solution containing methylseleninic acid was neutralized with sodium hydroxide; after adding hydrogen peroxide, the mixture was heated at 70 ℃ for 60 min for oxidation and conversion of methylseleninic acid into selenate. Subsequently, hydrogen chloride was added to reduce selenate and convert it into selenite (Table 2, Reaction 2). Finally, the reduction and conversion of ascorbic acid to elemental Se allowed the recovery of more than 99% pure Se (Table 2, Reaction 4).
Summary of wastewater treatment methods
When Se is physicochemically treated, contaminants may be easily included in the recovered Se compound due to non-specific reactions and therefore the purity of final product becomes low. On the contrary, microbial treatment can recover Se by specific reactions and less contaminants are therefore expected in the Se compound recovered; in addition, the purity of the final product becomes high. In particular, in the treatment using P. stutzeri NT-I, Se was recovered from wastewater by Se biomineralization and biovolatilization, and the recovered material was successfully recycled to elemental Se by further physicochemical treatment (Figure 2, Figure 3). However, microbial treatment takes longer than physicochemical treatment, which is a disadvantage of this method.
Different from the recycling of Se, research on the synthesis of high-value-added products by bacteria was also carried out. Kuroda et al., for example, synthesized bismuth selenide, which is used as a thermo-electroconversion element, by culturing P. stutzeri NT-I with selenite and bismuth mixed solutions [37]. Thus, instead of recovering only Se from wastewater, it is possible to simultaneously treat wastewater and synthesize bismuth selenide, a high-value-added product.
When Se wastewater is treated and the recovered Se is recycled, it is necessary to examine the final purified product morphology and the contaminants contained in Se wastewater via combined analyses.
Se treatment methods for contaminated soil
The difficult processing of selenate is also a social problem concerning soil treatment. In Japan, the revision of the soil contamination control law in 2010 implied acting on metal concentration in soil exceeding the environmental standard, even for naturally-derived metals including arsenic, boron, cadmium, chromium, fluorine, mercury, lead, and Se. In many cases, heavy metals of natural origin are detected in workers handling large amounts of soil and sand, and the prolongation of the construction period due to countermeasures together with the cost of treatments have become a problem. Selenate in particular has attracted attention because of its low reactivity and difficulty in treatment. Therefore, the following sections outline the treatment methods used in Japan for Se-containing soil and introduce a new soil treatment method currently being examined by the authors.
Water-deprivation containment
Soils containing Se have undergone "water-deprivation containment" in which water-deprivation sheets were laid around contaminated soils to achieve environmental standards [38–41]. Physically intercepting the contact between rainwater and contaminated soil using impermeable sheets also prevents the leaching of heavy metals in contaminated soil. This is an excellent technique that can respond to all concentrations of contamination because it prevents stormwater from entering the soil. At present, it is mainstream to lay a total of five layers (three layers of protective mats plus two layers of impervious sheets) but applying this arrangement to cover the bottom, side, and top of contaminated soil presents a high work burden and large amount of material used, which leads to high treatment costs. Water-soluble Se contained in the soil remains in situ in the same form even after containment but when water-deprivation sheets surrounding contaminated soils are damaged due to earthquakes or other natural occurrences, the water-soluble Se may leak. Thus, the water-deprivation containment method continues to pose the risk of Se leakage. Furthermore, if water-soluble Se remains in the soil, soil cannot be reused.
Insolubilization
As the cost of "water-deprivation containment" is high, the heavy metal insolubilization treatment, which has a 2-day curing period and low cost, has gained increasing attention. This insolubilization method can reduce the solubility of Se in soil by mixing drugs and other substances at concentrations adjusted to the amount of contaminated soil [42, 43]. In the case of selenite contamination, it has been shown to exerts a substantial effect. However, selenate in soil as well as in wastewater has low reactivity with drugs, and therefore a large amount of chemicals is required to meet environmental standards increasing the cost of soil treatment using this method. Although the insolubilization method can prevent the leaching of Se from contaminated soil, it poses the risk of re-elution of the Se remaining in the soil. Therefore, soils contaminated with Se and treated by insolubilization cannot be reused to prevent the spread of health hazards.
