Abstract
Background: Most children access early human immunodeficiency virus type 1 (HIV-1) diagnosis in Kenya. However, the detection frequency of HIV-1 drug-resistance mutations (DRMs) among the children, main cause of their antiretroviral therapy (ART) failure, has not been well known. This study aimed at investigating the DRM trends in newly HIV-1-diagnosed Kenyan children between 2014 and 2018.
Methods: Dried blood spots (DBS) were collected from children with HIV-1 under 18 months of age through the Kenya Early Infant Diagnosis program in 2014, 2017 and 2018 (n = 57, 70, and 50, respectively). HIV-1 proviral DNA was extracted from the DBS and analyzed genetically. DRMs were checked following the IAS-USA list and/or Stanford HIV-1DB PROGRAM algorithm.
Results: Among the Kenyan children with HIV-1, DRMs were detected in 57.9% [nucleoside reverse transcriptase inhibitors (NRTI)-DRM/non-NRTI (NNRTI)-DRM: 22.8%/57.9%] in 2014, 54.3% (11.4%/54.3%) in 2017, and 58.0% (14.0%/58.0%) in 2018. All children who had NRTI-DRMs had NNRTI-DRMs. As for NNRTI-DRMs, Y181C was found more in 2014 than 2017/2018 (28.1% vs. 7.1%/6.0%, p = 0.0002), whereas K103N/S more in 2017/2018 than 2014 (37.1%/34.0% vs. 17.5%, p = 0.026). Among the children with DRMs, 94.9% and 67.7% showed high-level resistance to nevirapine and efavirenz (NNRTI), respectively. The mother’s PMTCT history was significantly associated with the NNRTI-DRMs in all years.
Conclusion: Majority of newly HIV-1-diagnosed Kenyan children continuously harbored DRMs between 2014 and 2018, which probably originated from their mothers’ PMTCT. Checking DRMs before starting ART and/or using non-NNRTI-containing regimen for ART should be considered in children with HIV-1 in Kenya.
Introduction
Vertical transmission of human immunodeficiency virus type 1 (HIV-1) accounts for the majority of pediatric HIV-1 cases in the world1). In 2017, 180,000 [130,000–240,000] children less than 15 years of age were newly infected with HIV-12). More than 95% of these cases are via mother-to-child transmission (MTCT) with an estimated rate of 14–48% without intervention1,3,4), and 87% of them live in Sub-Saharan Africa4). MTCT of HIV-1 can occur in utero, intrapartum, and postpartum through breastfeeding1,3,5).
Prevention of mother-to-child transmission (PMTCT) programs have provided a range of services for women of reproductive age living with HIV-1 or at risk of acquiring HIV-1 to maintain their health and prevent their children from acquiring HIV-1. Effective interventions during pregnancy, labour, delivery, and breastfeeding have reduced the MTCT rates from 15–45% to below 5%1,3,5). Due to the global milestones achieved by PMTCT interventions and scale up, new pediatric HIV-1 infections have been declining, but not fully eliminated1,3,5,7). In developed countries, MTCT of HIV-1 is rarely seen anymore through measures such as combination antiretroviral therapy (cART), selective caesarian delivery, and avoidance of breastfeeding1,2,8,9). However, this is still a challenge in resource-limited settings such as Kenya in Sub-Saharan Africa, where the risk of transmission remains high, bearing the highest burden of HIV-1 infections 7,10,11).
In Kenya, PMTCT program is supplemented with the early infant diagnosis (EID) program for the infants who are born to mothers with HIV-1 to start ART as soon as possible after HIV-1 infection is diagnosed, according to the National AIDS & STI Control (NASCOP, the Kenya Ministry of Health) guidelines12). The EID program in Kenya has tested more than 56,000 children annually since its inception in 200513). The estimated newly diagnosed children with HIV-1 decreased from 33,000 in 2005 to 11, 000 in 2014 and further to 4,500 by 201814).
High genetic variants of HIV-1 are due to inherent properties in its reverse transcriptase (RT), which lacks proof-reading function and often makes mistakes during virus RNA reverse-transcription. Given the necessity of lifelong treatment for HIV-1 and fears of emergence of drug resistance mutations (DRMs), it is important to establish a robust and routine population-level surveillance and monitoring of HIV-1 DRMs. This is expected to improve health outcomes and minimize ART failure in the affected population, though sustainable and optimum adherence to ART is also important in ensuring treatment success11,15,16). In the pregnant women with HIV-1, HIV-strains with DRMs could be emerged by ART-driven selection, and vertically transmitted to their children17,18), though some studies suggested that the DRMs could be developed in children de novo18).
HIV-1 DRMs surveillance and monitoring systems and infrastructure are available for the improvement of PMTCT/pediatric care and treatment choice19,20). However, data on HIV-1 DRMs among newly diagnosed infants in Kenya remains scanty. In this study, we aimed at investigating the trends in the DRMs among Kenyan children newly diagnosed with HIV-1 infection in 2014, 2017, and 2018 to guide more successful ART in children.
Materials and Methods
Study Design
This was a cross-sectional study nested to the National Early Infant Diagnostic Program (EID) in Kenya to diagnose HIV-1 infection among children born to HIV-1-positive mothers. Remnant dried blood spots (DBS) samples collected from the children newly diagnosed with HIV-1 in 2014, 2017, and 2018 were used for genotypic analyses, such as HIV-1 DRMs and genotyping. Information on their demography, their mothers’ PMTCT interventions, and infant prophylaxis was abstracted from requisition forms accompanying the DBS. Sample collection was done after the parental or guardian consent.
