Abstract
INTRODUCTION: Pancreatic arteriovenous malformation is a rare disease characterized by abnormal vascular connections between arteries and veins. Despite the risk of increased intraoperative blood loss due to abundant blood flow, surgical resection remains the only curative modality for pancreatic arteriovenous malformation. We present a case of pancreatic arteriovenous malformation in which subtotal stomach-preserving pancreaticoduodenectomy was successfully performed following selective arterial embolization to reduce intraoperative blood loss.
CASE PRESENTATION: A 53-year-old Southeast Asian man was referred to our hospital with abdominal pain. Contrast-enhanced computed tomography revealed enhancement of the pancreatic head and superior mesenteric vein in the early arterial phase, suggesting the presence of an arteriovenous malformation. Maximum intensity projection images and angiography revealed arterial branches from the gastroduodenal artery and superior mesenteric artery to the arteriovenous malformation, subsequently draining into the portal venous circulation. We supposed that abdominal pain resistant to medical treatment was due to the pancreatic arteriovenous malformation, and surgical resection was deemed necessary. Subtotal stomach-preserving pancreaticoduodenectomy was safely performed on the day after arterial embolization of pancreatic arterial branches to reduce intraoperative blood loss. The procedure resulted in an intraoperative blood loss of 336g. The patient was discharged on the 16th postoperative day with no complications and has not experienced abdominal pain since.
CONCLUSIONS: Selective arterial embolization prior to pancreaticoduodenectomy against pancreatic arteriovenous malformation is a safe and feasible procedure to reduce intraoperative blood loss.
Abbreviations
AIPDA
anterior inferior pancreaticoduodenal artery
ASPDA
anterior superior pancreaticoduodenal artery
AVM
arteriovenous malformation
CT
computed tomography
GDA
gastroduodenal artery
MIP
maximum intensity projection
PIPDA
posterior inferior pancreaticoduodenal artery
PSPDA
posterior superior pancreaticoduodenal artery
SMA
superior mesenteric artery
SMV
superior mesenteric vein
SSPPD
subtotal stomach preserving pancreaticoduodenectomy
INTRODUCTION
Arteriovenous malformation (AVM) is a rare disease characterized by abnormal vascular connections between feeding arteries and veins. AVMs of the gastrointestinal system are most frequently observed in the cecum and right colon. In contrast, pancreatic AVMs, first reported by Halpern et al. in 1968,1) are relatively uncommon. AVM causes abdominal pain, upper gastrointestinal ulcers, gastrointestinal bleeding, and pancreatitis. Surgical resection is often necessary in patients resistant to medical treatment. However, the risk of excessive intraoperative blood loss due to abundant arterial blood flow into pancreatic AVMs should be considered. To mitigate this risk, it is important to reduce the abundant arterial blood flow by arterial embolization prior to surgical resection.