Soil washing
The soil washing technique can reduce the amount of heavy metals in contaminated soil by using water and/or solvents in the total amount of contaminated soil [44, 45]. By washing out water-soluble Se from the soil and further separating the fine granules that tend to elute rapidly, the residual amount of Se in the soil becomes lower than the environmental standard while the cleaning solution (water/solvent) contains a large amount of Se at low concentration. Because the water-soluble Se is removed by this process, the soil can be reused. Although not suitable for vegetation, this soil can be used industrially for embankments as organic components and other compounds are also washed out together with Se.
Adsorption layer method
The adsorption layer method can reduce the heavy metal concentration of leached water passed through an adsorption layer consisting of an adsorbent and a base material [46, 47]. For instance, when the leachate of excavation muck passes through an adsorption layer laid in the bottom of the contaminated soil due to rainfall, it can react with the adsorbent in the adsorption layer, which reduces the concentration of heavy metals in the leachate. In recent years, a sheet-like adsorption layer containing a standardized adsorbent has been developed, and an increasing number of treatment cases have used this method characterized by low work-load and relatively low cost because it is not necessary to treat the whole soil. As Se in the contaminated soil moves along with rainwater to the adsorption layer, water-soluble Se is also removed over time. However, as there is no method of evaluating if water-soluble Se has been completely removed from the contaminated soil, the excavation muck is not reused.
Biological metabolism method
Microbial metabolism-based (bioremediation) and plant metabolism-based (phytoremediation) methods have been attempted to insolubilize Se in selenate-contaminated soils that are difficult to treat using other methods [48–50]. However, these methods take longer to achieve the desired result when compared with existing engineering methods. Since P. stutzeri NT-I has a fast selenate reduction rate at 8.8×10-16 mol/h/cell, we considered using the bacterium as an alternative to non-specific drugs currently used for insolubilizing Se in soil. The experiments in which selenate in soils could be insolubilized by P. stutzeri NT-I are described below [51].
Tunnel excavation mucks were collected and used to simulate contaminated soil. The harvested excavation shears were crushed and partitioned to produce samples of 1−2 mm in diameter. When the soil dissolution test was carried out using these soil samples, the Se concentration did not exceed the environmental standard. According to the Ministry of the Environment, Se concentration in the eluted soil cannot exceed approximately 10 times the environmental reference value to be considered as natural Se contamination. Therefore, we added an aqueous sodium selenate solution to our soil samples so that the soluble Se concentration was less than ten times the environmental standard value (soil elution in mg/L); thus, simulating natural Se contamination.
To investigate Se mass balance in soil, a P. stutzeri NT-I suspension (optical density at 600 nm = 1.0; pH 7.0) was added at 100% to 100 g of simulated contaminated soil and allowed to stand at 38 °C. P. stutzeri NT-I reduces selenate to insoluble elemental Se in aqueous solution but also to DMDSe. As P. stutzeri NT-I reduces selenate to at least elemental Se in the soil. The phase change of soluble Se in the simulated contaminated soil after adding P. stutzeri NT-I was therefore investigated over time (Figure 4). When only sterile medium was added to the simulated contaminated soil, the Se concentration remained almost constant, but the environmental standard value was achieved in 72 h after P. stutzeri NT-I addition (Figure 4 (a)). In addition, a marked decrease in soluble Se concentration and increase in insoluble Se concentration was observed 24 h after P. stutzeri NT-I addition (Figure 4 (b)); 72 h after adding P. stutzeri NT-I, 94% of soluble Se was removed. This removal rate was the highest among the conditions tested so far. Analysis showed that approximately 5% of the initial concentration was detected as soluble Se and 49% as insoluble Se 72 h after P. stutzeri NT-I addition. Therefore, about 46% of the soluble Se in the initial soil was likely removed via P. stutzeri NT-I metabolism.