Study Population
The study samples were drawn from the EID central testing laboratories receiving samples from children born to HIV-1-positive mothers in health facilities throughout Kenya. The points of entry into the EID program include maternal and child health (MCH/PMTCT) clinics, maternity and pediatric wards, comprehensive care clinics/patient support center (CCC/PSC), and inpatient/outpatient units. Diagnosis of HIV-1 infection in the children was made using a rapid method as previously reported 21). We obtained the remnant DBS specimens of HIV-1 positive children under 18 months of age in 2014 (n = 57), 2017 (n = 70) and 2018 (n = 50) (Table 1).
Table 1.The factors for DRMs found in HIV-1-infected Kenyan children
| Factors |
2014 |
aOR (95% CI) |
| n |
NRTI-DRMs |
NNRTI-DRMs |
NRTI-DRMs |
NNRTI-DRMs |
| Overall |
57 |
13 (22.8) |
33 (57.9) |
|
|
| p value |
|
<0.001 |
|
|
|
| Gender |
|
|
|
|
|
| Female |
25 |
6 (24.0) |
14 (56.0) |
|
|
| Male |
32 |
7 (21.9) |
19 (59.4) |
|
|
| p value |
|
0.85 |
0.80 |
|
|
| HIV subtype |
|
|
|
|
|
| A |
38 |
9 (23.7) |
25 (65.8) |
|
|
| B |
2 |
0 |
0 |
|
|
| C |
6 |
0 |
2 (33.3) |
|
|
| D |
9 |
2 (22.2) |
4 (44.4) |
|
|
| G |
2 |
2 (100.0) |
2 (100.0) |
|
|
| p value (A vs. others) |
|
1.0 |
0.09 |
|
|
| Mothers’ PMTCT |
|
|
|
|
|
| Yes |
36 |
12 (33.3) |
26 (72.2) |
10.0 (1.2–83.7) |
5.2 (1.6–16.7) |
| No |
21 |
1 (4.8) |
7 (33.3) |
|
|
| missing data |
0 |
|
|
|
|
| p value |
|
0.02 |
0.004 |
|
|
| Breast feeding |
|
|
|
|
|
| Yes |
45 |
12 (26.7) |
28 (62.2) |
|
|
| No |
12 |
1 (8.3) |
5 (41.7) |
|
|
| p value |
|
0.26 |
0.20 |
|
|
| Age group |
|
|
|
|
|
| 1–6 months |
37 |
8 (21.6) |
21 (56.8) |
|
|
| 7–18 months |
20 |
5 (25.0) |
12 (60.0) |
|
|
| missing data |
0 |
|
|
|
|
| p value |
|
0.75 |
0.81 |
|
|
| Factors |
2017 |
aOR (95% CI) |
| n |
NRTI-DRMs |
NNRTI-DRMs |
NRTI-DRMs |
NNRTI-DRMs |
| Overall |
70 |
8 (11.4) |
38 (54.3) |
|
|
| p value |
|
<0.001 |
|
|
|
| Gender |
|
|
|
|
|
| Female |
31 |
4 (12.9) |
19 (61.3) |
|
|
| Male |
39 |
4 (10.3) |
19 (48.7) |
|
|
| p value |
|
1.0 |
0.29 |
|
|
| HIV subtype |
|
|
|
|
|
| A |
49 |
5 (10.2) |
26 (53.1) |
|
|
| B |
4 |
0 |
2 (50.0) |
|
|
| C |
5 |
0 |
3 (60.0) |
|
|
| D |
11 |
2 (18.2) |
6 (54.5) |
|
|
| G |
1 |
1 (100.0) |
1 (100.0) |
|
|
| p value (A vs. others) |
|
0.69 |
0.75 |
|
|
| Mothers’ PMTCT |
|
|
|
|
|
| Yes |
58 |
8 (13.8) |
37 (63.8) |
|
19.4 (2.3–160.8) |
| No |
12 |
0 |
1 (8.3) |
|
|
| missing data |
0 |
|
|
|
|
| p value |
|
0.33 |
<0.001 |
|
|
| Breast feeding |
|
|
|
|
|
| Yes |
54 |
6 (11.1) |
32 (59.3) |
|
|
| No |
16 |
2 (12.5) |
6 (37.5) |
|
|
| p value |
|
1.0 |
0.13 |
|
|
| Age group |
|
|
|
|
|
| 1–6 months |
35 |
6 (17.1) |
20 (57.1) |
|
|
| 7–18 months |
35 |
2 (5.7) |
18 (51.4) |
|
|
| missing data |
0 |
|
|
|
|
| p value |
|
0.26 |
0.63 |
|
|
| Factors |
2018 |
aOR (95% CI) |
| n |
NRTI-DRMs |
NNRTI-DRMs |
NRTI-DRMs |
NNRTI-DRMs |
| Overall |
50 |
7 (14.0) |
29 (58.0) |
|
|
| p value |
|
<0.001 |
|
|
|
| Gender |
|
|
|
|
|
| Female |
24 |
3 (12.5) |
12 (50.0) |
|
|
| Male |
26 |
4 (15.4) |
17 (65.4) |
|
|
| p value |
|
1.0 |
0.27 |
|
|
| HIV subtype |
|
|
|
|
|
| A |
41 |
7 (17.1) |
26 (63.4) |
|
|
| B |
2 |
0 |
0 |
|
|
| C |
0 |
0 |
0 |
|
|
| D |
5 |
0 |
3 (60.0) |
|
|
| G |
2 |
0 |
0 |
|
|
| p value (A vs. others) |
|
0.33 |
0.14 |
|
|
| Mothers’ PMTCT |
|
|
|
|
|
| Yes |
38 |
6 (15.8) |
25 (65.8) |
|
6.7 (1.2–37.2) |
| No |
10 |
0 |
2 (20.0) |
|
|
| missing data |
2 |
1 (50.0) |
2 (100.0) |