Herein, we present a case of pancreatic head AVM that was successfully treated with selective arterial embolization followed by subtotal stomach-preserving pancreaticoduodenectomy (SSPPD).
CASE PRESENTATION
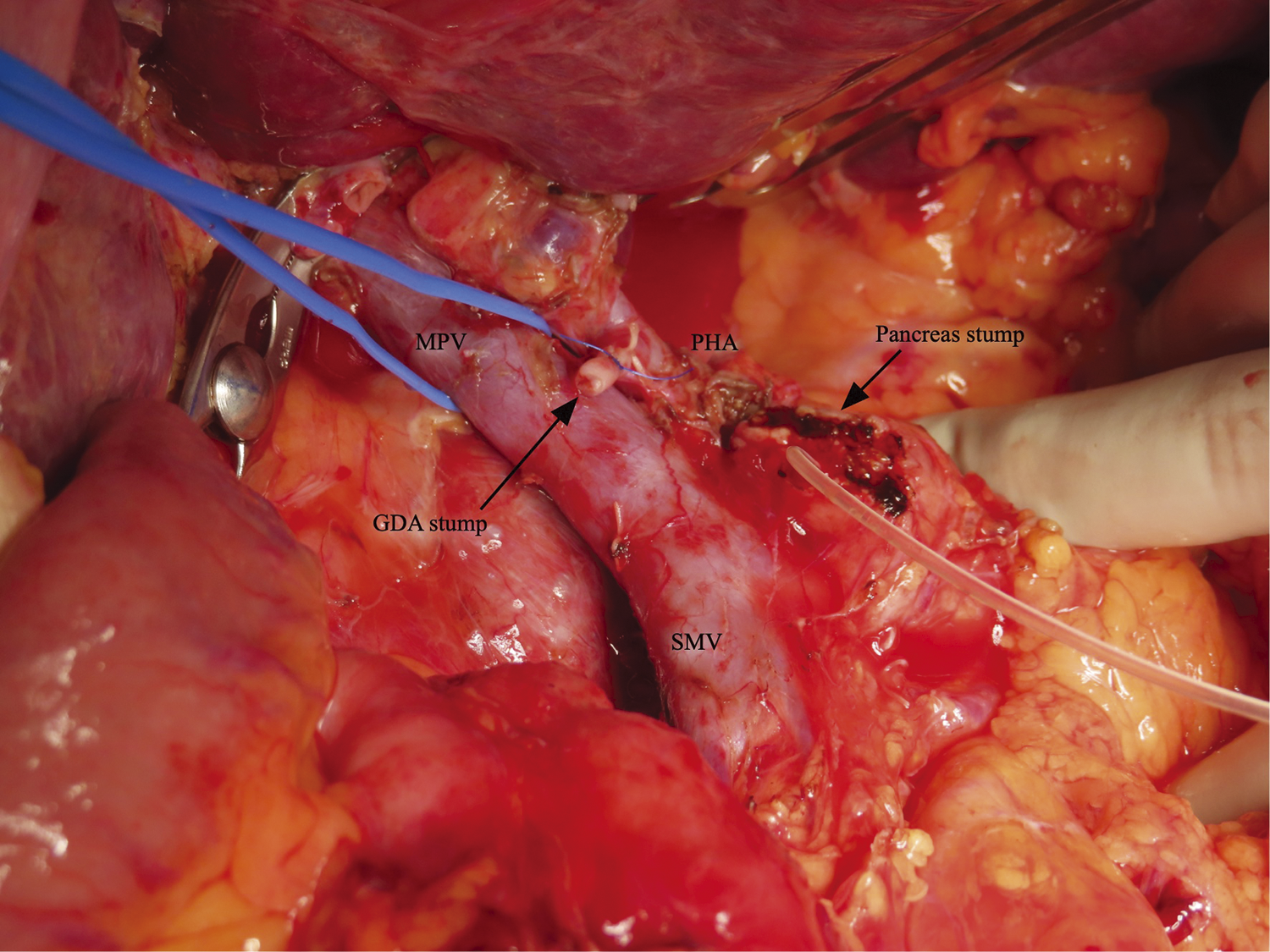
A 53-year-old Southeast Asian man presented to a local physician with abdominal pain. He had no specific previous medical history or prior treatments. Abdominal ultrasonography revealed hepatomegaly. Since his abdominal pain was resistant to any medication, he was referred to our hospital for a comprehensive examination and subsequent treatment. Upper gastrointestinal endoscopy revealed an ulcer in the duodenal bulb (Fig. 1), although he had been taking a histamine H2 antagonist. Helicobacter pylori infection was not detected in any examinations. Plain abdominal computed tomography (CT) revealed intrahepatic reticulated calcification (Fig. 2A), indicating the presence of Schistosomiasis japonica. Contrast-enhanced CT demonstrated early enhancement of the pancreatic head and superior mesenteric vein in the late arterial phase (Fig. 2B). Maximum intensity projection (MIP) images revealed blood flow from the gastroduodenal artery (GDA) and superior mesenteric artery (SMA) to tortuous vessels, subsequently draining into the portal venous circulation (Fig. 2C). Based on these results, we diagnosed the patient with pancreatic AVM. His abdominal pain persisted despite treatment with oral acetaminophen and proton pump inhibitors. Continuous intravenous fentanyl injection relieved his abdominal pain. However, the duodenal ulcer did not improve upon the second upper gastrointestinal endoscopic examination. We considered that the duodenal ulcer was likely caused by the abnormal peri-pancreatic circulation due to the pancreatic AVM, and surgical resection of the pancreatic head was needed to alleviate his symptoms. Considering the risk of increased intraoperative blood loss due to abundant arterial blood flow caused by the presence of the AVM in the pancreatic head, preoperative arterial embolization was scheduled for the day before surgery. Angiography revealed the existence of the AVM in the pancreatic head, which was supplied by the GDA and anterior/posterior pancreaticoduodenal artery (AIPDA/PIPDA) branching from the SMA (Figs. 3A and 3B). After selective arterial embolization of the GDA, APIDA, and PIPDA using gelatin sponges (Serescue; Nippon Kayaku, Tokyo, Japan), AVM was no longer visible under angiography of the celiac axis or SMA (Figs. 3C and 3D). We did not use coils because we were concerned that intra-arterial coils might hamper arterial ligation and division. No complications occurred after arterial embolization until the next day. Since there were no abnormal laboratory data just before surgery, SSPPD was performed. After laparotomy, GDA and peripancreatic arterial branches from SMA were firstly divided to reduce the pancreatic blood flow. The intraoperative findings did not demonstrate any impact of arterial embolization on the pancreatic parenchyma (Fig. 4). The operation time was 453 min, and the blood loss was 336g. The patient was discharged on the 16th postoperative day with no complications and has not experienced abdominal pain since.