The Se species in the gas phase of the tested soils were measured by gas chromatograph-mass spectrometer (GC-MS), but Se metabolites such as DMDSe were not detected. However, the previous findings suggest that the soluble Se in soil was reduced to DMDSe via the high Se vaporization capacity of P. stutzeri NT-I [35], which could convert 82% of the initial Se concentration in aqueous solution to DMDSe in 48 h. However, the synthesis of DMDSe by P. stutzeri NT-I is strongly influenced by aeration volume; the DMDSe recovery rate in the flask test without aeration was 76% but when forced aeration was used with a culture device the recovery rate increased to 82%. Therefore, forced aeration of contaminated soil by pumping may facilitate Se elimination by P. stutzeri NT-I. These results indicate that bacterial treatment of Se-contaminated soils not only insolubilizes the selenate in soil, but also removes Se from soils by reducing it to DMDSe. As DMDSe is synthesized in the 48 h after treatment with P. stutzeri NT-I and Se is removed, the soil can be reused as noncontaminated soil faster than when treated by the adsorption layer method. Because only Se is specifically removed from the soil after bacterial treatment, it can potentially be reused as a vegetation soil. Therefore, the removal of Se by P. stutzeri NT-I may be the most efficient treatment for naturally occurring Se-contaminated soils in Japan. However, several problems remain to be solved in the practical application of microbial treatment, such as the effect of the input microorganisms on the surrounding ecosystem.

(a) Time course of the selenium amount of soil elution. (Open circles): addition of P. stutzeri NT-I; (Cross marks): addition of sterile medium (without P. stutzeri NT-I).
(b) Mass balance (%). Black area: soluble selenium; shaded area: insoluble selenium
A new treatment method to enrich metal-contaminated soil has been proposed for treated soils that can be used for supporting vegetation. It consists of flower remediation, adding the value of landscape beautification to metal-treated soil [52, 53]. Treatment of heavy metal-contaminated soils, such as those contaminated by cadmium and lead, are currently being considered. Flower remediation of Se-contaminated soil may also be possible, as several plants capable of metabolizing Se have been reported, and therefore this treatment method should be carefully examined.
Summary of soil treatment methods
Selenium-containing soil treatment also has advantages and disadvantages. The impervious sheet method can physically enclose, but there is a risk of the selenium inside leaking out if the sheet avoids it. Although the insolubilization method has a low risk of selenium leakage, it requires the chemical to be mixed with the whole soil and the construction is complicated. Soil washing has a low risk of selenium leakage, but it also has a risk of secondary contamination from the low-concentration selenium solution produced by washing. The adsorbed layer method is easy to construct, but there is a risk of leakage if selenium above the assumed concentration is piled on the adsorbed layer. Treatment using biological metabolism is easy to install, but there are still issues to be overcome before commercialization. In construction sites where large amounts of soil are transferred, such as tunnel excavation sites, natural Se-contaminated soil is produced. Treatment conditions (season, amount of soil, area available for treatment, cost, and surrounding environment) vary from site to site, and therefore the treatments that can be applied also differ. Thus, not all naturally occurring Se-contaminated soils can be reused after treatment.
It has become established that selenium, in particular selenate, is difficult to treat, and the goal is to achieve environmental standards both for selenium-containing water and soil treatment. In addition, Se is cheaper than other rare metals and no significant profit can be expected when it is recycled. However, the recycling of rare metals is important to fulfill the SDGs established by the United Nations, and the recovery and reuse of Se is no exception. As outlined, there are several methods for Se treatment and further purification of the recovered Se compounds. At present, it is possible to retain the resource value of the recovered Se by selecting a suitable treatment method based on the properties of the Se-containing waste.
The authors declare no conflict of interest associated with this manuscript.