|
|
| p value |
|
0.32 |
0.013 |
|
|
| Breast feeding |
|
|
|
|
|
| Yes |
45 |
7 (15.6) |
28 (62.2) |
|
|
| No |
5 |
0 |
1 (20.0) |
|
|
| p value |
|
1.0 |
0.15 |
|
|
| Age group |
|
|
|
|
|
| 1–6 months |
29 |
3 (10.3) |
18 (62.1) |
|
|
| 7–18 months |
20 |
4 (20.0) |
11 (55.0) |
|
|
| missing data |
1 |
0 |
0 |
|
|
| p value |
|
0.42 |
0.62 |
|
|
| Factors |
Total |
aOR (95% CI) |
| n |
NRTI-DRMs |
NNRTI-DRMs |
NRTI-DRMs |
NNRTI-DRMs |
| Overall |
177 |
28 (15.8) |
100 (56.5) |
|
|
| p value |
|
<0.001 |
|
|
|
| Gender |
|
|
|
|
|
| Female |
80 |
13 (16.3) |
45 (56.3) |
|
|
| Male |
97 |
15 (15.5) |
55 (56.7) |
|
|
| p value |
|
0.89 |
1.0 |
|
|
| HIV subtype |
|
|
|
|
|
| A |
128 |
21 (16.4) |
77 (60.2) |
|
|
| B |
8 |
0 |
2 (25.0) |
|
|
| C |
11 |
0 |
5 (45.5) |
|
|
| D |
25 |
4 (16.0) |
13 (52.0) |
|
|
| G |
5 |
3 (60.0) |
3 (60.0) |
|
|
| p value (A vs. others) |
|
0.73 |
0.11 |
|
|
| Mothers’ PMTCT |
|
|
|
|
|
| Yes |
132 |
26 (19.7) |
88 (66.7) |
10.1 (1.3–76.5) |
6.4 (2.9–14.4) |
| No |
43 |
1 (2.3) |
10 (23.3) |
|
|
| missing data |
2 |
1 (50.0) |
2 (100.0) |
|
|
| p value |
|
0.01 |
<0.001 |
|
|
| Breast feeding |
|
|
|
|
|
| Yes |
144 |
25 (17.4) |
88 (61.1) |
|
2.8 (1.2–6.4) |
| No |
33 |
3 (9.1) |
12 (36.4) |
|
|
| p value |
|
0.24 |
0.01 |
|
|
| Age group |
|
|
|
|
|
| 1–6 months |
101 |
17 (16.8) |
59 (58.4) |
|
|
| 7–18 months |
75 |
11 (14.7) |
41 (54.7) |
|
|
| missing data |
1 |
0 |
0 |
|
|
| p value |
|
0.70 |
0.62 |
|
|
DRMs: HIV-1 drug resistance mutations; NRTI: nucleoside and nucleotide analogue reverse transcriptase inhibitors; NNRTI: Nonnucleoside analogue reverse transcriptase inhibitors; PMTCT: prevention of mother to child transmission; ( ): positive percentage; p value for each factor: from Chi-square or Fisher exact test; aOR: adjusted odds ratio, from multivariable logistic regression included all factors.
Extraction of DNA from DBS was carried out using the QIAamp® DNA Micro Kit (QIAGEN Ltd., Crawley, UK) according to the Protocol of isolation of genomic DNA from dried blood spots in the manufacturer’s Kit handbook. Three-millimeter diameter disks were punched from each DBS specimen card into a 1.5 ml micro centrifuge tube for DNA extraction. The downward steps included: lysis, binding, washing and elution of DNA. Purified DNA was stored at -30°C till PCR reaction.
Amplification and Sequencing
The partial pol gene encoding reverse transcriptase (RT) was amplified on a GeneAmp 9700 thermocycler (Applied Biosystems, Foster City CA, USA) using specifically designed primers. The HIV-1 pol-RT region was amplified using the primers: RT18 (5'-GGAAACCAAAAATGATAGGGGGAATTGGAGG-3') and KS104 (5'-TGACTTGCCCAATTTAGTTTTCCCACTAA-3') for the first PCR reaction and KS101 (5'-GTAGGACCTACACCTGTTCAACATAATTGGAAG-3') and KS102 (5'-CCCATCCAAAGAAATGGAGGAGGTTCTTTCTGATG-3') for nested reaction. The cycling conditions were one cycle at 94°C for 2 minutes, then 35 cycles at 98°C for 10 seconds, 45°C for 30 seconds, and 68°C for 90 seconds, with a final extension of 68°C for 5 minutes. Nested PCR was performed with one cycle at 94°C for 2 minutes, 35 cycles at 98°C for 10 seconds, 45°C for 30 seconds and 68°C for 90 seconds, and a last cycle of 68°C for 5 minutes as previously described22).
The PCR products were confirmed with gel electrophoresis. The purified HIV-1 RT-PCR positive fragments were then sequenced using BigDye Terminator v1.1 on a 3500XL sequencer (Applied Biosystems, USA) as previously described22).