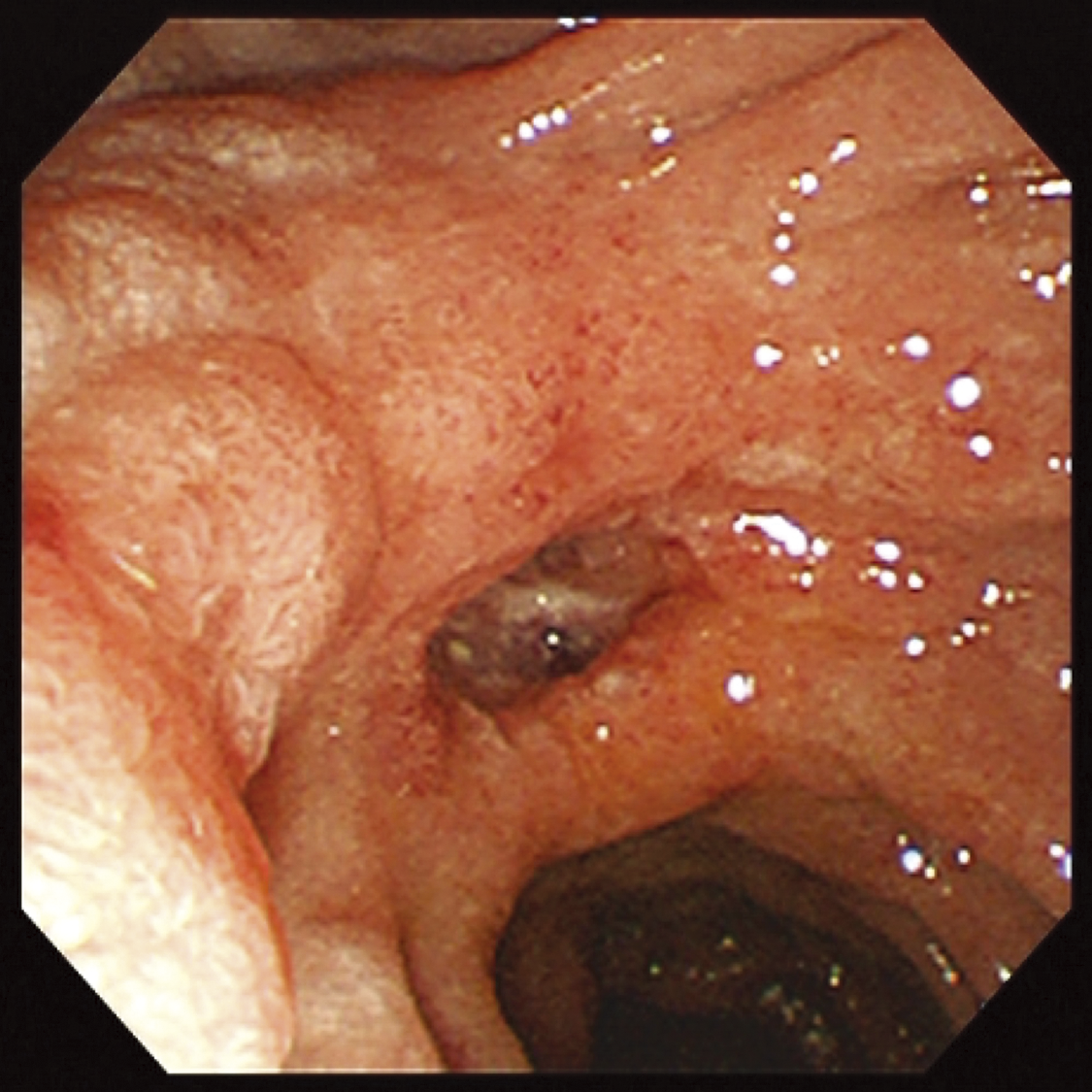
Fig. 1
Findings of upper gastrointestinal endoscopy. Upper gastrointestinal endoscopy revealed a large ulcer at the duodenal bulb.