Drug Resistance Mutation and HIV-1-Subtyping
The sequencing data were first analyzed using GENETYX Ver. 9.1.0 (GENETYX CORPORATION, Japan), and sent to the Stanford HIV-1DB PROGRAM for the analyses of DRMs, the mutation resistance scores, and HIV-1-subtyping as previously described23–25). HIV-1 genotypic drug resistance was defined as the presence of one or more resistance-related mutations as specified by the consensus mutation guidelines of the International AIDS Society-USA26). The estimated resistance levels to antiretroviral drugs such as nucleoside reverse transcriptase inhibitors (NRTI) and non-NRTI (NNRTI) were determined based on the recommendation by the Stanford HIVDB program24,25). HIV-1 subtyping was re-confirmed by CLUSTALW phylogenetic analysis in MEGA 6 after sequence alignment. The 23 references were selected from Los Alamos 2018 HIV Sequence Compendium and (http://hiv-eb.lanl.gov) and Stanford HIVdb and retrieved from Genbank.
Sequence Data
The generated sequences in this study were deposited in National Center for Biotechnology Information Genbank (https://www.ncbi.nlm.nih.gov/genbank/) under accession numbers KT213606–KT213662 and MT497617–MT497736.
Statistical Analysis
SPSS 25.0 was used for statistical analyses where statistical significance was defined as p < 0.05. Chi-square or Fisher’s Exact Probability Tests were used to compare the frequency of resistance mutation between years or groups of children within the same year. Mann-Whitney U test was used for comparing the numbers of DRMs and resistance scores. McNemar’s Test was used for comparing the frequency of NRTI and NNRTI resistance mutations in the same year and the relationship of DRMs and PMTCT with nevirapine- or efavirenz-containing regimen. Multivariable logistic regression was used for analysis of the potential factors for DRMs.
Study Approval
The protocol was approved by the Scientific and Ethics Review committee of the Kenya Medical Research Institute (SERU) under approval number KEMRI/SERU/CVR/006/3451. The remnant DBS specimens were anonymized with unlinked codes.
Results
Characteristics of the Study Participants
DBS were collected from a total of 177 children in 2014 [n = 57 (male/female: 32/25), median age: 3.0 months (range 1.5–15.0 months)], in 2017 [n = 70 (39/31), 7.0 months (1.0–16.0)], and in 2018 [n = 50 (26/24), 5.8 months (1.8–16.5)]. The gender proportion of the children did not significantly differ among the years (p = 0.894), whereas the age differed (p = 0.021). More children were born to mothers who were on PMTCT program in 2017 and 2018 than in 2014 (82.9% and 79.2% vs. 63.2%, p = 0.029), and more mothers received cART for their PMTCT in 2017 and 2018 than in 2014 (100.0% in 2017 and 2018, and 69.4% in 2014, p < 0.001). In 2017 and 2018, cART regimen used for mothers’ PMTCT was mainly zidovudine/lamivudine/efavirenz (AZT/3TC/EFV) or tenofovir (TDF)/3TC/EFV. The proportion of the children who received breast milk did not statistically differ among the three years (78.9%, 77.1%, and 90.0%, respectively, p = 0.174). The information of the children’s prophylaxis history was obtained only from 24 (48.0 %) children in 2018, thus this information was not included in further analyses (Table 1).
HIV-1 Subtypes
Based on HIV-1 RT sequences, five HIV-1 subtypes: A, B, C, D, and G, were found in this study. The most common subtype detected in all years was subtype A (66.7%, 70.0%, and 82.0% in 2014, 2017, and 2018, respectively), followed by subtype D (15.8%, 15.7%, and 10.0%). The distribution of HIV-1 subtypes did not significantly differ among the three years (p = 0.443) (Table 1, Supplementary Fig. 1–2).
HIV-1 Drug Resistance Mutations
Among the Kenyan children newly diagnosed with HIV-1 infection, DRMs were found in 57.9% (33/57), 54.3% (38/70) and 58.0% (29/50) in the years 2014, 2017, and 2018, respectively. DRM frequencies did not significantly differ among the three years (p = 0.60). NRTI associated DRMs were found in 22.8%, 11.4% and 14.0% of the children in 2014, 2017, and 2018, respectively (p = 0.20) (Fig. 1A). All children who had NRTI-DRMs had NNRTI-DRMs (Table 1).

Fig. 1.The HIV-1 drug resistance mutations (DRMs) found in Kenyan children. (A) The detection frequencies of the HIV-1 with resistance mutations to nucleoside and nucleotide analogue reverse transcriptase inhibitors (NRTI) and non-nucleoside analogue reverse transcriptase inhibitor (NNRTI) in 2014, 2017, and 2018. (B) The frequencies of major NRTI- (left panel) and NNRTI- (right panel) resistance-related mutations found in participants by year. p values from Chi-square test. (C) The number of resistance mutations harbored by children with DRMs in each year. Bold bar indicates the median numbers of the resistance mutations detected. p value from Mann-Whitney U test. (D) The proportions of the children with DRMs by their number. p values from Chi-square test when comparing the children with one and more than one DRMs.
As for NRTI-DRMs, M184I/V was found most in all three years (14.0%, 10.0%, and 6.0% in 2014, 2017, and 2018, respectively) (Fig. 1B left). As for NNRTI-DRMs, the frequency of Y181C was significantly higher in 2014 (28.1%) than in 2017 (7.1%) and 2018 (6.0%) (p = 0.002 and p = 0.003, respectively), whereas the frequency of K103N/S was significantly higher in 2017 (37.1%) and marginally in 2018 (34.0%) than in 2014 (17.5%) (p = 0.015 and p = 0.051, respectively) (Fig. 1B right).
We also compared NNRTI-DRMs found in the children with cART regimen used for their mothers’ PMTCT. In the children born to the mothers who received efavirenz-containing regimen, K103N/S was detected significantly more than Y181C (43.9% vs. 3.0%, p < 0.0001), whereas in the children born to the mothers who received nevirapine-containing regimen, Y181C was marginally more than K103N/S (25.0% vs. 18.8%) (p = 0.063) (Table 2, supplementary table 1).
Table 2.The relationship of mother’s PMTCT with K103N/S and Y181C mutation
| cART regimen used for mothers’ PMTCT |
n |
K103N/S |
Y181C |
K103N/S & Y181C |
McNemar test p value |
| nevirapine-containing |
16 |
3 (18.8) |
4 (25.0) |
0 |
0.063 |
| efavirenz-containing |
66 |
29 (43.9) |
2 (3.0) |
1 (1.5) |
<0.0001 |
| p value |
|
0.064 |
0.012 |
1.0 |
|
The mother’s PMTCT regimen was from those clearly reported PMTCT containing nevirapine or efavirenz; cART: combination of antiretroviral therapy; PMTCT: prevention of mother to child transmission.
In the children who had DRMs, the number of DRMs was found slightly more in 2014 (median: 2, range: 1–7) than in 2017 (1, 1–7, p = 0.094) and in 2018 (1, 1–4, p = 0.230), though the difference was not significant (Fig. 1C). Significantly more children had multiple DRMs in 2014 than in 2017 and 2018 (63.6% vs. 36.8% and 37.9%, p = 0.024 and 0.043, respectively) (Fig. 1D).
Resistance Mutation Scores and Estimated Resistance to NRTIs and NNRTIs
The total resistance mutation scores both against NRTI and NNRTI in the children with DRMs were significantly higher in 2014 [median 210 (range 85–485)] than in 2017 [130 (40–705), p = 0.047] and marginally higher than that in 2018 [130 (25–540), p = 0.057] (Fig. 2A).
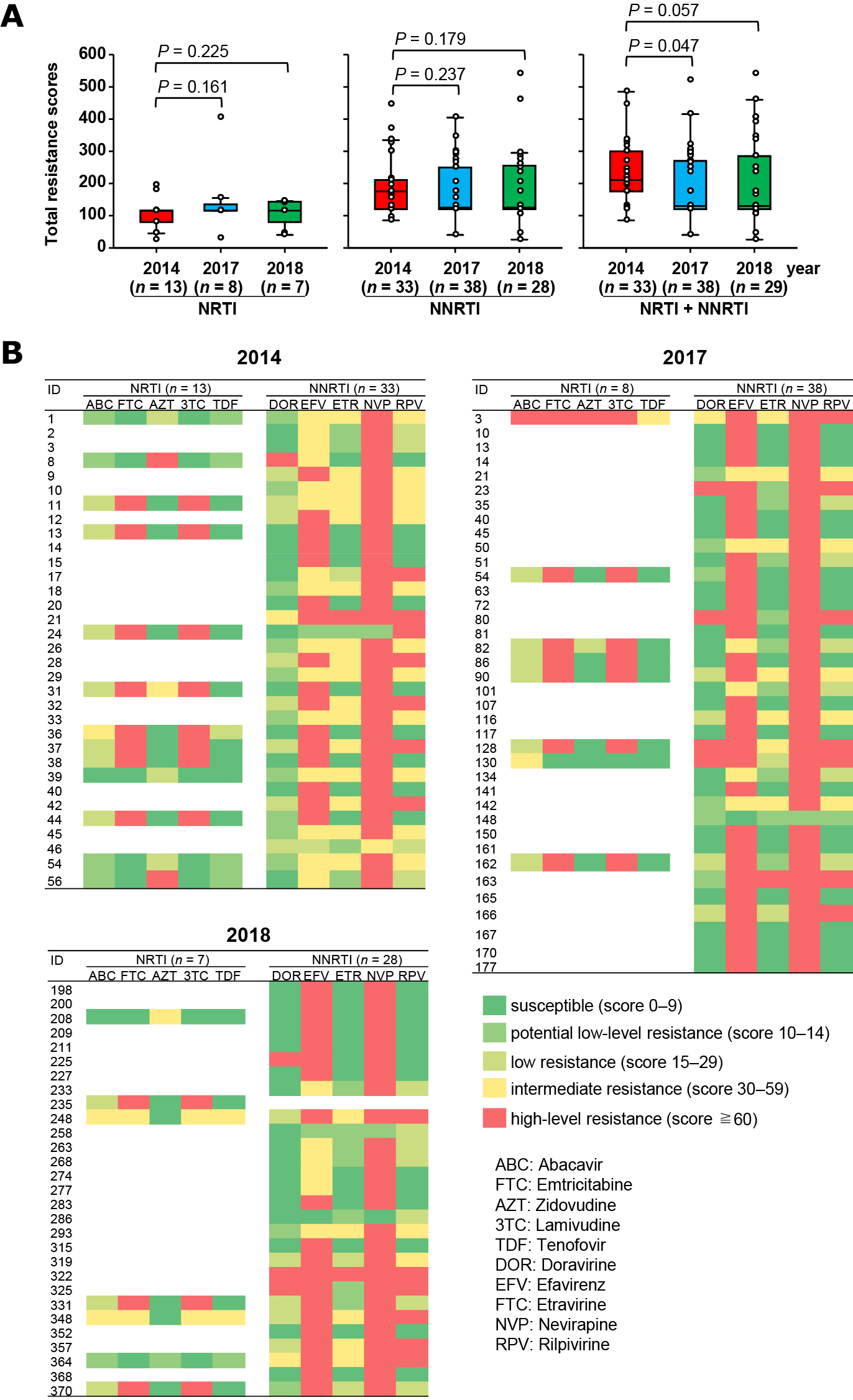
Fig. 2.The total resistance mutation scores and estimated level of resistance to NRTI and NNRTI in participants who harbored HIV-1 with drug resistance mutations. (A) The total resistance mutation scores to NRTI, NNRTI and both in 2014, 2017, and 2018. The resistance mutation scores obtained by inputting sequence to Stanford University HIV-1 drug resistance database program. The child (ID 235) in 2018 had minor NNRTI resistance mutation V179T which showed no scores to NNRTI, thus the number of the children for total resistance scores for NNRTI was 28, and that for NRTI + NNRTI was 29. p value: Mann-Whitney U test. (B) Heat map of the estimated level of resistance to NRTI and NNRTI in each child who had HIV-1 with resistance mutations. Resistance levels were defined by the Stanford University HIV-1 drug resistance database recommendation based on the resistance mutation scores.