Fig. 2
Findings of computed tomography (CT). (A) Non-enhanced CT showed the presence of intrahepatic reticulated calcification, indicating the presence of Schistosomiasis japonica. (B) Contrast-enhanced CT demonstrated the enhancement of the pancreatic head (arrow) and main portal vein (arrowhead) in the late arterial phase. (C) Maximum intensity projection images revealed blood flow from the gastroduodenal artery and superior mesenteric artery to the main portal vein.
Pathological examination revealed the presence of dilated vessels of unequal sizes, which was consistent with the features of pancreatic AVM (Fig. 5A). Eggs of Schistosoma japonicum were observed within pancreatic tissue and in the dissected lymph node around the pancreatic head (Fig. 5B).

Fig. 5
Pathological findings of resected specimens (hematoxylin and eosin staining). (A) Dilated vessels of unequal size were found in the pancreatic parenchyma, consistent with the finding of arteriovenous malformation. (B) Eggs of Schistosoma japonicum were observed in the resected pancreatic tissue.
DISCUSSION
AVM is a rare disease characterized by abnormal vascular connections between arteries and veins. Pancreatic AVM was first reported by Halpern et al. in 1968.1) Previous reports showed that pancreatic AVMs mainly occur in middle-aged Asian males. Although pancreatic AVMs occurred throughout the pancreatic parenchyma, 59.4% and 33.3% of pancreatic AVMs were found in the head and body/tail, respectively. In the remaining 7.2% of cases, pancreatic AVMs occupied the entire pancreatic parenchyma from head to tail.2,3) The primary cause of pancreatic AVMs is congenital origin (90.5%), including Osler–Weber–Rendu disease. Congenital AVMs are believed to be caused by abnormal formation of arteriovenous plexuses during embryonic development.2) The remaining 9.5% of this disease is thought to be acquired and caused by pancreatitis, trauma, and tumors.4) In this case, radiological and pathological examinations revealed the presence of Schistosoma japonicum in the hepatic and pancreatic parenchyma and peri-pancreatic lymph nodes. Vascular remodeling in the pancreatic parenchyma may be derived by the signaling cascade via several chemical mediators, such as interleukin-13 and transforming growth factor-β, triggered by Schistosoma antigen.5,6) Based on these findings, Schistosomiasis japonica may be a causal factor for the development of pancreatic AVMs.
Several radiological examinations have been performed to diagnose pancreatic AVMs. Recently, due to advancements in CT (time and spatial resolution) and image-analyzing software, contrast-enhanced multi-detector CT has become sufficient for diagnosing pancreatic AVMs.7) Three-dimensional analysis of contrast-enhanced multi-detector row CT images easily identifies the input arteries and the drainage vessels. Since we had already identified the AVM-associated vessels using enhanced multi-detector row CT images and computer-analyzed images, especially in MIP images,8) interventional angiography was easily performed.
Surgical resection and arterial embolization have been reported as treatment modalities for pancreatic AVMs. Although arterial embolization is a minimally invasive procedure, it is difficult to embolize all AVM-associated arteries. When conservative treatment and arterial embolization are ineffective, surgical resection should be considered. However, excessive intraoperative blood loss due to abundant peri-pancreatic arterial flow is a serious hazard. In the literature, 2 studies reported that intraoperative blood loss during pancreaticoduodenectomy for AVM in the pancreatic head without arterial embolization was 1260 and 1950g, respectively.9,10) Additionally, 2 reports described the effectiveness of preoperative arterial embolization in reducing the intraoperative blood loss during resection of AVM in the pancreatic head. Intraoperative blood loss during pancreaticoduodenectomy for AVM in the pancreatic head with preoperative arterial embolization of the GDA alone was 605g in 1 report,11) and another report did not describe the blood loss.12) In contrast to the 2 reports, we almost completely embolized AVM-related arteries via GDA, AIPDA, and PIPDA. After complete embolization, we considered the risks of arterial embolization, such as pancreatic necrosis or pancreatitis due to arterial ischemia.13) To avoid such risks associated with ischemia, we scheduled surgery for the day after arterial embolization. Fortunately, our patient did not show any complications related with arterial embolization until the next morning following embolization. As a result, SSPPD was safely performed, and the intraoperative blood loss was 336g. Thus, we strongly believe that the preoperative arterial embolization is safe and effective in reducing the intraoperative blood loss during resection of pancreatic AVMs. Moreover, we addressed the responsible arteries first to minimize the risk of massive bleeding as much as possible because the artery-first approach could reduce the intraoperative blood loss.14,15) Based on these points, we recommend planned arterial embolization before resection of pancreatic AVMs.