As shown in Fig. 2B, the children with NRTI-DRMs showed high-level resistance to emtricitabine [61.5% (8/13) in 2014, 87.5% (7/8) in 2017, and 42.9% (3/7) in 2018] and lamivudine [61.5% (8/13) in 2014, 87.5% (7/8) in 2017, and 42.9% (3/7) in 2018]. Most children with NNRTI-DRMs showed high-level resistance to nevirapine [93.9% (31/33) in 2014, 97.4% (37/38) in 2017, 92.9% (26/28) in 2018] and efavirenz [48.5% (16/33) in 2014, 81.6% (31/38) in 2017, 71.4% (20/28) in 2018]. Overall, 64.3% (18/28) of the children had HIV-1 with estimated high-level resistance to emtricitabine and/or lamivudine, respectively, accounting for 10.2% in all 177 children, and 94.9% (94/99) and 67.7% (67/99) children had HIV-1 with estimated high-level resistance to nevirapine and efavirenz, respectively, accounting for 53.1% and 37.9% in all the children.
The Factors Associated with Drug Resistance Mutations
As shown in Table 1, NNRTI-DRMs were found significantly more in children with their mothers’ PMTCT history than those without the history (72.2% vs. 33.3% in 2014, p = 0.004; 63.8% vs. 8.3% in 2017, p < 0.001; 65.8% vs. 20.0% in 2018, p = 0.013), and the same tendency was observed for NRTI-DRMs in 2014 (33.3% vs. 4.8%, p = 0.02). No significant difference was observed in the frequency of NRTI-DRMs and NNRTI-DRMs between female and male children, between the children infected with HIV-1 subtype A and other subtypes, between the children who were breast-fed or not, and between the children aged 1–6 months and above.
In multivariable logistic regression, mother’s PMTCT exposure was significantly related with NRTI-DRMs [adjusted odds ratio (aOR) 10.1, 95% confidence interval 1.2–83.7] and NNRTI-DRMs (aOR 5.2, 95% CI:1.6–16.7) in 2014, but only with NNRTI-DRMs in 2017 (aOR 19.4, 95% CI: 2.3–160.8) and 2018 (aOR 6.7, 95% CI: 1.2–37.2). When analyzed all children as a group, mother’s PMTCT was significantly related with both NRTI-DRMs (aOR 10.1, 95% CI:1.3–76.5) and NNRTI-DRMs (aOR 6.4, 95% CI:2.9–14.4), and breastfeeding with NNRTI-DRMs (2.8, 95% CI:1.2–6.4).
Discussion
In this study, we investigated the trend of HIV-1 DRMs among Kenyan children newly diagnosed with HIV-1 infection in 2014, 2017, and 2018 to guide more successful ART in the children. We found that more than half (54.3–58.0%) of the Kenyan children with HIV-1 continuously harbored DRMs between 2014 and 2018; all of the children with DRMs had NNRTI-DRMs and 11.4%–22.8% of them with NRTI-DRMs; most of the children with DRMs showed high-level resistance to NNRTI (94.9% and 67.7% to nevirapine and efavirenz, respectively); and NNRTI-DRMs of the children were significantly associated with their mother’s PMTCT history in all years.
In this study, overall 56.5% of the Kenyan children newly diagnosed with HIV-1 had NNRTI-DRMs, and 15.8% of them had NRTI-DRMs simultaneously in 2014, 2017, and 2018. The DRMs prevalence did not differ among these years, despite the decrease in the number of the children newly diagnosed with HIV-1 from 11, 000 in 2014 to 4,500 by 2018 in Kenya14). These results were comparable to those of Zambian study, in which 65.3% of the children newly diagnosed with HIV-1 had NNRTI-DRMs and 12.2% of them had NRTI-DRMs in 2015–201825). The frequency of HIV-1 DRMs in Kenya was also comparable to those reported in infants newly diagnosed with HIV-1 from other Sub-Saharan countries such as Malawi (NNRTI-DRMs/NRTI-DRMs: 68.0%/25.1% in 2016), Uganda (57.4%/18.4% in 2017), Cameroon (46.8%/10.0% in 2014), and Nigeria (49.0%/22.5% in 2016)27). Thus, high frequencies of DRMs, especially NNRTI-DRMs, continued to be found in newly HIV-diagnosed children in Sub-Saharan countries. Although it has been reported that NNRTI-DRMs before starting ART may not be associated with virologic failure28), this high frequency of the DRMs might have a negative impact on the ART in the children. Therefore, checking DRMs before starting ART and/or using non-NNRTI-containing regimen for ART should be considered in children with HIV-1 in Sub-Saharan countries.
At present, World Health Organization recommends abacavir/lamivudine (ABC/3TC: NRTIs) plus backbone anti-retroviral drug without NNRTI as the first- and second-line ART regimen for HIV-1-infected children29), and the Kenya HIV Prevention and Treatment Guidelines 2022 also recommends ABC/3TC plus dolutegravir (integrase inhibitor) as preferred regimen for children over 4 weeks age30). However, considering that 57.3% of the children and adolescents with HIV-1 were still treated with NNRTI-containing regimen in 2018–2020 in Kenya31), routine plasma viral load measurement would be needed to investigate the impact of the DRMs on ART as well as to monitor the effect of the treatment in the HIV-infected children in Kenya27).
In the current study, we found that mothers’ PMTCT was positively related with HIV-1 NNRTI-DRMs and NRTI-DRMs in the children newly diagnosed with HIV-1 infection in Kenya; that the appearance of HIV-1 strains with NNRTI-DRMs such as Y181C and K103N/S in the children was closely related with the usage of nevirapine and efavirenz, respectively, for their mothers’ PMTCT; and that breastfeeding was positively related with the NNRTI-DRMs. These findings suggest that HIV-1 with DRMs might be emerged by ART-driven selection in the pregnant women with HIV-1 and vertically transmitted to their children as previously reported17), and that HIV-1 strains with NNRTI-DRMs might be transmitted from mother to child more efficiently via breast milk than those with NRTI-DRMs. However, we could not ascertain the transmission of HIV-1 strains with DRMs from mother to child, since the mother’s blood samples were not available.
Due to insufficient information on children’s prophylaxis, we could not fully analyze the relationship of children’s prophylaxis with DRMs in this study. Twenty-three percent and 2.3% of the mothers whose children had HIV-1 strains with NNRTI-DRMs and NRTI-DRMs, respectively, had no history of PMTCT in this study. This result suggests that infant-prophylaxis may also play a critical role in the emergence of HIV-1 strain with DRMs. This is consistent with previous reports that the prevalence and pattern of DRMs were different between mothers and children25), and that most resistance mutations to nevirapine in children are selected de novo when the virus is exposed to nevirapine rather than being transmitted from their mothers32).
The study had some limitations. Incomplete demographics and clinical information made it difficult to link drug resistance findings to the aforementioned characteristics. In addition, PCR assays on remnant dried blood spots was a challenge due to compromised sample integrity and/or low viral load. Since this was a cross-sectional study, a longitudinal study would be needed to investigate the impact of the DRMs on the ART in these children.
Conclusion
Preventing the emergence of drug-resistant virus is one of the WHO’s strategic objectives of the Global Action Plan on HIV-1 to maintain efficacy of the limited pool of antiretroviral drugs currently available. The high levels of observed HIV-1 DRMs, especially the high proportion with high level resistance to nevirapine and efavirenz among newly diagnosed children in Kenya highlight the need for baseline DRMs screening prior to initiating ART in this population. In addition, routine monitoring of plasma viral load would be necessary to identify and subsequently predict early viral failure in the children on ART.
Acknowledgments
This study was supported by the Kenya goverment through Kenya Medical Research Institute Internal Research Grant (IRG/104/4) and Kanazawa University under support from JSPS. The authors acknowledge KEMRI Early Infant Diagnosis team for their technical support.
Author Contributions
SK conceptualized the idea, designed, carried out molecular genetics and drafted the manuscript. XB, QT, DO, JK, RL, VO, JM, MS and SC were involved in performing the laboratory experiments, data analysis and manuscript editing. MM was involved in EID sample access and data analysis. XB, ES, MT and HI were involved in the study design, coordination and editing the manuscript and final approval. The authors read and approved the final manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Purohit V, Rapaka RS, Shurtleff D. Mother-to-child transmission (MTCT) of HIV and drugs of abuse in post-highly active antiretroviral therapy (HAART) era. J Neuroimmune Pharmacol 5; 507–515: 2010. doi: 10.1007/s11481-010-9242-7.
- 2. UNAIDS DATA 2018. https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf (accessed 5th May 2021)
- 3. Coutsoudis A, Kwaan L, Thomson M. Prevention of vertical transmission of HIV-1 in resource-limited settings. Expert Rev Anti Infect Ther 8; 1163–1175: 2010. doi: 10.1586/eri.10.94.
- 4. Children, HIV and AIDS, Global snapshot, DECEMBER 2018. Unicef. https://data.unicef.org/resources/children-hiv-and-aids-global-and-regional-snapshots/2018/11/Global-snapshot-2018.pdf (accessed 5th May 2021)
- 5. WHO: Mother-to-child transmission of HIV. World Health Organization. https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/prevention/mother-to-child-transmission-of-hiv. (accessed 5th May 2021)
- 6. Salou M, Butel C, Konou AA, et al. High rates of drug resistance among newly diagnosed HIV-infected children in the national prevention of mother-to-child transmission program in Togo. Pediatr Infect Dis J 35; 879–885: 2016. doi: 10.1097/INF.0000000000001203.
- 7. UNAIDS: Miles to go: global AIDS update 2018. https://www.unaids.org/en/20180718_GR2018. (accessed 5th May 2021)
- 8. Mofenson LM, Munderi P. Safety of antiretroviral prophylaxis of perinatal transmission for HIV-infected pregnant women and their infants. J Acquir Immune Defic Syndr 30; 200–215: 2002. doi: 10.1097/00042560-200206010-00010.
- 9. Newell ML, Thorne C. Antiretroviral therapy and mother-to-child transmission of HIV-1. Expert Rev Anti Infect Ther 2; 717–732: 2004. doi: 10.1586/14789072.2.5.717.
- 10. John-Stewart GC. Infant feeding and prevention of mother-to-child transmission of HIV-1. Curr Opin HIV AIDS 3; 173–179: 2008. doi: 10.1097/COH.0b013e3282f50bc6.