CONCLUSIONS
In this case, selective arterial embolization before SSPPD for pancreatic head AVM was performed to reduce intraoperative blood loss. No complications were observed after arterial embolization or surgery. Selective arterial embolization prior to pancreaticoduodenectomy for pancreatic AVMs is a safe and feasible procedure to reduce intraoperative blood loss.
DECLARATIONS
Funding
This study did not receive any funding.
Authors’ contributions
RK was involved in the clinical practice, conception, design, and acquisition of data.
TS, JK, DS, YO, KK, and ShiotoS were involved in the clinical practice and conception and design.
TK, OJ, AU, AF, KM, and ShohachiS approved the final version of the manuscript.
All authors have read and approved the manuscript, and they are responsible for the manuscript.
Availability of data and materials
The datasets used in this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
This work does not require ethical considerations or approval.
Consent for publication
Informed consent was obtained from the patient for publication of this case report.
Competing interests
The authors declare that they have no competing interests.
REFERENCES
- 1) Halpern M, Turner AF, Citron BP. Hereditary hemorrhagic telangiectasia. An angiographic study of abdominal visceral angiodysplasias associated with gastrointestinal hemorrhage. Radiology 1968; 90: 1143–9.
- 2) Sharma M, Bedi MM, Mahesh S, et al. Arteriovenous malformation of the pancreatic head--difficulties in diagnosis and treatment. Indian J Gastroenterol 2011; 30: 46–8.
- 3) Song KB, Kim SC, Park JB, et al. Surgical outcomes of pancreatic arteriovenous malformation in a single center and review of literature. Pancreas 2012; 41: 388–96.
- 4) Nishiyama R, Kawanishi Y, Mitsuhashi H, et al. Management of pancreatic arteriovenous malformation. J Hepatobiliary Pancreat Surg 2000; 7: 438–42.
- 5) Graham BB, Kumar R. Schistosomiasis and the pulmonary vasculature (2013 Grover Conference series). Pulm Circ 2014; 4: 353–62.
- 6) Kolosionek E, King J, Rollinson D, et al. Schistosomiasis causes remodeling of pulmonary vessels in the lung in a heterogeneous localized manner: detailed study. Pulm Circ 2013; 3: 356–62.
- 7) Onozawa S, Miyauchi R, Takahashi M, et al. An Update of treatment of pancreatic arteriovenous malformation. Interv Radiol (Higashimatsuyama) 2023; 8: 49–55.
- 8) Endo K, Sata N, Shimura K, et al. Pancreatic arteriovenous malformation: a case report of hemodynamic and three-dimensional morphological analysis using multi-detector row computed tomography and post-processing methods. JOP 2009; 10: 59–63.
- 9) Abe T, Suzuki N, Haga J, et al. Arteriovenous malformation of the pancreas: a case report. Surg Case Rep 2016; 2: 6.
- 10) Hakoda H, Kawaguchi Y, Miyata Y, et al. Surgical resection of arteriovenous malformation of the pancreatic head with acute pancreatitis: a case report. J Surg Case Rep 2022; 2022: rjac427.
- 11) Fukami Y, Kurumiya Y, Mizuno K, et al. Pancreatic arteriovenous malformation with portal vein thrombosis. Surgery 2015; 157: 171–2.
- 12) Hosogi H, Ikai I, Hatano E, et al. Pancreatic arteriovenous malformation with portal hypertension. J Hepatobiliary Pancreat Surg 2006; 13: 344–6.
- 13) Wu W, An FD, Piao CL, et al. Management of pancreatic arteriovenous malformation: case report and literature review. Medicine (Baltimore) 2021; 100: e27983.
- 14) Nakao A, Takagi H. Isolated pancreatectomy for pancreatic head carcinoma using catheter bypass of the portal vein. Hepatogastroenterology 1993; 40: 426–9.
- 15) Weitz J, Rahbari N, Koch M, et al. The artery first approach for resection of pancreatic head cancer. J Am Coll Surg 2010; 210: e1–4.
 https://orcid.org/0000-0003-4530-377X
https://orcid.org/0000-0003-4530-377X
 https://orcid.org/0000-0002-6637-6997
https://orcid.org/0000-0002-6637-6997