- 11. Yah CS, Tambo E. Why is mother to child transmission (MTCT) of HIV a continual threat to new-borns in sub-Saharan Africa (SSA). J Infect Public Health 12; 213–223: 2019. doi: 10.1016/j.jiph.2018.10.008.
- 12. National AIDS & STI Control Program, Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya, 2018 Edition. https://www.chskenya.org/wp-content/uploads/2018/08/Kenya-ARV-Guidelines-2018.pdf. (accessed 5th May 2021)
- 13. Khamadi SA, Lihana RW, Osman S, et al. Genetic diversity of HIV type 1 along the coastal strip of Kenya. AIDS Res Hum Retroviruses 25; 919–923: 2009. doi: 10.1089/aid.2009.0005.
- 14. HIV ESTIMATES WITH UNCERTAINTY BOUNDS 1990-PRESENT, spreadsheet. UNAIDS. https://www.unaids.org/en/resources/fact-sheet. (accessed 6th November 2023)
- 15. Lel R, Ngaira J, Lihana R, et al. HIV-1 drug resistance mutations among infants born to HIV-positive mothers in Busia, Kenya. AIDS Res Hum Retroviruses 30; 236–238: 2014. doi: 10.1089/AID.2014.0158.
- 16. Ton Q, Frenkel L. HIV drug resistance in mothers and infants following use of antiretrovirals to prevent mother-to-child transmission. Curr HIV Res 11; 126–136: 2013. doi: 10.2174/1570162x11311020005.
- 17. Rojas Sánchez P, Holguín A. Drug resistance in the HIV-1-infected paediatric population worldwide: a systematic review. J Antimicrob Chemother 69; 2032–2042: 2014. doi: 10.1093/jac/dku104.
- 18. Compagno F, Naegele K, Kahlert CR, et al. The rate of mother-to-child transmission of antiretroviral drug-resistant HIV strains is low in the Swiss Mother and Child HIV Cohort Study. Swiss Med Wkly 149; w20059: 2019. doi: 10.4414/smw.2019.20059.
- 19. Bennett DE, Myatt M, Bertagnolio S, et al. Recommendations for surveillance of transmitted HIV drug resistance in countries scaling up antiretroviral treatment. Antivir Ther 13 Suppl 2; 25–36: 2008.
- 20. Jordan MR, Bennett DE, Bertagnolio S, et al. World Health Organization surveys to monitor HIV drug resistance prevention and associated factors in sentinel antiretroviral treatment sites. Antivir Ther 13 Suppl 2; 15–23: 2008.
- 21. Khamadi S, Okoth V, Lihana R, et al. Rapid identification of infants for antiretroviral therapy in a resource poor setting: the Kenya experience. J Trop Pediatr 54; 370–374: 2008. doi: 10.1093/tropej/fmn036.
- 22. Lihana RW, Lwembe RM, Bi X, et al. Efficient monitoring of HIV-1 vertically infected children in Kenya on first-line antiretroviral therapy. J Clin Virol 52; 123–128: 2011. doi: 10.1016/j.jcv.2011.06.014.
- 23. Tang MW, Liu TF, Shafer RW. The HIVdb system for HIV-1 genotypic resistance interpretation. Intervirology 55; 98–101: 2012. doi: 10.1159/000331998.
- 24. Hackett S, Teasdale CA, Pals S, et al. Drug resistance mutations among South African children living with HIV on WHO-recommended ART regimens. Clin Infect Dis 73; e2217–e2225: 2021. doi: 10.1093/cid/ciaa1068.
- 25. Bennett SJ, Chunda-Liyoka C, Poppe LK, et al. High nonnucleoside reverse transcriptase inhibitor resistance levels in HIV-1-infected Zambian mother-infant pairs. AIDS 34; 1833–1842: 2020. doi: 10.1097/QAD.0000000000002614.
- 26. Wensing AM, Calvez V, Ceccherini-Silberstein F, et al. 2022 update of the drug resistance mutations in HIV-1. Top Antivir Med 30; 559–574: 2022.
- 27. World Health Organization. HIV Drug Resistance Report 2021. https://www.who.int/publications/i/item/9789240038608. (accessed 18th December 2023)
- 28. Higa N, Pelz A, Birch D, et al. Association of virologic failure and nonnucleoside reverse transcriptase inhibitor resistance found in antiretroviral-naive children infected with human immunodeficiency virus and given efavirenz-based treatment. J Pediatric Infect Dis Soc 9; 261–264: 2020. doi: 10.1093/jpids/piz038.
- 29. Consolidated Guidelines on HIV prevention, teseting, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva, Switzerland: World Health Organization. https://www.who.int/news/item/16-07-2021-who-publishes-new-consolidated-hiv-guidelines-for-prevention-treatment-service-delivery-monitoring. (accessed 20th June 2023)
- 30. Kenya HIV Prevention and Treatment Guidelines, 2022. 2022 Edition. Ministry of Health Kenya. https://www.differentiatedservicedelivery.org/wp-content/uploads/Kenya-ARV-Guidelines-2022-Final-1.pdf. (accessed 18th December 2023)
- 31. Tsikhutsu I, Bii M, Dear N, et al. Prevalence and correlates of viral load suppression and human immunodeficiency virus (HIV) drug resistance among children and adolescents in south rift valley and Kisumu, Kenya. Clin Infect Dis 75; 936–944: 2022. doi: 10.1093/cid/ciac059.
- 32. Phan CT, Pham HV, Bi X, et al. Genetic analyses of HIV-1 strains transmitted from mother to child in Northern Vietnam. AIDS Res Hum Retroviruses 31; 797–805: 2015. doi: 10.1089/AID.2014.0335.


